Back to Journals » International Journal of Nanomedicine » Volume 13
Nanomedical studies of the restoration of nitric oxide/peroxynitrite balance in dysfunctional endothelium by 1,25-dihydroxy vitamin D3 – clinical implications for cardiovascular diseases
Authors Khan A, Dawoud H, Malinski T
Received 28 September 2017
Accepted for publication 1 December 2017
Published 19 January 2018 Volume 2018:13 Pages 455—466
DOI https://doi.org/10.2147/IJN.S152822
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Alamzeb Khan, Hazem Dawoud, Tadeusz Malinski
Department of Chemistry & Biochemistry, Nanomedical Research Laboratories, Ohio University, Athens, OH, USA
Background: Clinical studies indicate that vitamin D3 improves circulation and may have beneficial effects in hypertension. This study uses nanomedical systems to investigate the role of 1,25-dihydroxy vitamin D3 in the preservation/restoration of endothelial function in an angiotensin II (Ang II) cellular model of hypertension.
Methods: 1,25-dihydroxy vitamin D3-stimulated nitric oxide (NO) and peroxynitrite (ONOO-) concentrations were measured in situ with nanosensors (200–300 mm diameter with a detection limit of 1 nM) in human umbilical vein endothelial cells of African American (AA) and Caucasian American (CA) donors exposed to Ang II. The balance/imbalance between NO and ONOO- concentrations ([NO]/[ONOO-]) was simultaneously monitored and used as an indicator of endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction.
Results: [NO]/[ONOO-] imbalance in Ang II-stimulated dysfunctional endothelium was 0.20±0.16 for CAs and 0.11±0.09 for AAs. Uncoupled eNOS and overexpression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase contributed to high production of ONOO-. Vitamin D3 treatment reversed [NO]/[ONOO-] to 3.0±0.1 in CAs and 2.1±0.1 in AAs – exceeding that observed in normal endothelium. Vitamin D3 restored uncoupled eNOS and endothelial function by increasing cytoprotective NO and decreasing the cytotoxic ONOO-. The beneficial effect of vitamin D3 is associated with a favorable rate of NO and ONOO- release, restoration of the [NO]/[ONOO-] and the overall decrease in the overexpression of eNOS, inducible nitric oxide synthase and NADPH oxidase. This effect of vitamin D3 may prove to be beneficial in the treatment of hypertension and other cardiovascular diseases, including heart failure, myocardial infarction, vasculopathy, stroke and diabetes.
Keywords: vitamin D3, hypertension, nitric oxide, peroxynitrite, endothelial dysfunction, nanomedicine, restoration of endothelium
Introduction
There is a large body of observational data that links vitamin D3 active metabolite 1,25-dihydroxy vitamin D3 to the function of the cardiovascular system.1,2 This nonclassical effect of vitamin D3 is additional to the more classical role of vitamin D3 on the mineral-calcium metabolism in bone.3,4
Clinical studies suggest (somewhat inconsistently) that elevated vitamin D3 levels can lower arterial blood pressure.5 However, there is a strong correlation between insufficient serum levels of vitamin D3 that are observed in heart failure, myocardial infarction and elevation of arterial blood pressure.6 In cross-sectional studies (National Health and Nutrition Examination Survey III), a deficiency in vitamin D3 metabolites correlated with hypertension, diabetes mellitus, hyperglyceridemia and obesity.7
Studies carried out on vitamin D3 receptor (VDR) knockout and 1-α hydroxylase-deficient mouse models showed elevated arterial blood pressure.8,9 Other studies carried out on spontaneous hypertensive rats have indicated that vitamin D3 administration suppresses endothelium-dependent contraction of aorta in these models.10,11 Vitamin D3 has antihypertrophic, anti-inflammatory and antiproliferative properties and may reduce cardiac hypertrophy in spontaneous hypertensive rats.12 Furthermore, vitamin D3 has a direct effect on endothelial and smooth muscle cells and may decrease coagulation and increase re-endothelialization and fibrinolysis.12
The dysfunction of endothelium in the cardiovascular system is a common denominator of several diseases such as hypertension, diabetes, obesity and heart failure – all of which are diseases where an insufficient level of vitamin D3 is observed.13–15 The dysfunction of the endothelium is characterized by low production of bioavailable nitric oxide16 (NO) – a cytoprotective vasorelaxant – and a high concentration of cytotoxic vasoconstrictor, peroxynitrite (ONOO−).17 ONOO− is one of the most powerful oxidants in the biological milieu, and at high concentrations ONOO− can considerably shift the redox balance in endothelium and negatively affect vascular function and hemostasis.18,19
Endothelial peroxynitrite is produced in the rapid diffusion-controlled reaction of superoxide (O2−) with NO.20 There are two major sources of O2− in dysfunctional endothelium: NADPH oxidase and uncoupled endothelial nitric oxide synthase (eNOS). Partially uncoupled eNOS can concomitantly produce NO and O2−, in close proximity, potentially resulting in high levels of ONOO−. Therefore, uncoupled eNOS can be a very effective generator of ONOO−, which can trigger a cascade of redox events leading to endothelial dysfunction.
The study presented here uses nanomedical methods of measurement and analysis to elucidate the role of 1,25-dihydroxy vitamin D3 (abbreviated as vitamin D3 throughout the text) in the stimulation of NO and ONOO−. Normal and angiotensin II (Ang II)-induced dysfunctional human umbilical vein endothelial cells (HUVECs) of African Americans (AAs) and Caucasian Americans (CAs) were used in this study. Nanosensors were used for the in situ monitoring of the concentrations of NO and ONOO− after the treatment of dysfunctional endothelial cells with vitamin D3. We developed a unique nanosystem that can be used for the simultaneous direct measurement in situ of bioavailable NO and ONOO− in single endothelial cells.21,22 No other currently available methods of NO and ONOO− measurements (chemoluminescence, UV spectroscopy, fluorescence) are suitable for these kinds of measurements. The balance between NO concentration (NO) and ONOO− concentration (ONOO−) was used to evaluate the level of eNOS uncoupling and endothelial function/dysfunction. Vitamin D3 treatment can effectively restore [NO]/[ONOO−] to a level similar to normal endothelium for both CAs and AAs. We believe that the vitamin D3-stimulated improvement of endothelial function may directly benefit the treatment of the dysfunction of the cardiovascular system.
Methods
Reagents
Vitamin D3 (1,25-dihydroxy vitamin D3, calcitriol) was purchased from Cayman Chemicals. 7-(1,3-Benzoxazol-2-ylsulfanyl)-3-benzyl-3H-[1,2,3]triazolo[4,5-d]pyrimidine (VAS2870), an inhibitor of NADPH oxidase, and Ang II (human) were from Sigma Aldrich. Material for the preparation of NO and ONOO− sensors: Mn (III) meso-tetra (N-methyl-4-pyridyl) porphyrin pentachloride, TMHPPMn (CAS # 125565–45-9) and nickel (II) tetrakis (3-methoxy-4-hydroxy-phenyl) porphyrin, TNMPPNi (Cat # T40113) (Frontier Scientific). MCDB-131 complete medium (VEC Technologies). Bovine serum albumin, primary antibodies: rabbit polyclonal immunoglobulin G (IgG) anti eNOS (SC-654), rabbit polyclonal IgG anti-inducible nitric oxide synthase (iNOS) (SC-650), rabbit polyclonal IgG anti-Nox4 (SC-30141) and goat anti-rabbit IgG–horseradish peroxidase conjugate (SC-2004, secondary antibody) (Santa Cruz biotechnologies). BCA kit (Thermo Fisher Scientific).
Endothelial cell culture
HUVECs of AA (pooled, n=6, age 26±3 years) and CA (pooled, n=7, age 27±2 years) donors were purchased from Lonza (Walkersville, MD, USA). Cells were grown in T-75 flasks (Greiner bio-one, Cat # 658175) and incubated in MCDB-131 complete medium (VEC technologies) at 37°C under 5% CO2 and 95% air and passaged every 3–4 days. After reaching confluence, the monolayer cells were trypsinized and fully detached. MCDB-131 (10 mL) complete medium was then added to trypsinized cell suspension in order to inactivate trypsin. Cells were then centrifuged at 1,500 rpm for 5 minutes. The cell pellet was then resuspended in 3 mL of fresh MCDB-131 complete media. To maintain the cell culture passage, 1 mL of the cell suspension was transferred to each of the three T-75 flasks followed by addition of 11 mL of fresh MCDB-131 complete media. For measurement, cells were resuspended in MCDB-131 and 1×105 cells were seeded in each well of 24-well cell culture plates.
Preparation of electrochemical nanosensors
NO and ONOO− nanosensors (diameter 200–300 nm) were prepared according to the published procedure.21,22 Briefly, a carbon fiber (6 μm in diameter) was inserted into a glass capillary (80–100 μm in diameter). The tip (8–15 μm) of the capillary holding protruded carbon fiber was sealed with mixture of bee wax and rosin, and shortened/reduced to the diameter of 200–300 nm by heating in propane microburners. The active tip of the carbon fiber was then coated by electrochemical polymerization of monomeric TMHPPNi to form a conductive polymeric film. The polymeric TMHPPNi film was then coated with Nafion (1% in ethanol). Similarly, the ONOO− nanosensor was prepared by deposition of a conductive TNMPPMn polymeric film on the tip of the carbon fiber by cyclic voltammetry. The surface of the polymeric TNMPPMn sensor was then coated with poly(4-vinyl-pyridine). The sensors were stored at room temperature in a phosphate buffer (pH 7.4). The NO and ONOO− nanosensors have a detection limit of 1 and 3 nmol/L, respectively, and can be used separately or in tandem for the simultaneous measurements of NO and ONOO− concentrations. Each of the nanosensors sample a volume of about 3–10 picoliters.
The sensor’s selectivity is based on the potential of NO or ONOO− oxidation/reduction, very rapid electron transfer (high current) generated in this reaction, as well as the preventative barriers for negatively charged species (NO2−, NO3−, dopamine, etc.) for the NO sensor and a barrier for positively charged species for the ONOO− sensor. Additionally, the response selectivity for NO was confirmed in separate experiments in the presence of inhibitor(s) of eNOS (N(G)-nitro-L-arginine methyl ester [L-NAME]) or scavengers of ONOO− (Mn(III) tetrakis) (4-benzoic acid) porphyrin chloride. The detection limit of sensors is 1×10−9 mol/L for NO and 3×10−9 mol/L for ONOO−. The response time (limited by sensor response and analytical data collection system) is better than 5 μs and was estimated based on the response time of the sensor and processing/storage time of analytical (electrical current) signal.
Amperometric measurement of NO and ONOO−
Three-electrode system consisting of a platinum wire (counter electrode), Ag/AgCl (reference electrode) and a tandem of NO and ONOO− nanosensors (working electrodes) was used. Amperometric curves of current (proportional to concentration) versus time were measured using a GAMRY dual potentiostat, at a potential of 0.56 V (versus Ag/AgCl) for NO and −0.32 V (versus Ag/AgCl) for ONOO−. HUVECs cultured in a well were incubated under 5% CO2 and 95% O2 and allowed to grow for 1–2 days. Nanosensors were positioned at a well-defined and reproducible distance from the membrane of a single endothelial cell. X, Y and Z positions of the nanosensors were monitored according to the following procedure: first, the sensor was placed about 50 μm above endothelial cells, with the help of a stereotactic remote-controlled micromanipulator (Sexsapex, Finland) and gradually lowered closer to the membrane of the “sacrificial” single endothelial cell. When the sensor touched the membrane, a small signal (piezoelectric current) and a mechanically stimulated NO signal were recorded. The position of the sensor was assumed as zero distance from the cell membrane (axis Z=0). From Z=0, the sensor was raised about 5 μm and transferred along the X and Y axes (keeping the Z axis constant) and positioned above another endothelial cell, separated about 50–70 μm from the “sacrificial” cell. The “sacrificial” cell could not be used for further measurements because it was mechanically distorted by the sensor. The positioning of the nanosensor at the well-defined distance (5±2 μm) from the cell membrane is necessary to obtain reproducible results. NO concentration decreases with the increase of distance away from the membrane surface. A distance higher than 100 μm, NO can no longer be detected by the nanosensor.26
Vitamin D3 (concentration, 1 μmol/L in 0.1 mol/L phosphate buffer) was injected with a microinjector in a well and NO and/or ONOO− release was measured with a time response better than 5 μs. A stock solution of vitamin D3 was prepared as follows: vitamin D3 was initially dissolved in several microliters of dimethyl sulfoxide (DMSO) and subsequently diluted in water and an aqueous solution of 0.1 mol/L of phosphate buffer. The trace amounts of DMSO present in aqueous stock solution of vitamin D3 did not have any significant effect on NO or ONOO− release.
NO and ONOO− concentration was calculated by the standard addition method and/or by a standard calibration curve for each sensor before and after measurement. The nitric oxide nanosensor was calibrated by using a standard solution (range of about 20–600 nmol/L) prepared from stock solution of NO (saturated solution of NO 1.8 mM) in phosphate buffer (pH 7.4). A linear calibration curve was constructed from these measurements for each sensor. Also, a standard addition method was used to monitor the response of the sensor to subsequently added standard solution of NO. The concentration of NO standard was confirmed with UV-visible spectrophotometry (hemoglobin method) and/or coulometry.
The peroxynitrite nanosensor was also calibrated in amperometric mode by both, the calibration curve and the standard addition method. The absorbance of ONOO− was measured in standard solution using a UV-visible spectroscopy at a wavelength of 303 nm. The molar absorptivity coefficient for peroxynitrite is 1,670 M−1 cm−1. The electrochemical NO or ONOO− nanosensor measured the net concentration of diffusible NO and ONOO−.
In a separate set of experiments, cells were incubated with Ang II (1 μmol/L, 1 hour) to make them dysfunctional and vitamin D3-stimulated NO and ONOO− were measured. Additionally, dysfunctional (Ang II treated) endothelial cells were incubated with different concentrations of vitamin D3 before measuring NO and ONOO− in the presence or absence of L-arginine (eNOS substrate), VAS 2780 (NADPH oxidase inhibitor) or PEG-SOD (membrane-permeable dismutase of O2−).
Immunoassay
For detection of our targeted proteins (eNOS, iNOS, NADPH oxidase), the indirect enzyme-linked immunosorbent assay was performed as per the instructions of Abcam’s protocol and plates were read in a microplate reader (BioTek Synergy HT). Protein samples were analyzed in triplicate and the results were recorded from absorbance at 450 nm.
Statistical analysis and calculations
All data presented here are mean ± standard error, n=3–10. One-way analysis of variance with Student–Newman–Keuls multiple comparisons post hoc analysis was used to statistically analyze the mean difference between multiple comparisons. A P-value of <0.05 was considered statistically significant. Origin (v 6.1 for windows, originLab, Northampton, MA, USA) and GraphPad Prism were used to analyze and plot data.
Results
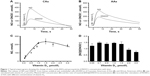
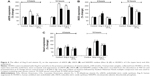
Typical amperograms showing changes of NO and ONOO− concentrations with time after stimulation of normal HUVECs with vitamin D3 (1 μmol/L) are shown in Figure 1A and B. Vitamin D3 stimulated NO release from endothelium. The maximal NO concentration of 370±15 nmol/L was reached after about 7 s for CAs. For AAs, the peak of 328±21 nmol/L was reached after about 6 s. NO release was accompanied by relatively low production of ONOO−, with maximal concentrations of 150±12 nmol/L for CAs and 190±17 nmol/L for AAs. A relationship between the maximal NO concentration and the concentration of vitamin D3 is shown in Figure 1C. A linear increase in NO was observed up to about 1 μmol/L concentration of vitamin D3. At concentrations of D3 higher than 1.5 μmol/L, the NO concentrations linearly decreased. A plot of the ratio of NO concentrations (NO) and ONOO− concentrations (ONOO−) versus the concentration of vitamin D3 is shown in Figure 1D. The [NO]/[ONOO−] ratio, which reflects the balance between these two molecules, increased slightly, but not significantly, with the increase of vitamin D3 levels. However, with concentrations of vitamin D3 higher than 1.5 μmol/L, the ratio of [NO]/[ONOO−] unfavorably shifted to low levels.
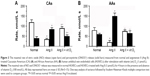
Figure 2A and B shows concentration changes of NO and ONOO− after stimulation by vitamin D3 in HUVECs treated with Ang II (1 μmol/L, 4 hrs) – cellular model of hypertension. For Ang II-treated CA endothelial cells, both maximal (NO) and maximal (ONOO−) changed significantly. NO decreased to 62±11 nmol/L, while ONOO− increased to 334±13 nmol/L. Maximal (NO) and (ONOO−) in AAs also changed considerably: (NO) decreased to 50±5 nmol/L and (ONOO−) increased to 430±19 nmol/L after treatment with Ang II and indicated significant eNOS uncoupling. The treatment of cells with vitamin D3, in this cellular model of hypertension, significantly improved the function of eNOS and endothelium (Figure 2C and D). Vitamin D3 restored (NO) and diminished (ONOO−) to levels similar to those observed for fully functional endothelial cells, in both AAs and CAs.
Vitamin D3 improves NO production in HUVECs of CAs and AAs
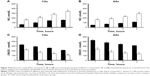
Vitamin D3 stimulated NO release, and the rate of NO release was moderate in normal HUVECs (Figure 3). The rate of NO release was faster in normal CA cells (120±12 nmol/L s) than in normal AA cells (80±13 nmol/L s). In contrast, the rate of ONOO− production was much faster in AA than in CA cells – about 55 and 30 nmol/L s, respectively. Ang II treatment slowed the rate of NO release by about 70%–75% for both CAs and AAs. As expected, the rate of ONOO− generation increased by about 80%. Vitamin D3 restored the rate of NO release and significantly decreased the rate of ONOO− production to a level similar to that observed in normal CA and AA cells. Vitamin D3 treatment significantly increased maximal NO concentration as compared to Ang II-treated HUVECs for both ethnic groups (Figure 4). [NO] increased linearly with the time of incubation with vitamin D3. The increase in the level of NO with vitamin D3 treatment was accompanied with a linear decrease of ONOO−. After 4 hours of incubation with vitamin D3, the concentration of NO increased by about 5 times, while there was an inverse fivefold decrease observed with ONOO− after the same incubation period. The beneficial effects of vitamin D3 was observed for both CA and AA HUVECs that were treated with Ang II (1 μmol/L).
Vitamin D3 improves the [NO]/[ONOO−] in HUVECs of CAs and AAs
To evaluate the status of endothelial function, we used the ratio of NO to ONOO− concentration [NO]/[ONOO−]. The ratio of [NO]/[ONOO−] reflects on the relative balance between cytoprotective NO and cytotoxic ONOO−.
In Ang II cellular model of hypertension, [NO]/[ONOO−] was 0.20±0.16 for CA and 0.11±0.09 for AA cells (Figure 5A and B). After 1 hour of treatment with vitamin D3, there was an increase in the [NO]/[ONOO−] ratio to 0.70±0.05 for both CAs and AAs. The increase in [NO]/[ONOO−] ratio was linear with vitamin D3 incubation time and reached a maximum of 3.0±0.1 for CAs and 2.10±0.11 for AAs after 4 hours, which exceeded the ratio observed in normal HUVECs. In normal HUVECs studied, the [NO]/[ONOO-] ratio was 2.5±0.2 and 1.7±0.2 for CA and AA cells, respectively (Figure 5C and D).
Effect of modulators of eNOS and NADPH pathway in Ang II cellular model of hypertension
We used the ratio of [NO]/[ONOO−] to elucidate the function of eNOS and NADPH in the Ang II cellular model of hypertension (Figure 5C and D). L-NAME, a nonselective eNOS inhibitor, does not have a significant effect on [NO]/[ONOO−]. However, at elevated concentrations of L-arginine (eNOS substrate) a substantial increase of the [NO]/[ONOO−] was observed. A similar increase in [NO]/[ONOO−] was also observed in the presence of sepiapterin, which is a precursor of tetrahydrobiopterin – an important cofactor in stabilizing eNOS dimer. An inhibitor of NADPH (VAS 2870) also significantly increased [NO]/[ONOO−] to the level observed in the presence of L-arginine or sepiapterin. The changes of [NO]/[ONOO−] due to cofactors, inhibitors and substrates were slightly more pronounced in CA cells than in AA cells.
Vitamin D3 downregulates expression of NADPH oxidase, eNOS and iNOS in cultured HUVECs of CAs and AAs
We hypothesized that the overexpression of NADPH oxidase, eNOS and iNOS may play a role in an unfavorable shift in the [NO]/[ONOO−] balance and endothelial function.
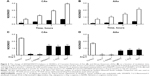
After 4 hours of incubation of HUVECs with Ang II, the eNOS expression was significantly upregulated in CAs and slightly in AAs (Figure 6A). Ang II further upregulated eNOS in HUVECs of both ethnic groups, and this effect was very significant after 12 hours of incubation. In contrast, vitamin D3 treatment downregulated eNOS expression in HUVECs of both ethnic groups. This effect was highly significant after 12 hours of incubation with vitamin D3.
iNOS expression can be upregulated due to oxidative and nitroxidative stresses and/or inflammation. Therefore, we estimated iNOS expression in both Ang II- and vitamin D3-treated HUVECs. Ang II significantly upregulated expression of iNOS in HUVECs of both ethnic groups versus control groups after 4 and 12 hours of incubation (Figure 6B). Treatment with vitamin D3 downregulated the expression of iNOS in HUVECs of both ethnic groups.
Ang II had minimal effect on the NADPH oxidase 4 expression after 4 hours of incubation; however, NADPH oxidase expression was upregulated after 12 hours (Figure 6C), indicating the effect of Ang II on the expression of enzymes responsible for O2−-induced oxidative stress. Vitamin D3 treatment significantly downregulated the expression of NADPH oxidase in HUVECs of both ethnic groups. The effect of vitamin D3 was more pronounced in HUVECs of AAs than in those of CAs. These results indicate that vitamin D3 may particularly protect endothelial cells from NADPH-generated O2−, and oxidative stress may reduce ONOO− formation and improve endothelial function.
Discussion
The nanomedical system utilized in these studies offers a unique opportunity to monitor in situ, the near real-time molecular changes of two signaling molecules, NO and ONOO−, produced by endothelial cells. The production of ONOO− increases with the increased rate of NO release. Therefore, any rapid stimulation of NO could be considered unfavorable for endothelial function due to the potential uncoupling of eNOS and enhanced ONOO− generation. Therefore, a modest rate of vitamin D3-stimulated NO release is of crucial importance to the proper function of endothelium and the cardiovascular system. This is one of the important findings of this study. Here, we used Ang II to produce dysfunctional endothelium, similar to that observed in hypertension. Treatment with vitamin D3, in this cellular model of hypertension, significantly restored bioavailable NO with the concomitant decrease in nitroxidative stress that is associated with high ONOO−. The moderate NO production by vitamin D3 does not cause eNOS uncoupling. As a net result, vitamin D3 maintains a favorably high ratio of [NO]/[ONOO−] in the endothelium with a relatively high concentration of cytoprotective vasorelaxant NO and a relatively low level of cytotoxic vasoconstrictor ONOO−.24 This favorable kinetics of NO generation and the subsequent low production of ONOO− after stimulation with vitamin D3 are crucial factors in the process of restoring dysfunctional endothelium.
In long-term (hours) treatment, vitamin D3 effectively reversed the imbalance between [NO] and [ONOO−] in Ang II cellular model of hypertension. Importantly, vitamin D3 restored eNOS coupling in dysfunctional HUVECs in both CAs and AAs as evidenced by increased NO bioavailability and reduced nitroxidative stress. The uncoupling of eNOS was significantly higher in AAs than in CAs, as indicated by [NO]/[ONOO−]. However, treatment with vitamin D3 produces proportional results for both ethnic groups. There was no significant difference in the % of [NO] and [ONOO−] changes between AAs and CAs versus control group. A decrease in the expression of eNOS, as well as NADPH oxidase, that occurs after vitamin D3 treatment of the Ang II model of hypertension is an important factor in the overall restoration of endothelial function. Even a small decrease in eNOS expression improved the availability of substrates (especially L-arginine) and other cofactors, and more effectively stabilized (coupled) eNOS dimer, preventing the generation of significant O2− by this enzyme.
The dominating product of coupled eNOS dimer is NO, while in the uncoupled dimer of eNOS, a rapid direct one-electron transfer to oxygen produces O2−. NO is the most effective scavenger of O2−, which produces ONOO− in a diffusion-controlled reaction.23,24 Therefore, vitamin D3 treatment actively reduced endothelial production of O2− from two major sources: NADPH oxidase and uncoupled eNOS. The efficiency of O2− production by uncoupled eNOS in dysfunctional endothelium can be comparable to that generated by NADPH. Vitamin D3 simultaneously decreases the level of O2− generated by these two sources, by reducing the level of NADPH on one side and then by limiting the generation of O2− from coupled eNOS dimer on the other side.
This effect accounts for the reduction of oxidative/nitroxidative stress and the overall increase in bioavailable NO – both a highly beneficial effect of vitamin D3 treatment on endothelium and the cardiovascular system. The process of restoring bioavailable NO production by vitamin D3 is more efficient and comprehensive than other possible pathways of the restoration of dysfunctional endothelium, like elevated levels of L-arginine, sepiapterin treatment, scavenging or dismutase of O2−. As shown here, L-arginine, sepiapterin or NADPH inhibition can only partially restore (20%–30%) endothelial function under physiologically acceptable concentrations.
In this study, we used the [NO]/[ONOO−] ratio as a precise indicator of eNOS coupling/uncoupling and endothelial function/dysfunction.25 In normal, functional endothelium, the [NO]/[ONOO−] ratio varies from 2 to 5.26 At an [NO]/[ONOO−] level of <2, the eNOS is considered partially uncoupled. When the [NO]/[ONOO−] ratio falls below 1, uncoupled eNOS become the dominant factor in the production of O2− and the highly efficient generator of ONOO−. Therefore, when the [NO]/[ONOO−] ratio is below 1, ONOO− is the main determinant of the oxidative/nitroxidative stress in endothelium. The nanomedical approach used in this study allowed us to directly measure in situ, with a time resolution of better than 5 ms, the concentration of NO and ONOO− in normal/fully functional and dysfunctional endothelium. In the HUVECs studied here, the initial [NO]/[ONOO−] was 2.5±0.2 and 1.7±0.2 for CAs and AAs, respectively. As we have shown in a previous study, the eNOS uncoupling is more advanced in AAs than in CAs.17 Treatment with Ang II further increased eNOS uncoupling, which was reflected by a decrease in [NO]/[ONOO−] ratio to 0.20±0.16 for CAs and 0.11±0.09 for AAs. This suggests that potential damage to endothelial function imposed by Ang II in hypertension is more severe in AAs than in CAs. It is interesting to note that the treatment of endothelial cells of AAs (Ang II cellular model of hypertension) with vitamin D3 significantly improved the [NO]/[ONOO−] ratio above the original levels observed in normal functioning HUVECs of CAs and AAs. The [NO]/[ONOO−] gradually increased in both groups after 1–4 hours of vitamin D3 treatment.
The balance/imbalance of [NO]/[ONOO−] is influenced by NO, O2− and ONOO−; coupled/uncoupled eNOS, iNOS and NADPH oxidase. All of these molecules and their sources may contribute to shift in oxidative/nitroxidative stress and bioavailability of NO. Upregulated expression of these three enzymes may lead to excessive oxidative stress in cellular environment, which effectively diminished the level of bioavailable NO. Furthermore, upregulation in the expression of iNOS, which is usually associated with inflammation, can contribute to this unfavorable effect. By design, iNOS is a secondary source of NO synthesis when there is a shortage of the NO generated by eNOS. High NO production by iNOS depletes L-arginine levels and accelerates the further uncoupling of eNOS and makes the recovery of eNOS dimer even more difficult. Additionally, the NO produced from iNOS contributes minimally to the overall level of bioavailable NO, but has a significant effect on the overall increase of ONOO− due to the scavenging of O2−. This further exposes the cellular environment to severe oxidative and nitroxidative stress and endothelial dysfunction. Also, excessive O2− and ONOO− can oxidize tetrahydrobiopterin, BH4, an important cofactor of eNOS and a stabilizer of eNOS dimer.27,28 In the absence of BH4, eNOS produces mostly O2−. Supplementation of L-arginine and/or sepiapterin (precursor of BH4) improved endothelial function by diminishing O2− and ONOO− concentrations.
Our study provides direct molecular insight to previously published observations that have suggested that vitamin D3 deficiency-induced hypertension is associated with vascular oxidative stress.29 It is well documented that oxidative stress is involved in vascular complications and development of hypertension.30 Oxidative and nitroxidative stress impairs endothelium-dependent relaxation of the blood vessels and is involved in the facilitation of smooth muscles contractions.31–33 It has been suggested that oxidative stress is the main source of endothelial damage, and that it is generated by NADPH oxidase – one of the key enzymes of facilitating oxidative stress. We found here that the contributions of NADPH to the total oxidative stress is dominant in the initial stage of endothelial dysfunction. However, in severely dysfunctional endothelium, the contribution of NADPH to the total O2− was less than that generated from uncoupled eNOS (40% and 60%, respectively). Results of the studies presented here are coherent with and complementary to the results of previous observations. Scavengers and inhibitors of oxidative and nitroxidative stress partially prevent the dysfunction of endothelium.34 It has been suggested that protective effect of AT1R blockers, the major class of antihypertensive drugs in patients with hypertension, is associated with their antioxidative action.35,36
In SHR, it has been found that vitamin D3 reduces the expression of NADPH oxidase in the vasculature.37 Vitamin D3 treatment of renal arteries of hypertensive patients showed improved endothelial function and reduction in oxidative stress.37 It was suggested that the downregulation of NADPH oxidase and the upregulation of SOD1 and SOD2 may be responsible for this effect.37 Our studies support and confirm this hypothesis.
Based on clinical studies, it has been suggested that vitamin D3 may improve NO, modulate vascular tone and lower blood pressure in patients with hypertension.29 However, none of these studies provided any direct proof of how vitamin D3 improves NO production and decreases nitroxidative stress. Our studies are not only coherent with previous observations, but provide new direct evidence, on the molecular level, why vitamin D3 can be highly beneficial for the treatment of a dysfunctional cardiovascular system. We proved here by direct NO measurements that vitamin D3 stimulated NO release within the first second of its exposure to endothelial cells. This rapid response of endothelium to vitamin D3 suggests that this process is most likely controlled by VDRs that are present on endothelial cell membrane,38 and is followed by the signal transduction through the calcium–calmodulin pathway. We suggest that the beneficial action of vitamin D3 on the cardiovascular system can be explained solely based on the enhanced effect on endothelial NO levels and its depreciative effect on ONOO− levels.
While the beneficial effects of vitamin D3 on dysfunctional eNOS and endothelium is well supported by this research, the effect of a high level of vitamin D3 on normal cells will require more studies to establish the potential for an unfavorable shift of [NO]/[ONOO−] to a level higher than 5. The ideal ratio of NO to ONOO− for fully functional endothelium varies from 3 to 5 and depends on the location of the endothelial cells in the vasculature. Generally, at [NO]/[ONOO−] higher than 7, the NO may become a dominating factor, which can potentially lead to excessive production of cyclic guanosine monophosphate, excessive smooth and cardiac muscle relaxation and a dramatic decrease in blood pressure. Therefore, one can expect that excessive vitamin D3 treatment of fully functional endothelial cells may produce risk for the cardiovascular system.
The studies presented here strongly indicate that vitamin D3 restores endothelial function by balancing [NO]/[ONOO−], increasing bioavailable NO and reducing oxidative and nitroxidative stress in HUVECs, thus confirming the potential role of vitamin D3 in the prevention and/or treatment of vascular complications. The improvement in the [NO]/[ONOO−] by vitamin D3 is mainly due to the restoration of eNOS coupling and decrease in eNOS, iNOS and NADPH expressions. Vitamin D3 does not significantly scavenge for superoxide or peroxynitrite but attenuates oxidative/nitroxidative stress and increases NO bioavailability by transcriptional regulation of the enzymes responsible for generating them. The net result is a decrease of the expression of iNOS and eNOS in the cellular environment. At low eNOS and iNOS expression, the available concentrations of major substrate, L-arginine and cofactor (BH4) become more available for eNOS coupling.
Therefore, these studies are the first to identify the molecular mechanism of vitamin D3-triggered restoration of the function of eNOS and the function of endothelial cells in the cardiovascular system. While these studies were performed using a cellular model of hypertension, the implications of the influence of vitamin D3 on dysfunctional endothelium is much broader. The dysfunction of endothelium is a common denominator of several cardiovascular diseases, particularly those associated with ischemic events. Therefore, we suggest that vitamin D3 treatment may be of clinical importance in the restoration of dysfunctional cardiac endothelium after heart ischemia, capillary endothelium after brain ischemia, hypovolemia, vasculopathy, diabetes and atherosclerosis. Our suggestion is strongly supported by several clinical studies indicating that vitamin D3, at doses significantly higher than those currently used for the treatment of bone diseases, can be highly beneficial for the treatment of the dysfunctional cardiovascular system.
Acknowledgments
Special thanks to Collin Arocho for all of his assistance in the preparation of this manuscript. Support for this research came from the Ita Pluta Plutowski endowment fund, the Ohio University Foundation, and the Marvin and Ann Dilley White Professorship endowment.
Disclosure
The authors report no conflicts of interest in this work.
References
Pilz S, Tomaschitz A, Ritz E, Pieber T. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol. 2009;6(10):621–630. | ||
Drechsler C, Pilz S, Obermayer-Pietsch B, et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010;31(18):2253–2261. | ||
St-Arnaud R. The direct role of vitamin D on bone homeostasis. Arch Biochem Biophys. 2008;473(2):225–230. | ||
Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–329. | ||
Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D3 and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women 1. J Clin Endocrinol Metab. 2001;86(4):1633–1637. | ||
Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174–1180. | ||
Martins D, Wolf M, Pan D. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition. Arch Intern Med. 2007;167(11):1159–1165. | ||
Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74(2):170–179. | ||
Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–238. | ||
Wong MS, Delansorne R, Man RY, Svenningsen P, Vanhoutte PM. Chronic treatment with vitamin D lowers arterial blood pressure and reduces endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2010;299(4):H1226–H1234. | ||
Wong M, Delansorne R. Vitamin D derivatives acutely reduce endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Hear Circ Physiol. 2008;295(1):H289–H296. | ||
Wu-Wong JR. Potential for vitamin D receptor agonists in the treatment of cardiovascular disease. Br J Pharmacol. 2009;158(2):395–412. | ||
Korda M, Kubant R, Patton S, Malinski T. Leptin-induced endothelial dysfunction in obesity. Am J Physiol Heart Circ Physiol. 2008;295(4):H1514–H1521. | ||
Mason RP, Corbalan JJ, Jacob RF, Dawoud H, Malinski T. Atorvastatin enhanced nitric oxide release and reduced blood pressure, nitroxidative stress and rantes levels in hypertensive rats with diabetes. J Physiol Pharmacol. 2015;66(1):65–72. | ||
Mason RP, Jacob RF, Dawoud H, Wagner MR, Mahmud FJ, Sherratt SCR. Eicosapentaenoic acid and atorvastatin active metabolite, alone or in combination, reversed glucose-and oxidized LDL-induced endothelial dysfunction measured ex vivo in rats. Vasc Med. 2016;67(13):2320. | ||
Mason RP, Kubant R, Jacob RF, Walter MF, Boychuk B, Malinski T. Effect of nebivolol on endothelial nitric oxide and peroxynitrite release in hypertensive animals: role of antioxidant activity. J Cardiovasc Pharmacol. 2006;48(1):862–869. | ||
Mason RP, Kalinowski L, Jacob RF, Jacoby AM, Malinski T. Nebivolol reduces nitroxidative stress and restores nitric oxide bioavailability in endothelium of black Americans. Circulation. 2005;112(24):3795–3801. | ||
Funovic P, Korda M, Kubant R, et al. Effect of beta-blockers on endothelial function during biological aging: a nanotechnological approach. J Cardiovasc Pharmacol. 2008;51(2):208–215. | ||
Huang X. Nitric Oxide/Peroxynitrite Balance in Kidney – Effect of Diabetes and Obesity. [master’s thesis]. Chemistry and Biochemistry. Ohio University; 2008:1–162. | ||
Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271(5 Pt 1):C1424–C1437. | ||
Malinski T, Taha Z. Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature. 1992;358(6388):676–678. | ||
Xue J, Ying X, Chen J, Xian Y, Jin L, Jin J. Amperometric ultramicrosensors for peroxynitrite detection and its application toward single myocardial cells. Anal Chem. 2000;72(21):5313–5321. | ||
Kelm M, Dahmann R, Wink D, Feelisch M. The nitric oxide/superoxide assay. Insights into the biological chemistry of the NO/O-2. interaction. J Biol Chem. 1997;272(15):9922–9932. | ||
Kalinowski L, Dobrucki LW, Brovkovych V, Malinski T. Increased nitric oxide bioavailability in endothelial cells contributes to the pleiotropic effect of cerivastatin. Circulation. 2002;105(8):933–938. | ||
Mason RP, Jacob RF, Corbalan JJ, Szczesny D, Matysiak K, Malinski T. The favorable kinetics and balance of nebivolol-stimulated nitric oxide and peroxynitrite release in human endothelial cells. BMC Pharmacol Toxicol. 2013;14(1):48. | ||
Burewicz A, Dawoud H, Jiang LL, Malinski T. Nitric oxide/peroxynitrite redox imbalance in endothelial cells measured with amperometric nanosensors. Am J Anal Chem. 2013;4(10):30–36. | ||
Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113(13):1708–1714. | ||
Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. 2003;111(8):1201–1209. | ||
Argacha J, Egrise D. Vitamin D deficiency-induced hypertension is associated with vascular oxidative stress and altered heart gene expression. J Cardiovasc Pharmacol. 2011;58(1):65–71. | ||
Münzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31(22):2741–2748. | ||
Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol. 2009;157(4):527–536. | ||
Vanhoutte PM, Feletou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144(4):449–458. | ||
Vanhoutte PM. Endothelium-dependent contractions in hypertension: when prostacyclin becomes ugly. Hypertension. 2011;57(3):526–531. | ||
Knight SF, Yuan J, Roy S, Imig JD. Simvastatin and tempol protect against endothelial dysfunction and renal injury in a model of obesity and hypertension. AJP Ren Physiol. 2010;298(1):F86–F94. | ||
Schmieder R. Mechanisms for the clinical benefits of angiotensin II receptor blockers. Am J Hypertens. 2005;18(5 Pt 1):720–730. | ||
Koh KK, Ahn JY, Han SH, et al. Pleiotropic effects of angiotensin II receptor blocker in hypertensive patients. J Am Coll Cardiol. 2003;42(5):905–910. | ||
Dong J, Wong SL, Lau CW, et al. Calcitriol protects renovascular function in hypertension by down-regulating angiotensin II type 1 receptors and reducing oxidative stress. Eur Heart J. 2012;33(23):2980–2990. | ||
Zehnder D, Bland R, Chana RS, et al. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13(3):621–629. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.