Back to Journals » International Journal of Nanomedicine » Volume 19
Nanoparticle-Based Combinational Strategies for Overcoming the Blood-Brain Barrier and Blood-Tumor Barrier
Authors Lim SH , Yee GT, Khang D
Received 5 December 2023
Accepted for publication 22 February 2024
Published 13 March 2024 Volume 2024:19 Pages 2529—2552
DOI https://doi.org/10.2147/IJN.S450853
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor R.D.K. Misra
Su Hyun Lim,1,2 Gi Taek Yee,3 Dongwoo Khang1,2,4
1Department of Health Sciences and Technology, GAIHST, Gachon University, Incheon, 21999, South Korea; 2Lee Gil Ya Cancer and Diabetes Institute, Gachon University, Incheon, 21999, South Korea; 3Department of Neurosurgery, Gil Medical Center, Gachon University, School of Medicine, Incheon, 21565, South Korea; 4Department of Physiology, School of Medicine, Gachon University, Incheon, 21999, South Korea
Correspondence: Dongwoo Khang, Department of Physiology, School of Medicine, Gachon University, Incheon, 21999, South Korea, Tel +82 32 899 1525, Email [email protected] Gi Taek Yee, Department of Neurosurgery, College of Medicine, Gil Medical Center, Gachon University, Incheon, 21565, South Korea, Tel +82-32-460-3304, Email [email protected]
Abstract: The blood-brain barrier (BBB) and blood-tumor barrier (BTB) pose substantial challenges to efficacious drug delivery for glioblastoma multiforme (GBM), a primary brain tumor with poor prognosis. Nanoparticle-based combinational strategies have emerged as promising modalities to overcome these barriers and enhance drug penetration into the brain parenchyma. This review discusses various nanoparticle-based combinatorial approaches that combine nanoparticles with cell-based drug delivery, viral drug delivery, focused ultrasound, magnetic field, and intranasal drug delivery to enhance drug permeability across the BBB and BTB. Cell-based drug delivery involves using engineered cells as carriers for nanoparticles, taking advantage of their intrinsic migratory and homing capabilities to facilitate the transport of therapeutic payloads across BBB and BTB. Viral drug delivery uses engineered viral vectors to deliver therapeutic genes or payloads to specific cells within the GBM microenvironment. Focused ultrasound, coupled with microbubbles or nanoparticles, can temporarily disrupt the BBB to increase drug permeability. Magnetic field-guided drug delivery exploits magnetic nanoparticles to facilitate targeted drug delivery under an external magnetic field. Intranasal drug delivery offers a minimally invasive avenue to bypass the BBB and deliver therapeutic agents directly to the brain via olfactory and trigeminal pathways. By combining these strategies, synergistic effects can enhance drug delivery efficiency, improve therapeutic efficacy, and reduce off-target effects. Future research should focus on optimizing nanoparticle design, exploring new combination strategies, and advancing preclinical and clinical investigations to promote the translation of nanoparticle-based combination therapies for GBM.
Keywords: glioblastoma, blood-brain barrier, blood-tumor barrier, nanoparticle, combination strategy, ultrasound-wave, magnetic field, intranasal drug delivery
Graphical Abstract:
Introduction
The blood-brain barrier (BBB), a highly specialized and selective biological barrier that serves as the interface between the body’s general blood circulation and the central nervous system (CNS). The BBB’s primary function is to regulate the influx of necessary substances like nutrients and oxygen to the brain, while restricting the entry of potentially harmful compounds, including many pharmaceuticals.1–3 Although the BBB is critical for maintaining the stable internal environment of the brain, it also poses significant challenges for treating neurological conditions, particularly brain tumors such as glioblastoma multiforme (GBM).4–6
GBM is the epitome of malignant primary brain tumors, characterized by its aggressive behavior and dismal prognosis, with median survival lingering around 15 months post standard interventions, surgery, radiotherapy, and chemotherapy.7 One of the major obstacles to successful treatment of glioblastoma is the limited efficacy of systemic drug delivery due to the intrinsic selectivity of the BBB and the additional blood-tumor barrier (BTB), which is formed by the abnormal vasculature and increased interstitial pressure within the tumor microenvironment.3,8 These barriers substantially limit the effective delivery of chemotherapeutic drugs to tumor sites, leading to poor treatment outcomes.9,10
To overcome the BBB and BTB and to improve the delivery of therapeutic agents in glioblastoma cells, various drug delivery strategies have been developed.11,12 These strategies include exploiting natural transcytosis mechanisms, intranasal routes that bypass the BBB, and, in particular, nanoparticle (NP)-based delivery systems.5,13
Drug delivery systems based on nanoparticles (NPs) are attracting significant attention due to their potential to improve both drug efficacy and safety. These systems offer promising advances in targeting and penetrating the complex environment of brain tumors, thereby improving therapeutic outcomes.14,15 These systems can be designed to overcome the physical and physiological barriers of the BBB and BTB and enable the selective delivery of therapeutic drugs to tumor cells while minimizing off-target effects on healthy tissues.8,16 Furthermore, NPs can be engineered to encapsulate multiple drugs, for use in combination therapies, which can improve the tumor treatment outcomes by targeting multiple signaling pathways and reducing drug resistance.14,17,18 The advantages of NP-based combination strategies include the combination of the properties of diverse types of NPs, enhancing the targeting ability and stability of each drug delivery system.19
However, despite the significant progress in developing drug delivery strategies for glioblastoma treatment, there remain several challenges and limitations that need to be addressed.20,21 These include the limited unique tumor microenvironment, the genetic and molecular heterogeneity of glioblastoma tumors.22 Progression of GBM alters the BBB, leading to the formation of the BTB, which further limits the efficacy of anti-tumor drugs. About 98% of small molecules and virtually all biological macromolecule drugs, including growth factors and monoclonal antibodies, are unable to penetrate the central nervous system to achieve therapeutic results.
This review aims to discuss the potential of NP-based combination strategy to overcome the BBB for the treatment of GBM. The review will provide an overview of the BBB and BTB, the challenges and limitations of conventional drug delivery strategies, and the potential advantages of NP-based drug delivery systems to enhance drug delivery across the BBB and highlight the opportunities and challenges of combining different drug delivery strategies for effective glioblastoma treatment.
Glioblastoma (GBM) and Blood Brain Barrier (BBB)
GBM is the most common and aggressive form of primary brain tumor diagnosed in adults, characterized by rapid progression and significant clinical challenges.23 It has a high mortality rate and limited treatment options due to the presence of the BBB, a selectively permeable membrane that separates the brain from the systemic circulation.24 The BBB is formed by endothelial cells lining the brain capillaries, which are tightly interconnected by tight junctions and surrounded by pericytes and astrocytes.5,25 The BBB restricts the entry of many substances, including chemotherapeutic agents, into the brain, thus limiting their efficacy in treating GBM.26 This section will provide an overview of GBM and the BBB and their interactions.
Glioblastoma
Glioblastoma is a malignant brain tumor that arises from glial cells, the supporting cells of the central nervous system, with median survival time less than 2 years. It is classified as a diffuse astrocytic glioma that is IDH-wild type and H3-wild type, tumors which originates from astrocytes, according to the World Health Organization (WHO) classification system.27 GBM is highly aggressive and invasive, even with current standard of care treatments, including surgery, radiation therapy, and chemotherapy.23,28 The prognosis for GBM patients is poor, and it is urgently needed for the development of novel drug delivery strategy enhance therapeutic efficacy.29
Blood-Brain Barrier (BBB)
The BBB is a highly selective barrier that regulates the exchange of substances between the brain and the bloodstream. The BBB is composed of specialized endothelial cells that line the brain capillaries and are interconnected by tight junctions, which prevent the paracellular diffusion of molecules.25 In addition to endothelial cells, pericytes and astrocytes also contribute to the structure and function of the BBB.25,30 Pericytes are contractile cells that surround the endothelial cells and regulate blood flow and vascular permeability, while astrocytes provide structural and metabolic support to the endothelial cells.30
The BBB is highly selective, allowing the entry of essential nutrients, such as glucose and amino acids, while preventing the entry of potentially harmful substances, such as pathogens and toxins. The BBB also restricts the entry of many drugs and chemotherapeutic agents into the brain, limiting their efficacy in treating GBM. The BBB’s ability to exclude drugs is due to the presence of efflux transporters, such as P-glycoprotein (P-gp), that actively transport drugs out of the brain.31 In addition, the tight junctions between endothelial cells prevent the paracellular diffusion of many drugs.32 The BBB is a major obstacle in the treatment of GBM, and strategies to overcome the BBB are needed to improve the efficacy of current therapies.
Blood-Brain Tumor Barrier (BTB)
The BTB is a specialized barrier that forms within brain tumors, specifically gliomas, to protect the tumor from the surrounding brain tissue. In glioblastoma, the BTB exhibits atypical features that affect its integrity, including diminished tight junctions (TJs) that normally tightly close endothelial cells, irregular coverage of blood vessels by pericytes, and the emergence of stem cell-derived pericytes. These abnormalities contribute to the degradation of the vascular structure as the tumor progresses. Furthermore, increased reactive astrocytes, accompanied by retraction of their endfeet, along with deterioration of the basal lamina, further undermine the stability of the BTB.3
The disruption of the BBB is evidenced by the higher drug accumulation in the brain tumor compared to the unaffected brain. It is also indirectly evidenced by the detection of brain tumor markers in the circulation, such as circulating tumor cells from gliomas. BBB disruption in gliomas is heterogeneous, occurs mainly in the tumor core, and is closely related to the stage of disease progression.10 The BTB exhibits several distinct features compared to the normal BBB. It is characterized by irregular vessel structure, enlarged endothelial cell gaps, increased permeability to macromolecules, and enhanced expression of certain transporters and receptors.23,28,33 A better understanding of the BTB is essential for the development of effective therapeutic strategies for brain tumors.
Interactions Between GBM and the BTB
GBM cells are known to interact with the BBB through a variety of mechanisms. One of the key mechanisms is through the expression of specific cell surface receptors that are able to bind to proteins present in the extracellular matrix of the BBB.34 For example, integrins such as αvβ3 and α5β1 are known to be overexpressed on GBM cells and can interact with laminin and fibronectin, respectively, which are present in the extracellular matrix of the BBB.35 Additionally, other cell surface receptors such as epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR) have also been shown to play a role in GBM-BBB interactions. These receptors can activate downstream signaling pathways that lead to increased angiogenesis and BBB disruption.36,37
GBM cells also secrete various factors that can affect the function of the BBB. For example, vascular endothelial growth factor (VEGF), which is overexpressed in GBM, has been shown to disrupt tight junctions between endothelial cells of the BBB and increase permeability.38 Other factors such as matrix metalloproteinases (MMPs) can degrade extracellular matrix proteins and basement membrane components of the BBB, leading to increased permeability.39
In addition to these direct interactions, GBM cells can also influence the function of the BBB through indirect mechanisms. For example, GBM cells can recruit immune cells such as microglia and macrophages to the brain, which can secrete cytokines and chemokines that can affect BBB function.40 As the glioma cells grow beyond the BBB, the demand for nutrients and oxygen increases, leading to angiogenesis, a process in which the tumor cells transform the existing microvasculature of the brain. Simultaneously, angiogenesis promotes the proliferation and survival of cancer cells by providing them with the necessary nutrients.41 During the process of angiogenesis, newly formed blood vessels typically exhibit an immature structure characterized by fenestrations and a lack of endothelial tight junction protein complexes. This structural immaturity leads to an increase in vascular permeability, allowing substances to pass through the vessel walls more easily. Compared to normal BBB capillaries, these immature capillaries are “leaky”. As the tumor lesion grows and expands, the tumor environment becomes increasingly hypoxic. In response to this hypoxic state, tumor cells increase the secretion of VEGF, a critical protein that stimulates angiogenesis.3,42 Differences between the BBB and BTB are shown in (Figure 1).
 |
Figure 1 Differences between the blood-brain barrier (BBB) of a healthy brain and the blood-brain tumor barrier (BTB) of glioblastoma. |
Overall, the interactions between GBM and the BBB are complex and multifactorial, involving both direct and indirect mechanisms. Understanding these interactions is crucial for the development of effective drug delivery strategies for the treatment of GBM.
Surgical resection remains the cornerstone of treatment for glioblastoma (GBM), the most aggressive form of brain cancer.43 The challenge with surgery is that it is difficult to eliminate all cancer cells, leaving patients vulnerable to recurrence. Post-operative treatments are therefore critical, but current methods are limited and affect long-term outcomes.7 Innovations in post-operative drug delivery systems, particularly those that utilize the precision and controlled release offered by nanotechnology, are under active research.8 The exploration of such novel strategies underscores the urgent need for improved therapeutic options in the post-surgical treatment landscape for GBM.43,44
Drug Delivery Strategies to Overcome the BBB
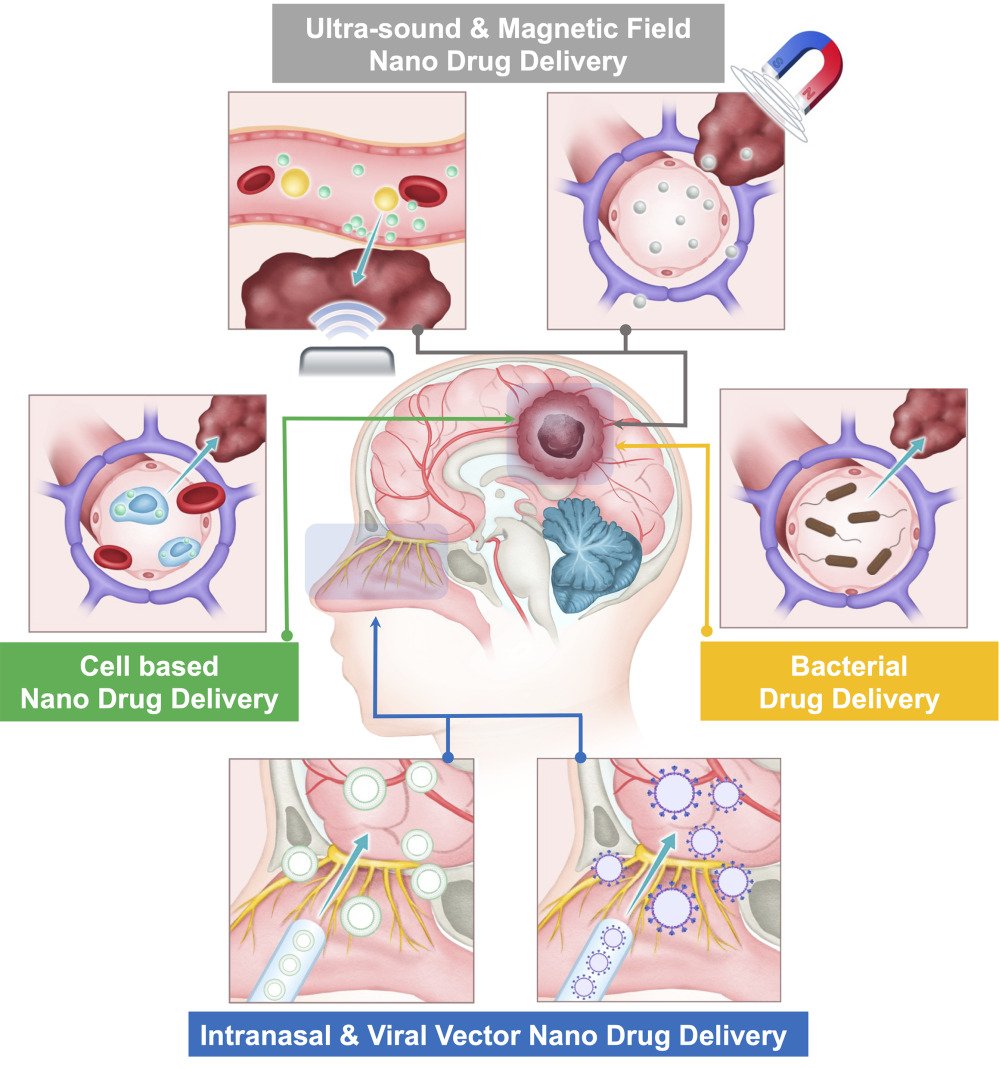
The blood-brain barrier (BBB) is characterized by its selective permeability, an essential feature for maintaining cerebral homeostasis by regulating the entry of substances into the brain. However, this selectivity also poses a significant challenge to the delivery of therapeutic agents to tumor tissues within the brain. With the growing understanding of the BBB and BTB, extensive research currently focusing on various diverse mechanisms to achieve precise drug delivery into the brain. The transport mechanism across the BBB can be divided into three categories, carrier-mediated transport, passive diffusion and transcytosis (Figure 2).
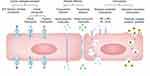 |
Figure 2 The transport mechanism across the BBB. |
Carrier-Mediated Transport
Carrier-mediated transport (CMT) is an energy-dependent mechanism that facilitates the active transport of various bioactive compounds, typically involving small hydrophilic molecules like glucose and amino acids.45 CMT proteins located in brain capillary endothelial cells can be expressed on the luminal, the abluminal side, or both.46 The specific localization and activity of these transporters regulate the passage of molecules across the BBB. They control whether molecules move unidirectionally, either as an influx into the brain or as an efflux back into the circulation, or bidirectionally, allowing solutes to move in both directions across the BBB in response to concentration gradients. This regulation is fundamental to maintaining the protective environment of the brain and its physiological homeostasis.47 Passive transporters, like the glucose transporter, allow solutes to move across cell membranes following their electrochemical gradient without requiring energy. Active transporters, like adenosine triphosphate (ATP)-binding cassette (ABC) transporters and ion pumps (ATPases), use energy to create gradients of solutes across membranes. These active transporters are involved in establishing solute gradients across cellular membranes, actively transporting solutes against their concentration gradient. This movement is powered by the energy released from the hydrolysis of ATP, facilitating the transfer of substances either out of the cell or into specific organelles.48,49 Solute transporters (SLCs) are the largest family of transmembrane transporters and are important in the exchange of various substances like nutrients, ions and metabolites through biological membranes. Most polar molecules cannot diffuse across cell membranes, so all cells express a significant amount of SLCs on the cell membrane cascade (Table 1).50,51 The endothelial cells of the brain that form the BBB express transport proteins that facilitate the movement of various solutes and nutrients into and out of the brain.52,53 Some of these transport proteins exhibit polarity in their expression, being found exclusively in either the luminal or abluminal membrane, while others are present in both membranes of the endothelial cells.54 The positioning of these transporters can result in selective substrate transport into or across the endothelial cell.55 The direction of this transport can be from the blood to the brain or from the brain to the blood. Another role of tight junctions within lateral cell membranes is to function as a partition, separating transport proteins and lipid rafts to the luminal or abluminal membrane domain. Such segregation restricts their independent movement between opposite sides of the endothelium, thereby preserving the polarization of the barrier.25,56,57
 |
Table 1 BBB Carrier-Mediated Transporters |
 |
Table 2 Examples of Receptor-Mediated and Adsorptive-Mediated Transcytosis of Complexes Across the BBB |
Transcytosis
Transcytosis is a process where molecules or particles are transported across the endothelial cells of the BBB via vesicular trafficking.45,47 This process is important for delivering drugs to the brain as the BBB limits the passive diffusion of many drugs, making them ineffective in treating neurological disorders and glioma.46 Transcytosis can occur through two mechanisms: receptor-mediated and adsorptive-mediated transcytosis.
Receptor-mediated transcytosis (RMT) is a highly efficient mechanism for targeted drug delivery across the BBB. In this process, the drugs are conjugated to ligands that bind to specific receptors expressed on the luminal side of the BBB endothelium.47 Upon binding, the receptor-ligand complex undergoes internalization and transcytosis across the endothelial cells to the abluminal side of the BBB, where the drug is released into the brain parenchyma.5
The most commonly used receptors for RMT include transferrin receptor (TfR), low-density lipoprotein receptor-related protein 1 (LRP1), insulin receptor (IR), and melanocortin receptor 1 (MCR1).60 Among these receptors, one of the most extensively studied receptors for RMT is the transferrin receptor. TfR is expressed on the surface of brain endothelial cells and binds to transferrin, a protein that transports iron across the BBB has been widely used for the delivery of therapeutic agents to the brain.61 The TfR-transferrin complex is internalized and transported across the endothelial cells via clathrin-mediated endocytosis. Once in the brain parenchyma, transferrin is released and can bind to its receptors on neurons and glial cells.47
One of the advantages of RMT is the high specificity and affinity of the ligands for their target receptors, which allows for selective drug delivery to the brain while minimizing off-target effects. Despite the advantages, RMT has some limitations for drug delivery across the BBB. One of the main limitations is the limited number of receptors available for targeting on the BBB endothelium, which can result in low drug delivery efficiency. Moreover, RMT is often limited to small molecule drugs due to the size limitations of the receptor-ligand complex. The size limitation is especially challenging for the delivery of macromolecules, such as proteins and nucleic acids, which have shown promise for the treatment of various brain diseases.47
Adsorptive-mediated transcytosis (AMT) is another strategy for drug delivery across the BBB. AMT relies on the principles of electrostatic interactions between the drug or NP and the BBB endothelial cells.52 The negatively charged plasma membrane of endothelial cells provides an opportunity for the adsorption of cationic or amphipathic molecules, facilitating their internalization and subsequent transcytosis. These interactions lead to the binding and subsequent internalization of the cargo, followed by transcytosis across the BBB. A variety of proteins or peptides can transport via AMT, such as peptides and cationic proteins.53
Peptides are short sequences of amino acids that can bind to specific receptors on the surface of BBB endothelial cells and facilitate transcytosis. One example of a peptide that has been studied for BBB transcytosis is the angiopep-2 peptide. Angiopep-2 is a 19 amino acid peptide that can bind to the low-density lipoprotein receptor-related protein 1 (LRP1) on BBB endothelial cells. Studies have shown that angiopep-2 can facilitate transcytosis of NPs across the BBB and enhance drug delivery to the brain.54 A list of various types of receptor and adsorptive-mediated transcytosis is presented in Table 2.25
Despite the potential of AMT for BBB drug delivery, there are still challenges to overcome. One major challenge is the limited number of specific peptide receptors on BBB endothelial cells. In addition, the peptide-conjugated drugs or NPs may also be subject to degradation by proteases in the BBB microenvironment.56 Further studies are needed to optimize peptide design and enhance the efficacy of peptide-mediated transcytosis for BBB drug delivery.
Cell-Based Nanoparticle Drug Delivery Strategies for Overcoming the BBB
NP-based drug delivery systems have shown great potential in overcoming the challenges imposed by the BBB for efficient drug delivery to the brain. However, to further enhance the targeting and therapeutic efficacy, NPs can be combined with cell-based drug delivery approaches.71 This synergistic strategy combines the advantages of NPs, such as enhanced drug encapsulation and controlled release, with the unique properties of various cell types that can actively transport the drug across the BBB and target specific cells within the brain. By using the unique properties and functions of different cell types, including mesenchymal stem cells (MSCs), neural stem cells (NSCs), monocytes/macrophages, and red blood cells (RBCs), NPs can be effectively transported across the BBB and targeted to specific cells within the brain (Figure 3).72,73
 |
Figure 3 The strategy of NP with cell-based drug delivery. |
Various Cell Types for NP Based Drug Delivery
Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells (MSCs) have gained significant attention as potential carriers for NP-based drug delivery to the brain due to their unique properties and therapeutic potential. Mesenchymal stem cells (MSCs) are characterized by their multipotency and can be derived from a variety of sources including bone marrow, adipose tissue, and umbilical cord tissue. These cells have significant potential due to their ability to differentiate into a variety of cell types. One of the key advantages of MSCs is their ability to migrate towards sites of injury and inflammation, including brain tumors, making them attractive candidates for targeted drug delivery to overcome the BBB. This property, known as homing, allows MSCs to actively target the diseased tissue and facilitate the delivery of therapeutic NPs. Moreover, MSCs possess immunomodulatory properties and can secrete various trophic factors that promote tissue regeneration and repair, further enhancing the therapeutic potential of the delivered NPs.74,75
Immune Cells
The immune system plays a critical role in defending the body against infections and diseases. Immune cells, including lymphocytes, monocytes, macrophages, and natural killer (NK) cells, have unique properties that can be harnessed for drug delivery across the BBB. These cells offer advantages such as their ability to interact with the BBB, their capacity for targeted delivery to specific brain regions, and their potential for modulating immune responses.76,77
Monocytes and macrophages are immune cells that have the ability to defense against pathogens and in tissue homeostasis. Monocytes are known to be recruited to glioma sites under the influence of tumor-associated chemokines and cytokines, which serve as chemical signals to guide their migration.78–80 Using monocytes as Trojan horses to deliver chemotherapeutic agents to brain tumors is a promising strategy. They could take up loaded chemotherapeutics encapsulated in or coated to nanoplatforms.81,82
The M1 macrophages, as carriers for delivering therapeutic NPs to glioma cells. The engineered M1 macrophages effectively internalized the PLGA NPs and migrated towards glioma cells. The NPs released the doxorubicin, anticancer drug, within the tumor microenvironment, resulting in enhanced drug accumulation and improved therapeutic efficacy. M1 macrophages exhibited tumor-targeting abilities, facilitated drug-loaded NP transport across the BBB, and promoted tumor-associated macrophage polarization toward the M1 phenotype, further contributing to antitumor activity.83 Lymphocytes are essential parts of the adaptive immune system, including T cells and B cells. These cells possess unique homing properties, allowing them to migrate to specific tissues and organs, including the brain. The potential of lymphocytes for targeted drug delivery to the brain has been explored in research.84
Neutrophils, a key component of leukocytes, are an essential part of the innate immune system, mainly involved in the early stages of the body’s defense against infection. They are the most prevalent white blood cells which are essential for fighting bacterial and fungal infections.85,86 Neutrophils are characterized by their multi-lobed nucleus and granules containing various antimicrobial molecules. In addition to their role in host defense, neutrophils have been identified as potential carriers for targeted drug delivery. The unique properties of neutrophils, such as their ability to migrate to infected and inflammatory sites, pathogens, and release antimicrobial molecules, make them attractive candidates for drug targeting.87,88 NPs accumulate at inflamed glioma sites following molecular targeting signals. The drug-loaded NPs are then released and taken up by infiltrating glioma cells, resulting in glioma treatment. Then, the drug-loaded NPs were released and taken up by infiltrating glioma cells, resulting in glioma treatment.89
NP Surface Modification
NP surface modification is a promising drug delivery strategy for enhancing the targeting and delivery of therapeutic agents to glioblastoma cells. Modifying the surface of NP can improve the binding, uptake, and retention of the therapeutic agent.14 Several types of NP surface modifications have been developed, including peptide conjugation, and antibody conjugation.
Peptide conjugation on NP is a widely used strategy that involves the attachment of peptides to the surface of NPs. Peptides are short chains of amino acids that can be synthesized to target specific cell surface receptors. The conjugation of peptides to the surface can improve the targeting of therapeutic agents to glioblastoma cells that overexpress the receptor of interest and enhance their ability to penetrate the BBB. For example, the cyclic peptide, cRGD, which binds to the αvβ3 and αvβ5 integrin receptors expressed on the surface of GBM cells. A study by Fang et al (2017) developed cRGD-conjugated PEG-PLA NPs for the targeted delivery of doxorubicin to GBM. The cRGD-conjugated NPs showed improved cellular uptake and cytotoxicity against GBM cells compared to non-targeted NPs and demonstrated enhanced accumulation in GBM xenografts in vivo.90
Antibody conjugation on NP is another approach for surface modification. Antibodies are proteins that can specifically recognize and bind to target molecules, making them an attractive tool for targeted drug delivery.91–93 Antibody conjugation involves attaching antibodies to the surface of NPs to enhance their selectivity and specificity for the targeted site. Antibody-mediated drug delivery has been shown to be effective in enhancing drug delivery across the BBB and improving therapeutic efficacy in preclinical models of brain diseases, including glioblastoma.94,95
One of the most commonly used antibodies for BBB drug delivery is the transferrin receptor (TfR) antibody. The TfR is highly expressed on the surface of brain endothelial cells and is involved in the transport of iron into the brain. TfR-targeted NPs have been shown to effectively cross the BBB and accumulate in brain tumors.96,97 Several of these antibodies have been reported to be effective in pre-clinical studies. Notably, OX26 and its variants, including 8D3 and RI7217, are some examples.97 Various types of NPs including metal NPs,98 liposomes,99 polymeric NPs100 have been used to carry the antibodies to deliver the drug payload to gliomas more effectively.
It is important to consider the protein corona effect, which refers to the rapid adsorption of proteins onto the NP surface upon exposure to biological fluids.101 This phenomenon can significantly alter the physicochemical properties of NPs and influence their interactions with biological systems. The composition and structure of the protein corona is dynamic and complex, depending on various factors such as NP size, shape, surface chemistry, and the protein composition of the surrounding biological fluid. The protein corona can influence NP functionality, biocompatibility, cellular uptake, and immune clearance, ultimately affecting the efficacy and safety of NP-based therapeutics.102 Therefore, strategies to control and manipulate the protein corona are essential to optimize NP surface modification and improve the performance of NP-based systems in biomedical applications.103
There are several limitations and challenges that must be addressed despite the potential benefits of antibody conjugation for BBB drug delivery. One major limitation is the potential for immunogenicity and antibody-mediated toxicity, which can result from the interaction between the antibody-conjugated NPs and the immune system. This can be particularly problematic in patients with pre-existing immune responses or in cases where the antibodies are derived from non-human sources.104
Another limitation is the potential for rapid clearance of the antibody-conjugated NPs by the reticuloendothelial system (RES) in the liver and spleen, which can limit their circulation time and accumulation in the brain. Strategies to overcome this limitation include the use of stealth coatings or the incorporation of ligands that can specifically target the RES.105
Intracellular Derived Vesicles NP
Intracellular derived vesicles, such as exosomes and membrane vesicles, are small membrane enveloped particles released by cells that are involved in intercellular communication. These vesicles have gained significant interest in recent years as potential drug delivery vehicles due to their ability to transport a variety of cargo, including proteins, nucleic acids, and lipids. Additionally, they have been shown to have inherent properties that make them an attractive option for drug delivery across the BBB, including their small size, ability to cross biological barriers, and low immunogenicity.106
Several studies have explored the potential of using exosomes as drug delivery vehicles for the treatment of glioblastoma. For instance, exosomes derived from dendritic cells (DCs) have been shown to be effective in delivering siRNA targeting the oncogene Bcl2-like protein 12 (Bcl2-L12) to glioblastoma cells.107 The study found that the exosomes were able to cross the BBB and deliver the siRNA to the tumor cells, resulting in reduced tumor growth and prolonged survival in a mouse model of GBM.108
Similarly, exosomes derived from mesenchymal stem cells (MSCs) have been shown to have therapeutic potential for glioblastoma. A study found that MSC-derived exosomes loaded with miR-124, a tumor suppressor miRNA, were able to inhibit the growth of glioblastoma cells in vitro and in vivo.109
Exosomes derived from neural stem cells (NSCs) have also been explored for the treatment of glioblastoma. A study found that exosomes derived from NSCs loaded with doxorubicin, a chemotherapy drug, were able to cross the BBB and deliver the drug to the tumor cells, resulting in reduced tumor growth and increased survival in a mouse model of glioblastoma.110 Another study found that exosomes derived from NSCs loaded with the chemotherapeutic agent paclitaxel were able to inhibit the growth of glioblastoma cells both in vitro and in vivo.111
In addition to their potential as drug delivery vehicles, exosomes derived from various cell types have also been shown to have intrinsic anti-tumor effects. For instance, exosomes derived from glioblastoma stem-like cells (GSCs) were found to inhibit the growth of glioblastoma cells in vitro and in vivo.112
Despite their potential, there are several challenges associated with the use of exosomes as drug delivery vehicles. One challenge is the difficulty in producing large quantities of exosomes with consistent quality and purity. Another challenge is the potential for off-target effects, as exosomes can be taken up by cells other than the intended target cells.113,114 Furthermore, the heterogeneity of exosomes derived from different cell types can make it difficult to compare and interpret results from different studies.
Nanoparticle-Based Combination Strategies for Overcoming the BBB
Ultrasound Wave Responsive NP
Ultrasound-mediated drug delivery is a non-invasive strategy that uses focused ultrasound waves to temporarily disrupt the BBB and facilitate the delivery of therapeutic drugs to the brain.115–117 Ultrasound waves can be used alone or in combination with microbubbles, which are small gas-filled bubbles that oscillate in response to the ultrasound waves, causing mechanical disruption of the endothelial cells in the BBB.115,116 The use of ultrasound in combination with NPs has been shown to further enhance the delivery of therapeutic agents to the brain, as NPs can act as carriers for the drugs and facilitate their transport across the disrupted BBB.115,117,118 Low-frequency focused ultrasound (FUS) is an innovative and non-invasive approach for temporarily and reversibly opening the BBB. This strategy, guided by magnetic resonance imaging (MRIgFUS), uses low-frequency ultrasound waves in concert with gas-filled microbubbles (MBs) to specifically disrupt the BBB.119 The MBs, which are echogenic in nature and generally consist of lipid-encapsulated perfluorocarbon gas, range from 1 to 5 μm in diameter.120 They are widely used as contrast agents in ultrasound imaging and aid in the precise targeting and disruption of the BBB for therapeutic purposes.121 The pressure amplitudes utilized in FUS to open the BBB are safe for human application, as they remain comparable to those employed in diagnostic ultrasound, ie, less than 1.5–2 MPa. The exact physical processes leading to FUS-induced BBB opening are not fully understood, although it is hypothesized that ultrasound waves cause MBs to engage with nearby brain tissue primarily by two methods, stable and inertial cavitation. Stable cavitation occurs when microbubbles (MBs) rhythmically contract and expand in a low-pressure environment. This action generates microstreaming in the fluid surrounding the MBs, which applies mechanical forces to endothelial cells and transiently disrupts tight junction proteins.122 Under high-pressure conditions, inertial cavitation leads to the collapse or disruption of microbubbles (MB), which generates robust mechanical forces like shock waves and microjets. These forces can perforate cell membranes and increase permeability in blood vessels (Figure 4).123
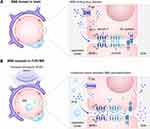 |
Figure 4 The strategy of NP with responsive ultrasound wave (A) BBB formed in brain capillary (B) brain capillaries exposed to FUS (focused ultrasound beam) with MB (micro bubble). |
One of the most widely studied NPs in combination with ultrasound is liposomes. Liposomes, spherical vesicles composed of a lipid bilayer, have the ability to encapsulate both hydrophilic and hydrophobic drugs. This unique structural feature makes them exceptionally versatile as carriers for a wide range of therapeutic agents. When combined with ultrasound, liposomes can be rapidly and non-invasive delivered to the brain, significantly improving the efficiency of drug delivery and consequently therapeutic efficacy.124–126 In the specific context of glioma treatment, magnetic resonance image-guided microbubble-enhanced low-intensity pulsed focused ultrasound (LIFU) is used to transiently open the blood-brain barrier (BBB). This technique facilitates the delivery of a novel liposome-loaded small molecule, an MGMT inactivator, to mice with temozolomide-resistant gliomas.127 This strategy demonstrates the potential of combining liposomal drug carriers to more effectively target and treat challenging brain tumors.
In addition to liposomes, other NPs such as polymeric NPs and dendrimers have also been investigated in combination with ultrasound for BBB permeabilization. Polymeric NPs are composed of biocompatible polymers such as poly (lactic-co-glycolic acid) (PLGA) or polyethylene glycol (PEG), which can be engineered to encapsulate a variety of drugs and target specific cells or tissues.128 When combined with ultrasound, polymeric NPs have been shown to enhance the delivery of therapeutic agents to the brain and improve treatment outcomes.129
The use of ultrasound in combination with NPs for BBB permeabilization has several advantages over other strategies (Table 3). First, ultrasound is a non-invasive and safe technology that can be easily applied to the target region without causing tissue damage.130 Second, NPs can be designed to target specific cells or tissues, allowing for selective delivery of therapeutic agents to the brain.115 Finally, the combination of ultrasound with NPs can enhance the delivery of therapeutic agents to the brain and improve treatment outcomes.123
 |
Table 3 NP-Based Combination Strategies |
Despite the potential of ultrasound-mediated drug delivery, there are several challenges that need to be addressed for its widespread clinical application (Table 3). One major challenge is optimizing the ultrasound parameters to achieve sufficient disruption of the BBB without causing tissue damage or inducing an immune response. Another challenge is ensuring that the therapeutic agents are delivered specifically to the tumor site and not to healthy brain tissue, which could cause neurological damage.117,118
Recent advances in ultrasound technology, such as the development of high-frequency ultrasound and the use of imaging techniques to monitor drug delivery in real-time, have shown promise in addressing some of these challenges. Additionally, research is ongoing to investigate the use of ultrasound-mediated drug delivery in combination with other strategies, such as immunotherapy, to enhance the therapeutic efficacy of glioblastoma treatment.116,118
Magnetic Field Responsive NP
Magnetic targeting and guidance are a non-invasive strategy that uses magnetic fields to guide magnetic NPs (MNPs) to the site of GBM. This technique harnesses magnetic nanoparticles (MNPs), which can be functionalized with therapeutic compounds, and employs an external magnetic field to direct these particles across the BBB and into glioblastoma (GBM) tissues (Figure 5).131,132 Prior to systemic administration, these MNPs are conjugated with therapeutic molecules. When administered into the bloodstream, these MNPs circulate systemically until they are subjected to an externally applied magnetic field concentrated at the GBM site.131–133 This field acts as a focusing point, pulling the MNPs into the brain tumor, which can significantly increase the local drug concentration and enhance the therapeutic efficacy.134 To ensure circulatory stability and evade immune recognition, these MNPs are often coated with biocompatible that prevent premature opsonization or aggregation within the vascular system.135 When these MNPs are directed by the magnetic field to localize to the region of the cerebral tumor, they can accelerate the deposition of therapeutic agents within the GBM, thereby increasing the concentration of the drug at the target site while decreasing the systemic exposure.131,133
 |
Figure 5 The strategy of NP with responsive magnetic field. |
Superparamagnetic iron oxide nanoparticles (SPIONs) exhibit unique magnetic properties, primarily due to their iron oxide core and a surrounding polymer coating. This design not only imparts magnetic responsiveness to the SPIONs but also ensures crucial biological characteristics such as biocompatibility, internalization, and viability. Taking advantage of these magnetic properties, a promising therapeutic approach involves the use of an external magnetic field to direct SPIONs specifically to tumor regions.136
One study investigated the use of SPIONs conjugated with the chemotherapy drug temozolomide (TMZ) for the treatment of GBM. The SPIONs-TMZ conjugates were able to cross the BBB and accumulate in the tumor site, resulting in improved efficacy compared to free TMZ.137 Despite these advancements, magnetic targeting is not without its intricacies and limitations. The introduction of a magnetic field to bodily tissues can inadvertently cause an increase in temperature, a phenomenon known as magnetic hyperthermia, which has the potential to damage healthy surrounding tissues.138 Moreover, there is a risk that MNPs might distribute to off-target tissues or organs, thereby causing unwanted side effects. To mitigate these risks, meticulous optimization of the magnetic field’s parameters, including its strength and exposure time, is critical.139 Moreover, a profound understanding of the MNPs’ physicochemical properties, such as their magnetic responsiveness, size, surface charge, and biodegradability, is essential. These factors play a decisive role in the MNPs’ circulation time, biodistribution, and the precision of their targeting capability.132,140
As research in this area progresses, there is a growing emphasis on developing sophisticated MNP systems that exhibit controlled drug release mechanisms, targeted payload delivery, and minimal off-target accumulation.141 This would entail the integration of stimuli-responsive elements into the MNP design, allowing for the controlled release of drugs in response to specific physiological signals at the tumor site.131 Magnetic field strategies to overcome the blood-brain barrier (BBB) offer a blend of advantages and limitations that are important for their application in medical treatments (Table 3).132,138,139 Major advantages include the non-invasive nature of the technique, which makes it safer and more comfortable for patients. It allows for the unique ability to remotely trigger drug release, providing precise control over when and where the drug is activated.138 In addition, because the magnetic nanoparticles are localized in the brain, systemic side effects are minimized, and the treatment is more targeted and less disruptive to other body systems.139 On the other hand, the limitations of this approach cannot be overlooked. It is only suitable for a limited range of drug types tailored to work with magnetic nanoparticles. The depth of penetration of the magnetic field into brain tissue is limited, which may limit its effectiveness for deeper brain regions.118 In addition, the use of high-intensity magnetic fields raises concerns about potential tissue heating and damage, posing a risk to patient safety.138,139 Therefore, while magnetic field strategies represent a promising avenue for crossing the BBB, their application must be carefully balanced with these inherent limitations.
Intranasal NP Drug Delivery
Intranasal delivery is an attractive non-invasive route for delivering therapeutics to the brain. The olfactory and trigeminal nerves in the nasal cavity provide direct access to the brain, bypassing the BBB.142,143 Additionally, intranasal delivery is a convenient and painless method of drug administration, especially for chronic or repeated dosing. However, only small molecules and lipophilic drugs can efficiently penetrate the nasal mucosa and reach the brain, limiting the types of therapeutics that can be delivered by this route. NPs have been explored as a means of enhancing intranasal delivery of drugs to the brain.144,145
The first step in the NP drug delivery pathway using intranasal delivery is the administration of NPs as a nasal spray or droplets. When administered intranasally, the NPs are dispersed in the nasal cavity. The large surface area and rich blood supply of the nasal mucosa facilitate efficient drug absorption.144
The NPs can deposit in different regions of the nasal cavity, including the olfactory region and the respiratory region (Figure 6). The olfactory region is important region for brain drug delivery as it provides a direct pathway to the brain through the olfactory epithelium and the olfactory nerves. The NPs can directly access the olfactory bulb and subsequently diffuse into other brain regions. In the respiratory region, the NPs can be absorbed into the bloodstream and reach the brain through systemic circulation.144,145
 |
Figure 6 The strategy of NP in combination with intranasal drug delivery. |
Upon deposition in the nasal cavity, the NPs interact with the nasal mucosa. The surface properties of the NPs play a crucial role in their interaction with the mucosal tissues. NPs can penetrate the nasal mucosa through various mechanisms, including diffusion, endocytosis, and paracellular or transcellular transport.146 The NPs can diffuse across the nasal epithelial cells, taking advantage of their small size and favorable physicochemical properties. Additionally, some NPs can be taken up by the nasal epithelial cells through endocytosis, allowing them to bypass the mucosal barrier and enter the systemic circulation.146,147
NP-based intranasal delivery has been shown to enhance brain delivery of drugs that are otherwise unable to overcome the BBB. Polymeric NPs, such as PLGA and chitosan, have been used to improve the intranasal delivery of various drugs to the brain. Additionally, solid lipid NPs (SLNs) have also been investigated for intranasal drug delivery due to their ability to improve drug solubility and stability, as well as their potential to enhance drug transport across the nasal mucosa.148–150
NP-based intranasal drug delivery offers rapid drug action through direct access to the GBM and rapid absorption through the highly vascularized mucosa, ensuring rapid therapeutic effects (Table 3). In addition, it is easy to administer and can be particularly effective for low molecular weight drugs, increasing their bioavailability. However, the limitations of this approach are significant. It is only suitable for a limited range of drug types, and rapid mucociliary clearance in the nasal cavity can lead to rapid elimination of the drug.142,143 There’s also a risk of mechanical dose loss due to improper administration techniques. In addition, some patients may experience nasal irritation or discomfort, and there’s a limit to the amount of drug that can be effectively delivered by this route.143 This combination of factors makes it critical to consider both the advantages and disadvantages when choosing intranasal drug delivery for the treatment of diseases such as GBM. Further studies are needed to optimize the design of NP systems for intranasal drug delivery and to evaluate their efficacy in clinical and safety.144
Viral Vector Drug Delivery
Virus-Like NP
Virus-like nano particle (VLP) drug delivery has emerged as an promising approach in the treatment of GBM, primarily due to its potential to effectively cross the BBB. VLPs, which mimic the structure of viruses but lack viral genetic material, offer a promising platform for the targeted delivery of therapeutic agents.151 These particles providing a unique approach to transport therapeutic agents across the BBB and deliver them to GBM cells (Figure 7). VLPs are known for their structural similarity to viruses, allowing them to utilize the natural cellular entry pathways used by viruses without the associated risk of viral replication or infection.151,152 This property makes VLPs an attractive vehicle for drug delivery, as they can encapsulate a wide range of therapeutic agents, including small molecules, nucleic acids and proteins.151 VLPs are renowned for their structural similarity to viruses, allowing them to leverage the natural cellular entry pathways used by viruses, without the associated risk of viral replication or infection.153 This feature makes VLPs an attractive vehicle for drug delivery, as they can encapsulate a wide range of therapeutic agents, including small molecules, nucleic acids, and proteins.154 Recent advancements have focused on understanding the complicated pathways employed by viruses to overcome the BBB.152 There have been reports indicating that SARS-CoV-2 can affect several cranial nerves, such as the olfactory, trigeminal, optic, and vagus nerves. In addition, SARS-CoV-2 can spread to the systemic circulatory system, after a respiratory tract infection that is typical of the virus.155 Upon reaching the BBB, SARS-CoV-2 can penetrate endothelial cells through interaction with the angiotensin-converting enzyme 2 (ACE2) receptor, alteration of tight junction proteins formed by BBB endothelial cells, or phagocytosis by immune cells. transcellular pathway, paracellular pathway, and the Trojan horse pathway.156 The entry of JC VLPs (John Cunningham virus-like particles) into brain endothelial cells was mediated by clathrin-dependent mechanisms, and exocytosis or cellular and paracellular transcytosis of VLPs across the BBB was observed in vitro. VLPs were found in mouse brain endothelia through Intravenous (IV) and Internal carotid artery (ICA) injection.157
The ability of VLPs to cross the BBB is a critical aspect of their functionality in GBM treatment. Researchers have been investigating various strategies to enhance BBB penetration, such as modifying the surface properties of VLPs to exploit receptor-mediated transcytosis pathways.158 Research on brain tumor treatment using VLPs is still in its early stages, but it holds promise as various viruses capable of crossing the BBB have been reported, suggesting a potential avenue for exploration.159
The safety and biocompatibility of VLPs are paramount for their use in clinical applications. Despite the potential of VLPs in GBM treatment, challenges remain in translating these technologies from the laboratory to the clinic. Issues such as the scalability of VLP production, the stability of drug encapsulation, and the long-term safety of these delivery systems need to be thoroughly addressed. Ongoing research and clinical trials are essential to refine VLP technology, assess its effectiveness in treating GBM, and ultimately integrate VLP-based drug delivery systems into standard GBM treatment regimens.152
Bacteria Engineering NP
Bacteria have shown promising results in cancer therapy. Bacterial engineering approaches for cancer treatment are independent of genetic variations, offering distinct advantages over traditional treatments due to their inherent tumor-targeting ability, capacity for tissue penetration, and gene delivery capabilities.160 Research has shown that bacteria can cross the BBB through transcellular, paracellular, or phagocyte-mediated mechanisms (Figure 7). This discovery has provided the evidence for using bacteria in the treatment of GBM. However, the use of live bacteria for GBM therapy is limited due to challenges such as imprecise drug release control, inadequate immune response stimulation, and potential bacterial toxicity, such as bacteremia. There is growing interest in developing hybrid systems that combine bacteria and nanoparticles for drug delivery in various cancer types.161
A study developed a Trojan bacteria system for delivering therapeutics to combination therapy photothermal immunotherapy for GBM. The therapeutic agents used were glucose polymer (GP)-conjugated and indocyanine green (ICG)-loaded silicon nanoparticles (GP-ICG-SiNPs). Facultatively anaerobic bacteria, such as attenuated Salmonella typhimurium VNP20009 (VNP) and Escherichia coli 25,922 (EC), efficiently internalize these nanoparticles via the bacteria-specific ATP-binding cassette (ABC) transporter, forming the Trojan bacteria system. Compared to free agents, which have difficulty crossing the BBB and reaching GBM tissues, the engineered Trojan bacteria can transport therapeutics across the BBB, target GBM and penetrate GBM tissues more effectively. The Trojan bacteria system utilizes photothermal effects induced by ICG-loaded SiNPs under 808-nanometer irradiation to destruct tumor cells, release tumor-associated antigens and promote immune responses.162
Conclusion and Future Perspective
The presence of the blood-brain barrier (BBB) and the blood-tumor barrier (BTB) is a major impediment to the treatment of glioblastoma (GBM), one of the most aggressive malignant forms of brain cancer.3,5 The selective permeability of physiological barriers impedes the passage of therapeutic molecules, posing a challenge to delivering drugs to the brain and tumor microenvironment. Large and hydrophilic molecules are unable to cross these barriers, resulting in lower therapeutic concentrations at the site of action and reduced treatment efficacy for patients, ultimately impacting survival rates.163,164 To improve treatment outcomes in GBM, it is crucial to advance new approaches that can overcome these barriers.
Various strategies have been explored to overcome the BBB and BTB, and among them, NP-based drug delivery systems have shown great potential. Surface modifications, such as receptor-mediated targeting and adsorptive-mediated transcytosis, have demonstrated enhanced NP transport across the barriers.15,22 The use of NP-based combination strategies holds immense potential.165 The use of NPs with ultrasound waves or magnetic fields represents a developed strategy to enhance drug delivery to tumor sites.115,132 This approach takes advantage of the increased permeability and localized targeting, thereby improving the efficiency and precision of therapeutic agent delivery to the targeted area. Furthermore, the integration of cell-based drug delivery systems with NPs offers a unique approach to enhance drug delivery to GBM. Mesenchymal stem cells (MSCs), monocytes/macrophages, and other immune cells have been explored as carriers for delivering NPs to the tumor site. These cells possess inherent tropism for GBM and can be genetically modified or loaded with NPs to enhance their therapeutic efficacy.75
The combination of NP-based drug delivery systems with cell-based carriers not only improves the delivery of therapeutic agents but also offers additional advantages such as immunomodulation, tumor targeting, and the potential for personalized medicine.14 The use of genetically modified cells or the engineering of living cells with functionalized NP provides exciting avenues for tailored and targeted therapies. In recent years, the development of NP based combination therapy approaches has gained significant attention in the treatment of cancer.16,166 By combining NP-based strategies with other modalities, such as ultrasound-wave, magnetic field, or intranasal delivery, the synergistic effects can lead to improved drug penetration and therapeutic efficacy. These strategies offer promising opportunities for non-invasive and targeted drug delivery to GBM, ultimately improving patient outcomes. The rationale behind combination therapy lies in the complementary mechanisms of action employed by different modalities. The use of ultrasound-wave or magnetic field can enhance the permeability of the BBB and facilitate the transport of NPs across the barrier.115,118
Similarly, intranasal delivery offers a direct route to bypass the BBB and deliver therapeutics to the brain.146 By combining these approaches with NP-based drug delivery systems, the potential for achieving higher drug concentrations within the tumor site increases significantly. Moreover, NP based combination therapy has the advantage of addressing the inherent heterogeneity of GBM. The tumor microenvironment is characterized by diverse cell populations, each with distinct vulnerabilities and resistance mechanisms. By targeting multiple pathways simultaneously, combination therapy can overcome drug resistance and improve treatment efficacy. Additionally, combination approaches can enhance therapeutic selectivity, reducing off-target effects and minimizing systemic toxicity.167
Viral vector drug delivery holds significant potential due to their inherent properties such as specific targeting capabilities and surface modifiability. Research has shown that virus and bacteria can cross the BBB through transcellular, paracellular, or Trojan horse mechanisms.159 To advance the development and clinical translation of bacterial nanoparticle-based therapies for glioblastoma, it is essential to resolve challenges related to immunogenicity, scalability, target specificity and microbiome interactions.161
However, successful clinical application of combination therapy requires careful consideration and optimization of several factors. These include the selection of appropriate therapeutic agents, dosage regimens, and treatment schedules to maximize synergistic effects. Furthermore, the design and engineering of NPs must be tailored to ensure optimal drug loading, stability, and controlled release. Safety considerations, including potential toxicity and long-term effects, should also be thoroughly evaluated.168
Furthermore, the clinical translation of nanoparticle-based strategies for overcoming the blood-brain barrier (BBB) is entering a promising phase, propelled by recent research. These trials aim to validate the safety, efficacy, and optimal dosing of these innovative systems.157 Personalized medicine, using detailed patient profiles to tailor nanoparticle properties, is expected to refine treatment efficacy further.158 The convergence of nanotechnology with emerging fields such as genomics and proteomics is expected to open new avenues for personalized medicine.169,170 Understanding individual patient profiles could lead to the development of custom-tailored nanoparticle systems, optimizing treatment efficacy while minimizing side effects.159 Ethical considerations and conflicts of interest are essential in research involving human participants. This includes ensuring informed consent through informed consent forms that detail the scope and potential risks of the study.168 Institutional Review Board (IRB) documents play a critical role in ensuring the ethical conduct of the study, including the protection of participants’ rights and welfare. These documents must be carefully prepared and approved prior to the start of the research to ensure adherence to ethical standards and resolve any potential conflicts of interest to maintain the integrity of the study.171
In conclusion, NP-based combination therapy strategies have the potential to overcome the challenges of BBB and BTB for the treatment of GBM. The combination of NP-based drug delivery systems with complementary strategies such as ultrasound, magnetic field or intranasal delivery will greatly enhance the potential for improved drug penetration and therapeutic efficacy. The ability to target multiple pathways and overcome drug resistance further underscores the importance of combination therapy for the treatment of GBM. Continued research and development, coupled with personalized treatment approaches, can provide promising potential for overcoming GBM and can significantly improve lifetime of patient.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF- 2022M3A9G8018189), and by the Gachon University Research Fund of 2020 (GGU- 202008430004).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Abbott NJ, Ronnback L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi:10.1038/nrn1824
2. Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17(1):69. doi:10.1186/s12987-020-00230-3
3. Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20(1):26–41. doi:10.1038/s41568-019-0205-x
4. Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788(4):842–857. doi:10.1016/j.bbamem.2008.10.022
5. Wu D, Chen Q, Chen X, Han F, Chen Z, Wang Y. The blood-brain barrier: structure, regulation, and drug delivery. Signal Transduct Target Ther. 2023;8(1):217. doi:10.1038/s41392-023-01481-w
6. Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol. 2018;15(7):422–442. doi:10.1038/s41571-018-0003-5
7. Rong L, Li N, Zhang Z. Emerging therapies for glioblastoma: current state and future directions. J Exp Clin Cancer Res. 2022;41(1):142. doi:10.1186/s13046-022-02349-7
8. Cui J, Xu Y, Tu H, et al. Gather wisdom to overcome barriers: well-designed nano-drug delivery systems for treating gliomas. Acta Pharm Sin B. 2022;12(3):1100–1125. doi:10.1016/j.apsb.2021.08.013
9. Wadajkar AS, Dancy JG, Hersh DS, et al. Tumor-targeted nanotherapeutics: overcoming treatment barriers for glioblastoma. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(4):e1439.
10. Steeg PS. The blood–tumour barrier in cancer biology and therapy. Nat Rev Clin Oncol. 2021;18(11):696–714. doi:10.1038/s41571-021-00529-6
11. Guyon J, Chapouly C, Andrique L, Bikfalvi A, Daubon T. The normal and brain tumor vasculature: morphological and functional characteristics and therapeutic targeting. Front Physiol. 2021;12:622615. doi:10.3389/fphys.2021.622615
12. Terstappen GC, Meyer AH, Bell RD, Zhang W. Strategies for delivering therapeutics across the blood-brain barrier. Nat Rev Drug Discov. 2021;20(5):362–383. doi:10.1038/s41573-021-00139-y
13. Dong X. Current strategies for brain drug delivery. Theranostics. 2018;8(6):1481–1493. doi:10.7150/thno.21254
14. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi:10.1038/s41573-020-0090-8
15. Duan L, Li X, Ji R, et al. Nanoparticle-based drug delivery systems: an inspiring therapeutic strategy for neurodegenerative diseases. Polymers. 2023;15(9):2196.
16. Zhao Y, Yue P, Peng Y, et al. Recent advances in drug delivery systems for targeting brain tumors. Drug Deliv. 2023;30(1):1–18. doi:10.1080/10717544.2022.2154409
17. Ma L, Kohli M, Smith A. Nanoparticles for combination drug therapy. ACS Nano. 2013;7(11):9518–9525. doi:10.1021/nn405674m
18. Gurunathan S, Kang MH, Qasim M, Kim JH. Nanoparticle-mediated combination therapy: two-in-one approach for cancer. Int J Mol Sci. 2018;19:3264.
19. Mottaghitalab F, Farokhi M, Fatahi Y, Atyabi F, Dinarvand R. New insights into designing hybrid nanoparticles for lung cancer: diagnosis and treatment. J Control Release. 2019;295:250–267. doi:10.1016/j.jconrel.2019.01.009
20. Cha GD, Kang T, Baik S, et al. Advances in drug delivery technology for the treatment of glioblastoma multiforme. J Control Release. 2020;328:350–367. doi:10.1016/j.jconrel.2020.09.002
21. Cha GD, Jung S, Choi SH, Kim DH. Local drug delivery strategies for glioblastoma treatment. Brain Tumor Res Treat. 2022;10(3):151–157. doi:10.14791/btrt.2022.0017
22. Pandey N, Anastasiadis P, Carney CP, et al. Nanotherapeutic treatment of the invasive glioblastoma tumor microenvironment. Adv Drug Deliv Rev. 2022;188:114415.
23. Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro-Oncology. 2014;16(7):896–913. doi:10.1093/neuonc/nou087
24. Wu W, Klockow JL, Zhang M, et al. Glioblastoma multiforme (GBM): an overview of current therapies and mechanisms of resistance. Pharmacol Res. 2021;171:105780. doi:10.1016/j.phrs.2021.105780
25. Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi:10.1016/j.nbd.2009.07.030
26. Mo F, Pellerino A, Soffietti R, Ruda R. Blood-brain barrier in brain tumors: biology and clinical relevance. Int J Mol Sci. 2021;22(23):12654.
27. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK; World Health O, International Agency for Research on C. WHO Classification of Tumours of the Central Nervous System. World Health Organization classification of tumours. International Agency For Research On Cancer; 2016:408.
28. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med. 2005;352(10):987–996. doi:10.1056/NEJMoa043330
29. Aldoghachi AF, Aldoghachi AF, Breyne K, Ling KH, Cheah PS. Recent advances in the therapeutic strategies of glioblastoma multiforme. Neuroscience. 2022;491:240–270. doi:10.1016/j.neuroscience.2022.03.030
30. Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:7323):557–U231. doi:10.1038/nature09522
31. Miller DS, Bauer B, Hartz AMS. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60(2):196–209. doi:10.1124/pr.107.07109
32. Tachibana K, Iwashita Y, Wakayama E, Nishino I, Nishikaji T, Kondoh M. Tight junction modulating bioprobes for drug delivery system to the brain: a review. Pharmaceutics. 2020;12(12):1236.
33. Rathi S, Griffith JI, Zhang W, et al. The influence of the blood-brain barrier in the treatment of brain tumours. J Intern Med. 2022;292(1):3–30. doi:10.1111/joim.13440
34. Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci. 2020;21(24):9739.
35. Ellert-Miklaszewska A, Poleszak K, Pasierbinska M, Kaminska B. Integrin signaling in glioma pathogenesis: from biology to therapy. Int J Mol Sci. 2020;21(3):888.
36. An Z, Aksoy O, Zheng T, Fan QW, Weiss WA. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. 2018;37(12):1561–1575. doi:10.1038/s41388-017-0045-7
37. Pearson JRD, Regad T. Targeting cellular pathways in glioblastoma multiforme. Signal Transduct Target Ther. 2017;2:17040. doi:10.1038/sigtrans.2017.40
38. Wen L, Tan Y, Dai S, et al. VEGF-mediated tight junctions pathological fenestration enhances doxorubicin-loaded glycolipid-like nanoparticles traversing BBB for glioblastoma-targeting therapy. Drug Deliv. 2017;24(1):1843–1855. doi:10.1080/10717544.2017.1386731
39. Rempe RG, Hartz AMS, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: versatile breakers and makers. J Cereb Blood Flow Metab. 2016;36(9):1481–1507. doi:10.1177/0271678X16655551
40. Ma J, Chen CC, Li M. Macrophages/microglia in the glioblastoma tumor microenvironment. Int J Mol Sci. 2021;22(11):5775.
41. Monteiro AR, Hill R, Pilkington GJ, Madureira PA. The role of hypoxia in glioblastoma invasion. Cells-Basel. 2017;6(4):45.
42. Blethen KE, Arsiwala TA, Fladeland RA, et al. Modulation of the blood-tumor barrier to enhance drug delivery and efficacy for brain metastases. Neurooncol Adv. 2021;3(Suppl 5):v133–v143. doi:10.1093/noajnl/vdab123
43. Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242(3):326–341. doi:10.1097/01.sla.0000179621.33268.83
44. Chojak R, Kozba-Gosztyla M, Slychan K, et al. Impact of surgical resection of butterfly glioblastoma on survival: a meta-analysis based on comparative studies. Sci Rep. 2021;11(1):13934. doi:10.1038/s41598-021-93441-z
45. Ayloo S, Gu C. Transcytosis at the blood-brain barrier. Curr Opin Neurobiol. 2019;57:32–38. doi:10.1016/j.conb.2018.12.014
46. Li J, Zheng M, Shimoni O, et al. Development of novel therapeutics targeting the blood-brain barrier: from barrier to carrier. Adv Sci. 2021;8(16):e2101090. doi:10.1002/advs.202101090
47. Pulgar VM. Transcytosis to cross the blood brain barrier, new advancements and challenges. Front Neurosci. 2018;12:1019. doi:10.3389/fnins.2018.01019
48. Begley DJ. ABC transporters and the blood-brain barrier. Curr Pharm Des. 2004;10(12):1295–1312. doi:10.2174/1381612043384844
49. Gomez-Zepeda D, Taghi M, Scherrmann JM, Decleves X, Menet MC. ABC transporters at the blood-brain interfaces, their study models, and drug delivery implications in gliomas. Pharmaceutics. 2020;12(1):20.
50. Hong M. Biochemical studies on the structure-function relationship of major drug transporters in the ATP-binding cassette family and solute carrier family. Adv Drug Deliv Rev. 2017;116:3–20. doi:10.1016/j.addr.2016.06.003
51. Puris E, Fricker G, Gynther M. Targeting transporters for drug delivery to the brain: can we do better? Pharm Res. 2022;39(7):1415–1455. doi:10.1007/s11095-022-03241-x
52. Lu W. Adsorptive-mediated brain delivery systems. Curr Pharm Biotechnol. 2012;13(12):2340–2348. doi:10.2174/138920112803341851
53. Tuma P, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83(3):871–932. doi:10.1152/physrev.00001.2003
54. Habib S, Singh M. Angiopep-2-modified nanoparticles for brain-directed delivery of therapeutics: a review. Polymers. 2022;14(4):712.
55. Uchida Y, Ohtsuki S, Katsukura Y, et al. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem. 2011;117(2):333–345. doi:10.1111/j.1471-4159.2011.07208.x
56. Lalatsa A, Schatzlein AG, Uchegbu IF. Strategies to deliver peptide drugs to the brain. Mol Pharm. 2014;11(4):1081–1093. doi:10.1021/mp400680d
57. Visser CC, Voorwinden LH, Crommelin DJ, Danhof M, de Boer AG. Characterization and modulation of the transferrin receptor on brain capillary endothelial cells. Pharm Res. 2004;21(5):761–769. doi:10.1023/b:pham.0000026425.69874.8e
58. Shawahna R, Uchida Y, Decleves X, et al. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm. 2011;8(4):1332–1341. doi:10.1021/mp200129p
59. Al-Majdoub ZM, Al Feteisi H, Achour B, et al. Proteomic quantification of human blood-brain barrier SLC and ABC transporters in healthy individuals and dementia patients. Mol Pharm. 2019;16(3):1220–1233. doi:10.1021/acs.molpharmaceut.8b01189
60. Zhang W, Liu QY, Haqqani AS, et al. Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human. Fluids Barriers CNS. 2020;17(1):47. doi:10.1186/s12987-020-00209-0
61. Predescu SA, Predescu DN, Malik AB. Molecular determinants of endothelial transcytosis and their role in endothelial permeability. Am J Physiol-Lung C. 2007;293(4):L823–L842. doi:10.1152/ajplung.00436.2006
62. Talukder MJ, Takeuchi T, Harada E. Receptor-mediated transport of lactoferrin into the cerebrospinal fluid via plasma in young calves. J Vet Med Sci. 2003;65(9):957–964. doi:10.1292/jvms.65.957
63. Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490(1–3):5–12. doi:10.1016/j.ejphar.2004.02.040
64. Zlokovic BV, Skundric DS, Segal MB, Lipovac MN, Mackic JB, Davson H. A saturable mechanism for transport of immunoglobulin G across the blood-brain barrier of the Guinea pig. Exp Neurol. 1990;107(3):263–270. doi:10.1016/0014-4886(90)90144-h
65. Banks WA, Niehoff ML, Martin D, Farrell CL. Leptin transport across the blood-brain barrier of the Koletsky rat is not mediated by a product of the leptin receptor gene. Brain Res. 2002;950(1–2):130–136. doi:10.1016/s0006-8993(02)03013-5
66. Herz J, Marschang P. Coaxing the LDL receptor family into the fold. Cell. 2003;112(3):289–292. doi:10.1016/S0092-8674(03)00073-4
67. Gaillard PJ, Visser CC, de Boer AG. Targeted delivery across the blood-brain barrier. Expert Opin Drug Deliv. 2005;2(2):299–309. doi:10.1517/17425247.2.2.299
68. Pan W, Kastin AJ. TNFα transport across the blood-brain barrier is abolished in receptor knockout mice. Exp Neurol. 2002;174(2):193–200. doi:10.1006/exnr.2002.7871
69. Kamalinia G, Khodagholi F, Shaerzadeh F, et al. Cationic albumin-conjugated chelating agent as a novel brain drug delivery system in neurodegeneration. Chem Biol Drug Des. 2015;86(5):1203–1214. doi:10.1111/cbdd.12586
70. Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. Studies on the internalization mechanism of cationic cell-penetrating peptides. J Biol Chem. 2003;278(33):31192–31201. doi:10.1074/jbc.M303938200
71. Ding S, Khan AI, Cai X, et al. Overcoming blood-brain barrier transport: advances in nanoparticle-based drug delivery strategies. Mater Today. 2020;37:112–125. doi:10.1016/j.mattod.2020.02.001
72. Li YJ, Wu JY, Liu J, et al. From blood to brain: blood cell-based biomimetic drug delivery systems. Drug Deliv. 2021;28(1):1214–1225. doi:10.1080/10717544.2021.1937384
73. Le QV, Lee J, Lee H, Shim G, Oh YK. Cell membrane-derived vesicles for delivery of therapeutic agents. Acta Pharm Sin B. 2021;11(8):2096–2113. doi:10.1016/j.apsb.2021.01.020
74. Ruiz-Garcia H, Alvarado-Estrada K, Krishnan S, Quinones-Hinojosa A, Trifiletti DM. Nanoparticles for stem cell therapy bioengineering in glioma. Front Bioeng Biotechnol. 2020;8:558375. doi:10.3389/fbioe.2020.558375
75. Guo Y, Hu D, Lian L, et al. Stem cell-derived extracellular vesicles: a promising nano delivery platform to the brain? Stem Cell Rev Rep. 2023;19(2):285–308. doi:10.1007/s12015-022-10455-4
76. Buzas EI. The roles of extracellular vesicles in the immune system. Nat Rev Immunol. 2023;23(4):236–250. doi:10.1038/s41577-022-00763-8
77. Sedgwick AJ, Ghazanfari N, Constantinescu P, Mantamadiotis T, Barrow AD. The role of NK cells and innate lymphoid cells in brain cancer. Front Immunol. 2020;11:1549. doi:10.3389/fimmu.2020.01549
78. Wei J, Chen P, Gupta P, et al. Immune biology of glioma-associated macrophages and microglia: functional and therapeutic implications. Neuro Oncol. 2020;22(2):180–194. doi:10.1093/neuonc/noz212
79. Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21(11):799–820. doi:10.1038/s41573-022-00520-5
80. Buonfiglioli A, Hambardzumyan D. Macrophages and microglia: the cerberus of glioblastoma. Acta Neuropathol Commun. 2021;9(1):54. doi:10.1186/s40478-021-01156-z
81. Ou A, Wang Y, Zhang J, Huang Y. Living cells and cell-derived vesicles: a trojan horse technique for brain delivery. Pharmaceutics. 2023;15(4):1257.
82. Wang C, Li K, Li T, et al. Monocyte-mediated chemotherapy drug delivery in glioblastoma. Nanomedicine (Lond). 2018;13(2):157–178. doi:10.2217/nnm-2017-0266
83. Pang L, Zhu Y, Qin J, Zhao W, Wang J. Primary M1 macrophages as multifunctional carrier combined with PLGA nanoparticle delivering anticancer drug for efficient glioma therapy. Drug Deliv. 2018;25(1):1922–1931. doi:10.1080/10717544.2018.1502839
84. Choi A, Javius-Jones K, Hong S, Park H. Cell-based drug delivery systems with innate homing capability as a novel nanocarrier platform. Int J Nanomed. 2023;18:509–525. doi:10.2147/IJN.S394389
85. Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:324475.
86. Malech HL, DeLeo FR, Quinn MT. The Role of Neutrophils in the Immune System: an Overview. Methods Mol Biol. 2020;2087:3–10. doi:10.1007/978-1-0716-0154-9_1
87. Wang HJ, Zang J, Zhao ZH, Zhang Q, Chen SJ. The advances of neutrophil-derived effective drug delivery systems: a key review of managing tumors and inflammation. Int J Nanomed. 2021;16:7663–7681. doi:10.2147/Ijn.S328705
88. Zhao Y, Zhang HG, Zhang QX, Tao H. Research progress of neutrophil-mediated drug delivery strategies for inflammation-related disease. Pharmaceutics. 2023;15(7):1881.
89. Wu M, Zhang H, Tie C, et al. MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat Commun. 2018;9(1):4777. doi:10.1038/s41467-018-07250-6
90. Fang Y, Jiang Y, Zou Y, et al. Targeted glioma chemotherapy by cyclic RGD peptide-functionalized reversibly core-crosslinked multifunctional poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelles. Acta Biomater. 2017;50:396–406. doi:10.1016/j.actbio.2017.01.007
91. Jin SJ, Sun YP, Liang X, et al. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduction Tar. 2022;7(1). doi:10.1038/s41392-021-00868-x
92. Kumari M, Acharya A, Krishnamurthy PT. Antibody-conjugated nanoparticles for target-specific drug delivery of chemotherapeutics. Beilstein J Nanotechnol. 2023;14:912–926. doi:10.3762/bjnano.14.75
93. Fu ZW, Li SJ, Han SF, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduction Tar. 2022;7(1):93.
94. Bajracharya R, Caruso AC, Vella LJ, Nisbet RM. Current and emerging strategies for enhancing antibody delivery to the brain. Pharmaceutics. 2021;13(12):2014.
95. Teixeira MI, Lopes CM, Amaral MH, Costa PC. Surface-modified lipid nanocarriers for crossing the blood-brain barrier (BBB): a current overview of active targeting in brain diseases. Colloids Surf B Biointerfaces. 2023;221:112999. doi:10.1016/j.colsurfb.2022.112999
96. Ramalho MJ, Loureiro JA, Coelho MAN, Pereira MC. Transferrin receptor-targeted nanocarriers: overcoming barriers to treat glioblastoma. Pharmaceutics. 2022;14(2):279.
97. Koneru T, McCord E, Pawar S, Tatiparti K, Sau S, Iyer AK. Transferrin: biology and use in receptor-targeted nanotherapy of gliomas. ACS Omega. 2021;6(13):8727–8733. doi:10.1021/acsomega.0c05848
98. Paris-Robidas S, Emond V, Tremblay C, Soulet D, Calon F. In vivo labeling of brain capillary endothelial cells after intravenous injection of monoclonal antibodies targeting the transferrin receptor. Mol Pharmacol. 2011;80(1):32–39. doi:10.1124/mol.111.071027
99. Kang Y-S, Jung H-J, Oh J-S, Song D-Y. Use of PEGylated immunoliposomes to deliver dopamine across the blood–brain barrier in a rat model of parkinson’s disease. CNS Neurosci Ther. 2016;22(10):817–823. doi:10.1111/cns.12580
100. Gomes MJ, Kennedy PJ, Martins S, Sarmento B. Delivery of siRNA silencing P-gp in peptide-functionalized nanoparticles causes efflux modulation at the blood–brain barrier. Nanomedicine. 2017;12(12):1385–1399. doi:10.2217/nnm-2017-0023
101. Lee YK, Choi E-J, Webster TJ, Kim S-H, Khang D. Effect of the protein Corona on nanoparticles for modulating cytotoxicity and immunotoxicity. Int J Nanomed. 2015;10:97–113. doi:10.2147/IJN.S72998
102. Lima T, Bernfur K, Vilanova M, Cedervall T. Understanding the lipid and protein corona formation on different sized polymeric nanoparticles. Sci Rep. 2020;10(1):1129. doi:10.1038/s41598-020-57943-6
103. Marei HE. Multimodal targeting of glioma with functionalized nanoparticles. Cancer Cell Int. 2022;22(1):265. doi:10.1186/s12935-022-02687-8
104. Lu R-M, Hwang Y-C, Liu I-J, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1):1. doi:10.1186/s12929-019-0592-z
105. Fam SY, Chee CF, Yong CY, Ho KL, Mariatulqabtiah AR, Tan WS. Stealth coating of nanoparticles in drug-delivery systems. Nanomaterials. 2020;10(4):787. doi:10.3390/nano10040787
106. Kong J, Zou R, Law G-L, Wang Y. Biomimetic multifunctional persistent luminescence nanoprobes for long-term near-infrared imaging and therapy of cerebral and cerebellar gliomas. Sci Adv. 2022;8(10):eabm7077. doi:10.1126/sciadv.abm7077
107. Cui YX, Sun JJ, Hao WY, et al. Dual-target peptide-modified erythrocyte membrane-enveloped PLGA nanoparticles for the treatment of glioma. Front Oncol. 2020;10. doi:10.3389/fonc.2020.563938
108. Allami P, Heidari A, Rezaei N. The role of cell membrane-coated nanoparticles as a novel treatment approach in glioblastoma. Front Mol Biosci. 2023;9:1083645.
109. Sharif S, Ghahremani MH, Soleimani M. Delivery of exogenous miR-124 to glioblastoma multiform cells by wharton’s jelly mesenchymal stem cells decreases cell proliferation and migration, and confers chemosensitivity. Stem Cell Rev Rep. 2018;14(2):236–246. doi:10.1007/s12015-017-9788-3
110. Zhang C, Song J, Lou L, et al. Doxorubicin-loaded nanoparticle coated with endothelial cells-derived exosomes for immunogenic chemotherapy of glioblastoma. Bioeng Transl Med. 2021;6(3):e10203. doi:10.1002/btm2.10203
111. Zhong L, Wang J, Wang P, et al. Neural stem cell-derived exosomes and regeneration: cell-free therapeutic strategies for traumatic brain injury. Stem Cell Res Ther. 2023;14(1):198. doi:10.1186/s13287-023-03409-1
112. Lee H, Bae K, Baek AR, et al. Glioblastoma-Derived Exosomes as Nanopharmaceutics for Improved Glioma Treatment. Pharmaceutics. 2022;14(5):1002.
113. Karami Fath M, Azami J, Masoudi A, et al. Exosome-based strategies for diagnosis and therapy of glioma cancer. Cancer Cell Int. 2022;22(1):262. doi:10.1186/s12935-022-02642-7
114. Li J, Wei Y, Zhang C, et al. Cell-membrane-coated nanoparticles for targeted drug delivery to the brain for the treatment of neurological diseases. Pharmaceutics. 2023;15(2):621.
115. Burgess A, Shah K, Hough O, Hynynen K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert Rev Neurother. 2015;15(5):477–491. doi:10.1586/14737175.2015.1028369
116. Dasgupta A, Liu M, Ojha T, Storm G, Kiessling F, Lammers T. Ultrasound-mediated drug delivery to the brain: principles, progress and prospects. Drug Discov Today Technol. 2016;20:41–48. doi:10.1016/j.ddtec.2016.07.007
117. Zhang S, Zhang S, Luo S, et al. Ultrasound-assisted brain delivery of nanomedicines for brain tumor therapy: advance and prospect. J Nanobiotechnology. 2022;20(1):287. doi:10.1186/s12951-022-01464-z
118. Barzegar-Fallah A, Gandhi K, Rizwan SB, Slatter TL, Reynolds JNJ. Harnessing ultrasound for targeting drug delivery to the brain and breaching the blood-brain tumour barrier. Pharmaceutics. 2022;14:2231.
119. Martinez P, Nault G, Steiner J, et al. MRI-guided focused ultrasound blood-brain barrier opening increases drug delivery and efficacy in a diffuse midline glioma mouse model. Neurooncol Adv. 2023;5(1):vdad111. doi:10.1093/noajnl/vdad111
120. Phillips LC, Dhanaliwala AH, Klibanov AL, Hossack JA, Wamhoff BR. Focused ultrasound-mediated drug delivery from microbubbles reduces drug dose necessary for therapeutic effect on neointima formation--brief report. Arterioscler Thromb Vasc Biol. 2011;31(12):2853–2855. doi:10.1161/ATVBAHA.111.238170
121. Wu SY, Sanchez CS, Samiotaki G, Buch A, Ferrera VP, Konofagou EE. Characterizing focused-ultrasound mediated drug delivery to the heterogeneous primate brain in vivo with acoustic monitoring. Sci Rep. 2016;6:37094. doi:10.1038/srep37094
122. Arsiwala TA, Sprowls SA, Blethen KE, et al. Ultrasound-mediated disruption of the blood tumor barrier for improved therapeutic delivery. Neoplasia. 2021;23(7):676–691. doi:10.1016/j.neo.2021.04.005
123. Brighi C, Salimova E, de Veer M, Puttick S, Egan G. Translation of focused ultrasound for blood-brain barrier opening in glioma. J Control Release. 2022;345:443–463. doi:10.1016/j.jconrel.2022.03.035
124. Wang S, Chen Y, Guo J, Huang Q. Liposomes for tumor targeted therapy: a review. Int J Mol Sci. 2023;24(3):2643.
125. Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid nanoparticles horizontal line from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–17015. doi:10.1021/acsnano.1c04996
126. Kim YS, Ko MJ, Moon H, et al. Ultrasound-responsive liposomes for targeted drug delivery combined with focused ultrasound. Pharmaceutics. 2022;14(7):1314.
127. Papachristodoulou A, Signorell RD, Werner B, et al. Chemotherapy sensitization of glioblastoma by focused ultrasound-mediated delivery of therapeutic liposomes. J Control Release. 2019;295:130–139. doi:10.1016/j.jconrel.2018.12.009
128. Zhang X, Hu J, Zhao G, et al. PEGylated PLGA-based phase shift nanodroplets combined with focused ultrasound for blood brain barrier opening in rats. Oncotarget. 2017;8(24):38927–38936. doi:10.18632/oncotarget.17155
129. Wang F, Dong L, Liang SM, et al. Ultrasound-triggered drug delivery for glioma therapy through gambogic acid-loaded nanobubble-microbubble complexes. Biomed Pharmacother. 2022;150:113042.
130. Hersh AM, Bhimreddy M, Weber-Levine C, et al. Applications of focused ultrasound for the treatment of glioblastoma: a new frontier. Cancers. 2022;14(19):4920.
131. Mody VV, Cox A, Shah S, Singh A, Bevins W, Parihar H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl Nanosci. 2014;4(4):385–392. doi:10.1007/s13204-013-0216-y
132. Yang HW, Hua MY, Liu HL, Huang CY, Wei KC. Potential of magnetic nanoparticles for targeted drug delivery. Nanotechnol Sci Appl. 2012;5:73–86. doi:10.2147/NSA.S35506
133. Huang J, Li Y, Orza A, et al. Magnetic nanoparticle facilitated drug delivery for cancer therapy with targeted and image-guided approaches. Adv Funct Mater. 2016;26(22):3818–3836. doi:10.1002/adfm.201504185
134. Spoiala A, Ilie CI, Motelica L, et al. Smart magnetic drug delivery systems for the treatment of cancer. Nanomaterials-Basel. 2023;13(5):876.
135. Zeng S, Tang Q, Xiao M, et al. Cell membrane-coated nanomaterials for cancer therapy. Mater Today Bio. 2023;20:100633. doi:10.1016/j.mtbio.2023.100633
136. Wahajuddin Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomed. 2012;7:3445–3471. doi:10.2147/Ijn.S30320
137. Marino A, Camponovo A, Degl’Innocenti A, et al. Multifunctional temozolomide-loaded lipid superparamagnetic nanovectors: dual targeting and disintegration of glioblastoma spheroids by synergic chemotherapy and hyperthermia treatment. Nanoscale. 2019;11(44):21227–21248. doi:10.1039/c9nr07976a
138. Wlodarczyk A, Gorgon S, Radon A, Bajdak-Rusinek K. Magnetite nanoparticles in magnetic hyperthermia and cancer therapies: challenges and perspectives. Nanomaterials. 2022;12(11):1807.
139. Chang D, Lim M, Goos J, et al. Biologically targeted magnetic hyperthermia: potential and Limitations. Front Pharmacol. 2018;9:831. doi:10.3389/fphar.2018.00831
140. Michael JS, Lee BS, Zhang MQ, Yu JS. Nanotechnology for treatment of glioblastoma multiforme. J Transl Intern Med. 2018;6(3):128–133. doi:10.2478/jtim-2018-0025
141. Wankhede M, Bouras A, Kaluzova M, Hadjipanayis CG. Magnetic nanoparticles: an emerging technology for malignant brain tumor imaging and therapy. Expert Rev Clin Pharmacol. 2012;5(2):173–186. doi:10.1586/ecp.12.1
142. Henkin RI. Intranasal delivery to the brain. Nat Biotechnol. 2011;29(6):480. doi:10.1038/nbt.1866
143. Hanson LR, Frey WH. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9(Suppl 3):S5. doi:10.1186/1471-2202-9-S3-S5
144. Jeong SH, Jang JH, Lee YB. Drug delivery to the brain via the nasal route of administration: exploration of key targets and major consideration factors. J Pharm Invest. 2023;53(1):119–152. doi:10.1007/s40005-022-00589-5
145. Formica ML, Real DA, Picchio ML, Catlin E, Donnelly RF, Paredes AJ. On a highway to the brain: a review on nose-to-brain drug delivery using nanoparticles. Appl Mater Today. 2022;29:101631.
146. Lee D, Minko T. Nanotherapeutics for nose-to-brain drug delivery: an approach to bypass the blood brain barrier. Pharmaceutics. 2021;13(12):2049.
147. Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52. doi:10.1016/j.lfs.2017.12.025
148. Shah P, Sarolia J, Vyas B, Wagh P, Ankur K, Kumar MA. PLGA nanoparticles for nose to brain delivery of clonazepam: formulation, optimization by 32 factorial design, in vitro and in vivo evaluation. Curr Drug Deliv. 2021;18(6):805–824. doi:10.2174/1567201817666200708115627
149. Xinchen Y, Jing T, Jiaoqiong G. Lipid-based nanoparticles via nose-to-brain delivery: a mini review. Front Cell Dev Biol. 2023;11:1214450. doi:10.3389/fcell.2023.1214450
150. Hong SS, Oh KT, Choi HG, Lim SJ. Liposomal formulations for nose-to-brain delivery: recent advances and future perspectives. Pharmaceutics. 2019;11(10):540.
151. Kim KR, Lee AS, Kim SM, Heo HR, Kim CS. Virus-like nanoparticles as a theranostic platform for cancer. Front Bioeng Biotechnol. 2022;10:1106767. doi:10.3389/fbioe.2022.1106767
152. Mohsen MO, Bachmann MF. Virus-like particle vaccinology, from bench to bedside. Cell Mol Immunol. 2022;19(9):993–1011. doi:10.1038/s41423-022-00897-8
153. Nooraei S, Bahrulolum H, Hoseini ZS, et al. Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J Nanobiotechnology. 2021;19(1):59. doi:10.1186/s12951-021-00806-7
154. Ikwuagwu B, Tullman-Ercek D. Virus-like particles for drug delivery: a review of methods and applications. Curr Opin Biotechnol. 2022;78:102785. doi:10.1016/j.copbio.2022.102785
155. Zhang L, Zhou L, Bao L, et al. SARS-CoV-2 crosses the blood-brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct Target Ther. 2021;6(1):337. doi:10.1038/s41392-021-00719-9
156. Kim KS. Investigating bacterial penetration of the blood-brain barrier for the pathogenesis, prevention, and therapy of bacterial meningitis. ACS Infect Dis. 2020;6(1):34–42. doi:10.1021/acsinfecdis.9b00319
157. Ye D, Zimmermann T, Demina V, et al. Trafficking of JC virus-like particles across the blood-brain barrier. Nanoscale Adv. 2021;3(9):2488–2500. doi:10.1039/d0na00879f
158. Nowak I, Madej M, Secemska J, Sarna R, Strzalka-Mrozik B. Virus-based biological systems as next-generation carriers for the therapy of central nervous system diseases. Pharmaceutics. 2023;15(7):1931.
159. Chen YL, Bao CJ, Duan JL, Xie Y, Lu WL. Overcoming biological barriers by virus-like drug particles for drug delivery. Adv Drug Deliv Rev. 2023;203:115134. doi:10.1016/j.addr.2023.115134
160. Rizvi SAA, Saleh AM. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J. 2018;26(1):64–70. doi:10.1016/j.jsps.2017.10.012
161. Mehta N, Lyon JG, Patil K, Mokarram N, Kim C, Bellamkonda RV. Bacterial carriers for glioblastoma therapy. Mol Ther Oncolytics. 2017;4:1–17. doi:10.1016/j.omto.2016.12.003
162. Sun R, Liu M, Lu J, et al. Bacteria loaded with glucose polymer and photosensitive ICG silicon-nanoparticles for glioblastoma photothermal immunotherapy. Nat Commun. 2022;13(1):5127. doi:10.1038/s41467-022-32837-5
163. Pardridge WM. Drug transport across the blood-brain barrier. J Cerebr Blood F Met. 2012;32(11):1959–1972. doi:10.1038/jcbfm.2012.126
164. Pardridge WM. CSF, blood-brain barrier, and brain drug delivery. Expert Opin Drug Deliv. 2016;13(7):963–975. doi:10.1517/17425247.2016.1171315
165. Kucuksayan E, Bozkurt F, Yilmaz MT, Sircan-Kucuksayan A, Hanikoglu A, Ozben T. A new combination strategy to enhance apoptosis in cancer cells by using nanoparticles as biocompatible drug delivery carriers. Sci Rep-Uk. 2021;11(1):13027.
166. Fang RH, Gao W, Zhang L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat Rev Clin Oncol. 2023;20(1):33–48. doi:10.1038/s41571-022-00699-x
167. Zhao M, van Straten D, Broekman MLD, Preat V, Schiffelers RM. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics. 2020;10(3):1355–1372. doi:10.7150/thno.38147
168. Gupta S, Smith TR, Broekman ML. Ethical considerations of neuro-oncology trial design in the era of precision medicine. J Neurooncol. 2017;134(1):1–7. doi:10.1007/s11060-017-2502-0
169. Alghamdi MA, Fallica AN, Virzi N, Kesharwani P, Pittala V, Greish K. The promise of nanotechnology in personalized medicine. J Pers Med. 2022;12(5):673.
170. Gil PR. Nanotechnology opens the landscape of personalized medicine. Curr Med Chem. 2018;25(35):4552. doi:10.2174/092986732535181026145729
171. Chehelgerdi M, Chehelgerdi M, Allela OQB, et al. Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation. Mol Cancer. 2023;22(1):169. doi:10.1186/s12943-023-01865-0
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

