Back to Journals » International Journal of Nanomedicine » Volume 15
Nanomaterial-Based Tumor Photothermal Immunotherapy
Received 11 February 2020
Accepted for publication 18 June 2020
Published 19 November 2020 Volume 2020:15 Pages 9159—9180
DOI https://doi.org/10.2147/IJN.S249252
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Peng Xu, Feng Liang
The State Key Laboratory of Refractories and Metallurgy, Coal Conversion and New Carbon Materials Hubei Key Laboratory, School of Chemistry and Chemical Engineering, Wuhan University of Science and Technology, Wuhan 430081, People’s Republic of China
Correspondence: Feng Liang Email [email protected]
Abstract: In recent years, photothermal therapy (PTT) particularly nanomaterial-based PTT is a promising therapeutic modality and technique for cancer tumor ablation. In addition to killing tumor cells directly through heat, PTT also can induce immunogenic cell death (ICD) to activate the whole-body anti-tumor immune response, including the redistribution and activation of immune effector cells, the expression and secretion of cytokines and the transformation of memory T lymphocytes. When used in combination with immunotherapy, the efficacy of nanomaterial-based PTT can be improved. This article summarized the mechanism of nanomaterial-based PTT against cancer and how nanomaterial-based PTT impacts the tumor microenvironment and induces an immune response. Moreover, we reviewed recent advances of nanomaterial-based photothermal immunotherapy and discussed challenges and future outlook.
Keywords: nanomaterials, cell death mechanism of PTT, tumor microenvironment, immunogenic cell death, photothermal immunotherapy
Introduction
Cancer has become one of the most serious threats to human health in the last few decades. According to estimates from the International Cancer Research Agency (ICRA) in 2018, there were over 18.1 million new cancer cases and 9.6 million cancer deaths in the globe.1 Tumor metastases are the main reason for nearly 90% of all cancer-related deaths. For many patients, when they were diagnosed with cancer, metastasis has already occurred. It was noticed that over 80% of patients diagnosed with lung cancer present with metastatic disease.2–6 Chemotherapy, hormonal therapy and radiation therapy serve palliative purposes in the metastatic cancers and offer a modest extension of survival. However, the therapeutic effect is still unsatisfactory.3
The human immune system has a powerful mechanism to eliminate abnormal cells by constantly examining “self-labeling” or “non-self-antigens” on the cell surface.7–9 The broad definition of immunotherapy is the use of the body’s natural defenses against disease. Cancer immunotherapy aims to activate host immune system to fight cancer cells.10–13 In the past decade, cancer immunotherapy has made great progress and has been applied to clinical studies, including monoclonal antibodies,14 dendritic cell (DC)-based vaccine,15,16 chimeric antigen receptor (CAR) T cells,17,18 whole-cell vaccine19,20 and immune checkpoint inhibitors.21–26 However, several recent studies have revealed that these therapeutic methods exhibited inconsistent therapeutic responses to different patients. In addition, the cascading effects of inflammatory media, organ toxicity, hematopoietic system dysfunction and other side effects also limit the clinical optimization of these methods.27–31
A satisfactory tumor therapy strategy cannot only eliminate the primary tumors but also activate the host immune system and eliminate the metastatic and residual tumor cells. Combining local therapy with immunotherapy is a good choice to improve the therapeutic effect while making full use of the benefits of immunotherapy.32–36
Recent years, photothermal therapy (PTT), employing tumor site targeted photothermal conversion nanomaterials to convert light energy into heat under near-infrared (NIR) light irradiation to kill tumor cells, has been recognized to be an effective and minimally invasive therapeutic strategy for treating primary tumors.37,38 By local administration of photosensitizers and minimally invasive NIR radiation, hyperthermia prompted by PTT can be controlled to minimize the damage to non-targeted tissues. Interestingly, recent revisions have shown that hyperthermia can induce dying tumor cells to release antigens, pro-inflammatory cytokines, and immunogenic intracellular substrates, thus promoting immune activation. Nanomaterials with a photothermal effects are widely used to enhance PTT, including gold nanoparticles such as gold nanoshells (GNShs),39–41 gold nanorods (GNRs),42–44 gold nanocages (GNCs),45–47 gold nanostars (GNSs),48–50 carbon nanomaterials, such as single-walled carbon nanotubes (SWCNTs),51–53 graphene;54–56 semiconductor nanoparticles, such as copper sulfide (CuS),57–59 molybdenum disulfide (MoS2)60–62 organic NIR dyes such as indocyanine green (IGC),63–68 IR780,69–71 IR82072–74 as well as other PTT nanomaterials. In addition to direct killing effect, it is recognized that a key role caused by PTT is immunogenic cell death (ICD).75–79 During ICD, dendritic cells (DCs) capture the released damaged-associated molecular patterns (DAMPs) and tumor-associated antigens (TAAs), then processed and presented to adaptive immune cells to activate specific immune response.76,79–81
Researchers have recognized the potential benefits of PTT when it was introduced to compensate for some inherent drawbacks of immunotherapy. Nanomaterials for PTT can be further modified with immunostimulants or other immune drugs to enhance the whole body’s anti-tumor immune responses. This nanomaterial-based PTT cannot only directly ablation of tumors but also induce continuous antitumor immune effects, known as photothermal immunotherapy. This article summarized the mechanism of nanomaterial-based PTT against cancer, recent advances of nanomaterial-based photothermal immunotherapy, and also discussed challenges and future outlook.
Nanomaterial-Based PTT for Tumor Treatment
It is well known that temperature is one of the most important parameters determining the dynamics and viability of organisms,82–84 temperature-induced changes at the cellular level are determined by the intensity and duration of the temperature increment.85,86 Depending on the magnitude of the temperature increment, the effects on tumor cells can be classified as follows:87 (1) when the temperature rises slightly to 41°C, the transmembrane diffusion rate and blood flow speed of the cells will be accelerated; (2) at the temperature of 41–48°C, proteins will aggregate, and increases sensitivity to radiation and chemotherapy. At this temperature of more than 60 minutes, will cause irreversible damage to cells; (3) at the temperature of 48–60°C, even 4–6 minutes will cause irreversible damage to the cells, resulting in a severe denaturation of proteins and serious damage to DNA. This thermal response behavior of cells is the basis for cancer treatment using PTT.
Nanomaterial-based PTT is a new type of nanotechnology, using the photothermal conversion ability of nanomaterials to convert light energy into heat energy to kill tumor cells (Figure 1A)88. Compared with the existing mainstream treatment methods, PTT is a spatiotemporal controlled, minimally invasive, high selectivity to localized cancer, good anti-cancer effect treatment. Besides, PTT is suitable for the treatment of solid tumors, particularly for treating superficial tumors, as the poor blood supply in cancerous tissue leading poorer heat resistance than normal tissues.89–91 Usually, PTT chooses 650–900 nm NIR light as the light source because tissue absorption in this area is weak, resulting in deep tissue penetration and low tissue damage.92,93 In addition, the satisfactory photothermal reagents used in PPT should be characterized by low or non-toxic, high NIR absorption, and high photothermal conversion efficiency.94–96 Nanomaterials with a photothermal effects are widely used to enhance PTT and have achieved encouraging therapeutic results.97 Photothermal agents mainly include gold nanomaterials, like GNShs, GNRs, GNCs, GNSs;39–50 carbon nanomaterials such as SWCNTs, graphene, fullerene,51–56,98–100 semiconductor nanomaterials such as MoS2, WS2, Bi2Se3;60–62,101–104 organic NIR dyes such as IGC, IR780, IR820 (Figure 1B).63–74
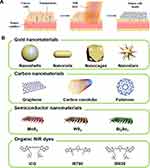 |
Figure 1 (A) Schematic illustration of nanoparticle-based PTT. Reproduced from Riley RS, Day ES. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(4):1449. Copyright 2017, John Wiley and Sons.88 (B) Four major photothermal agents for PTT, including gold nanoparticles, carbon nanomaterials, semiconductor nanomaterials and organic NIR dyes.Reprouduced from Kim H, Beack S, Han S, et al. Multifunctional photonic nanomaterials for diagnostic, therapeutic, and theranostic applications. Adv Mater. 2018;30(10):1701460.Copyright 2018, John Wiley and Sons.97 |
However, there are still some ambiguity in PTT. Many studies on PTT have found that PTT can effectively kill tumor cells and eliminate tumors, but the specific mechanism of cell death is understudied. It is necessary to understand the path of PTT-induced cell death, so that the response of tumors to different PTT can be understood. Then, by regulating the parameters of treatment to achieve the desired effect, moreover, more appropriate and effective treatments can be designed for each case.
How PTT Induces Cell Death
Even though PTT is already in clinical trials,105,106 we know little that how PTT induces cell death. According to the published articles on PTT for cancer treatment, it is difficult to find a universal cell death mechanism. The cell death mechanism caused by PTT is associated with a variety of factors, such as the properties of tumors, the time of irradiation, the heat absorbed by the cell, the level of internalization of the nanomaterials.107 Therefore, the cellular response to PTT is complex. Following, the mechanism of PTT-induced cell death is analyzed, showing the complexity of this process.
PTT and Apoptosis/Necrosis
Cancer hyperthermal therapy refers to the treatment that raises the cell temperature to 41–48°C, which leads to a range of cell damage, including cell membrane damage, intracellular protein degeneration, damage of RNA/DNA synthesis, and eventually leads to cell apoptosis.82,108–111 PTT also has a similar effect to hyperthermia. However, whether PTT induces apoptosis or necrosis remains unclear, and maybe a single mechanism is insufficient to describe the effects of PTT.
Recent studies demonstrate that the cell death mechanism of PTT can be influenced by the laser type, nanomaterials location, photothermal dose. Huang et al112 investigated the mechanism of PTT to human oral squamous cell carcinoma (HSC-3) cells, using cytoplasm-targeted peptide (RGD) or nuclear-targeted peptide (NLS) functionalized gold nanosphere. PTT studies showed that nanosecond pulsed lasers irradiation can only cause necrosis, regardless of where the nanomaterials were located in the cell. When treating cells with the continuous wave (CW) laser, cell death was attributed to apoptosis with gold nanosphere located in the nucleus; however, when the gold nanospheres were in the cytoplasm, higher energy led to necrosis, while lower energy caused apoptosis. This indicated that the effects of localization and laser type are quite complex and may affect the mechanism of cell death in PTT. The effect of nanomaterials location using folate-functionalized gold nanorods (F-GNRs) was described by Tong et al in 2007.113 Malignant human oral epidermoid cancer (KB) cells were incubated with the F-GNRs for 6 h and 17 h to ensure that the nanoparticles were delivered to the cell surface or the perinuclear region, respectively. PTT carried out by CW laser irradiation, the results showed that when F-GNRs were inside the cell, it need 60mW laser intensity to kill the cell, but only need 6mW when F-GNR were on the cell surface. This indicated that the location of nanomaterials was important for the effectiveness of PTT, and F-GNRs on the cell surface had a better heat transfer effect. Further analysis of the cell death mechanism found that F-GNRs-mediated cavitation caused the rupture of the cell membrane. Membrane rupture led to the influx of extracellular Ca2+ followed by degradation of the actin network, resulting in a large number of blebbing response. Finally, the mode of cell death was thought to be apoptosis. Ali et al114 developed a mild PTT strategy to induce cancer cell apoptosis, by fixing the GNRs concentration (7.5 nM), laser power (5.8 w/cm2) and adjusting the laser exposure time. They irradiated the human breast cancer (MCF-7) cells and found that after 2 min laser exposure, the cells mainly apoptosis (42.7% apoptosis and 2.89% necrosis), while 5 min laser exposure led to a significant increase in necrosis cells (20.17% apoptosis and 15.5% necrosis). This demonstrated that high-dose of PTT can lead to necrosis, while low-dose of PTT can promote apoptosis. Li and Gu115 reported the effects of changes in laser energy during PTT on cervical cancer (HeLa) cells. After incubation with GNRs for 6 h, HeLa cells received a femtosecond pulsed scanning laser irradiated. When the laser power was 27.8 w/cm2, 10 scans induced apoptosis, while increasing it to 30 times led to necrosis. When the laser power was reduced to 13.9 w/cm2, multiple scans only induced apoptosis. However, the laser power was increased to 55.6 w/cm2, only one scan can lead to necrosis. This suggested that laser power could affect the pathway of cell death.
To better understand the specific mechanisms involved, the apoptotic cell death was further investigated by examining biomarkers of the apoptotic pathways. Pérez-Hernández et al116 have found that PTT-specific-induced apoptosis was mediated by the intrinsic mitochondrial pathway via the activation of Bid (Figure 2A). Mocan et al117 established that hyperthermia after PPT can induce the depolarization of mitochondrial membrane, thus activating the free radical flux and oxidation state within the cell, and regulating cell damage through the apoptotic pathway. Ali et al118 found that the metabolism of phenylalanine after PTT was disrupted and activated key proteins in several apoptosis pathways, such as HADHA and ACAT1. Additionally, this group119 also found that cytochrome c and p53-related apoptosis mechanisms were associated with PTT-induced apoptosis. Proteomics analysis identified a variety of apoptosis pathways, such as caspase cascade, granzyme B signal, BAD protein phosphorylation.
 |
Figure 2 (A) Mechanism of apoptosis after NIR laser-irradiation. Reproduced with permission from Pérez-Hernández M, Del Pino P, Mitchell SG, et al. Dissecting the molecular mechanism of apoptosis during photothermal therapyusing gold nanoprisms. ACS Nano. 2015;9(1):52–61. .116 Copyright 2015, American Chemical Society. (B) Schematic illustration of HSP70 inhibitor optimized PTT. Reproduced with permission from Ali MRK, Ali HR, Rankin CR, et al. Targeting heat shock protein70 using gold nanorods enhances cancer cell apoptosis in lowdose plasmonic photothermal therapy. Biomaterials. 2016;102:1–8. .128 Copyright 2016, Elsevier. |
PTT and Heat Shock Proteins
In the PTT process, many functional biological macromolecules were expressed to assist a variety of biological processes, such as the folding of new polypeptides, the formation of protein complexes, and the promotion of intracellular protein transport, to protect cells from adverse conditions. Heat shock proteins (HSPs) refer to a group of proteins produced by cells under the induction of stressors, especially at high temperatures. They are also chaperonins that bind to denatured proteins during heat stress and preventing them from aggregating, and then promote refolding of the protein after temperature recovery. Therefore, HSPs are associated with PTT-induced cell death mechanism.120–124
Some studies supported the idea that inhibiting various HSPs, including HSP60, HSP70, and HSP90, could reduce the heat resistance of tumor cells, enhance tumor sensitivity to PTT and improve PTT efficiency.125–136 Wang et al134 developed a novel vanadium oxide composite loaded with ICG (VO2-ICG). In acidic tumor microenvironment, VO2 NPs were decomposed and VO2+ released simultaneously, which could not only inhibit the function of HSP60 but also generate hydroxyl radical (•OH) by catalyzing H2O2 in tumor. In addition, the VO2 NPs also released ICG during decomposition, allowing for the NIR luminescence imaging and PTT. The inhibition of HSP60 reduced the heat resistance of cells and the production of •OH increased oxidative stress in cells, which further improved the efficiency of PTT. Small interfering RNA (siRNA) and small molecular inhibitors have been used to inhibit the HSPs expression and enhanced the PTT efficiency. An effective and versatile PTT platform based on hollow GNShs functionalized with siRNA against HSP70 was designed by Wang et al.129 Efficient downregulation of HSP70 after laser irradiation was achieved in vitro and in vivo. Finally, sensitized cancer cells to heat, and improved PTT efficiency. Ali et al128 investigated and applied GNRs to test how different cell lines responded to PTT and correlated their response to PTT to their HSP70 levels. Three different epithelial origin cancer cell lines: HSC (oral), Huh7.5 (liver) and MCF-7 (breast) were heated to the same temperature. Compared with HSC and MCF-7 cells, the apoptosis of Huh7.5 cells increased significantly after PTT. HSP70 is known to enhance the cells’ resistance to heat, so they measured relative HSP70 levels in these cells after PTT. The results showed that the HSP70 level of Huh7.5 cell was 10 times lower than that of HSC and MCF-7 cells. Then, the expression of HSP70 was down-regulated by siRNA, and all three cell lines showed a significant increase in apoptosis after PTT. To enhance PTT, they further conjugated GNRs with HSP70 inhibitor quercetin, and obtained results similar to siRNA, the conjugation of HSP70 inhibitor enhanced the effect of PTT (Figure 2B). Jiang et al133 synthesized a flowerlike Lu:Nd@NiS2 core-shell nanoparticles loaded with a natural HSP90 inhibitor-phenolic epigallocatechin 3-gallate (EGCG). Under laser irradiation, EGCG was released from Lu:Nd@NiS2 and bound to HSP90 to reduced cell’s resistance to heat, resulting in better therapeutic effect at the same temperature rise. However, the role of HSPs should not be overlooked in the immune system, such as HSPs being able to present antigens to DCs and induce anti-tumor immune response.137 Therefore, HSP inhibitors are not suitable for all treatments.
PTT Impacts the Tumor Microenvironment and Immune Response
PTT in Tumor Microenvironment
Tumor microenvironment (TME) plays a vital role in cancer development and metastasis. It consists of extracellular matrix (ECM), various cell types, blood vessels, interstitial fluid and other tumor-related components (Figure 3).138–140 In many types of solid tumors, dense ECM and tumor matrix supply the development of the tumor, also as a natural barrier to the effective spread of therapeutic drugs. Additionally, the rampant proliferation of tumor cells and the extension of dysfunctional blood vessels can cause tumors to form a hypoxia and acidification microenvironment, significantly affecting the therapeutic effect of various therapies.141 Moreover, cancer cells can recruit a large number of immunosuppressive cells, including regulatory T cells, tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs), to form immunosuppressive TME. Which can suppress anti-tumor immune effects, protect tumor cells from attack, and promote tumor growth.142,143
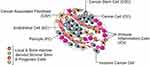 |
Figure 3 The Cells in the Tumor Microenvironment. Reproduced with permission from Hanahan D, Weinberg RA. Hallmarks of cancer: the nextgeneration. Cell. 2011;144(5):646–674.140 Copyright 2011, Elsevier. |
Research results show that nanomaterials can have a great impact on TME. It has been well known that PTT can increase the temperature of local tumors, resulting in increased blood flow inside the tumor and improved hypoxia in the TME. Thus, many recent studies have shown that the combination of PTT with photodynamic therapy or radiation therapy can significantly improve the synergetic effects by effectively alleviating the hypoxia in the tumor.144–147 Additionally, increased tumor blood flow due to PTT can also lead to increased microvascular permeability, which may widen the therapeutic window for poorly permeable cancers and enhance targeted drug delivery.148,149 Moreover, gold nanoparticles are inherently capable of regulating TME. Saha et al150 demonstrated that bare gold nanoparticles can regulate the secretome of cells to reduce the growth of desmoplastic tissue and inhibit tumor growth, thus interfering with crosstalk between pancreatic cancer cells and pancreatic stellate cells.
PTT Induction of Immune Response
Immunotherapy is a promising treatment of cancer that supports body immune system fight cancer. The generation of an anti-tumor immune response involves some key steps: Initially, TAAs released from tumor cells are captured by DCs, which can efficiently phagocytose, process and deliver tumor antigens. Then, mature DCs migrate to the lymph nodes to present the tumor antigens to T cells, activating the effector T cell responses to tumor-specific antigens. Finally, activated effector T cells migrate and infiltrate to the tumor region, where they specifically identify and kill cancer cells. Furthermore, the killed cancer cells release more TAAs, increasing the breadth and depth of the immune response during the cycle’s subsequent.151,152
To induce a specific anti-tumoral immune response, TAAs are required in cancer vaccines. However, it is difficult to screen out a generic TAAs for multiple types of cancers. Surprisingly it has been noticed that ablative cancer treatments, like PTT, can cause immunogenic death of tumor cells.75,76 This immunogenic cell death (ICD) is characterized by the release of TAAs, DAMPs, and pro-inflammatory cytokines from the dead cells that help present TAAs to immune cells, resulting in an antigen-specific immune response.77,78 The advantage of PTT as part of immune therapy is that localized tumor ablation can release TAAs to form a broad spectrum in situ cancer vaccine.
During ICD, TAAs and DAMPs are released by dying tumor cells. TAAs can enhance the antigen-specific T cell response, while the release of DAMPs can serve as the “dangerous” signal of immune stimulation to enhance the engulfment of tumor antigens by DCs, induce DCs maturation and activate T-cells, thereby initiating anti-tumor immune response. Many of the immunogenic factors involved in the death of apoptotic cells have been identified as DAMPs, such as calreticulin (CRT),153,154 ATP,155,156 high mobility group box 1 (HMGB-1)157,158 and HSPs.159,160 ATP acts as a chemical attractor to recruit antigen present cells (APCs); CRT acts as an “eat-me” signal, facilitating APCs to engulf dying tumor cells and their fragments.161 HMGB-1 and HSPs stimulate antigen presentation to T cells (Figure 4).162,163
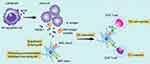 |
Figure 4 Characteristics of immunogenic cell death (ICD). Reproduced with permission from Duan X, Chan C, LinW. Nanoparticle-mediated immunogenic celldeath enables and potentiates cancer immunotherapy. Angew ChemInt Ed Engl. 2019;58(3):670–680.163 Copyright 2019, John Wiley and Sons. |
It is important to note that PTT-induced ICD requires an appropriate thermal dose, “more is better” do not apply to every situation. Sweeney et al164 explored the thermal “window” of ICD by Prussian blue nanoparticle-based PTT (PBNP-PTT) in neuroblastoma animal model. To determine whether a cell is in the process of ICD, the criteria is that the intracellular ATP and HMGB1 should be decreased while the surface CRT should be increased. They found that ICD markers were highly expressed within an optimal temperature window (63.3–66.4°C), however, higher (83.0–83.5°C) or lower (50.7–52.7°C) temperatures both produced lower expression (Figure 5). This finding was subsequently confirmed by vaccination studies in neuroblastoma models. The tunability of the ICD presented in this work may also apply to other nanomaterial-based PTT, and perhaps the “more is better” approach is not suitable for these therapies, and there may be the optimal window for treatment.
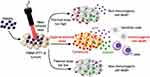 |
Figure 5 PTT generates ICD when an optimal thermal dose is administered to tumors. Reproduced with permission from Sweeney EE, Cano-Mejia J, Fernandes R. Photothermal therapy generatesa thermal window of immunogenic cell death in neuroblastoma. Small. 2018;14(20):1800678..164 Copyright 2018, John Wiley and Sons. |
Nanomaterial-Based Photothermal Immunotherapy
Cancer immunotherapy has achieved remarkable clinical responses owing to its unique advantages, with specific anti-tumor induction and long-term immuno-memory response. However, the response rate is low, and the potential side effects are still the major barriers to the widespread use of immunotherapy in the clinic.28,29,31
Recent researches mainly have focused on the immune response after nanomaterial-based PTT. On one hand, nanodrugs can be actively or passively targeted to the tumor site, and then tumor cells are killed by PTT. On the other hand, TAAs and cell fragments are released, activating the systemic immunity and further killing residual or metastatic tumors. Furthermore, the generation of immune-memory could prevent cancer recurrence. This nanomaterial-based PTT can directly eliminate tumors and induce sustained antitumor immune effects called photothermal immunotherapy.165
However, the tumor fragments produced by PTT may not be sufficient to effectively activate the anti-tumor response. Therefore, nanomaterials for PTT can be further combined with immunostimulants or other immune drugs to enhance the whole body’s anti-tumor immune responses.
Photothermal Immunotherapy Combined with Immunoadjuvant
Numerous researches have established that immuno-stimulation induced by PTT alone was insufficient. One effective way is to add immunoadjuvants, which are a class of substances that act as an auxiliary or enhanced substance in the immune response. Nanotechnologies are very effective in immune responses, mainly when nanoparticle-based PTT combined with immunoadjuvants, it can trigger the immune responses, increasing the infiltration of immune cell in the tumor site. This effect cannot only inhibit the recurrence of primary tumor but also inhibit or eradicate metastatic tumors.
In the past few years, PTT in conjunction with an immunoadjuvant has become an epidemic strategy to enhance the immune response to cancer. As attractive photothermal agents, gold nanoparticles with different morphologies like GNRs, GNShs have been investigated combine with immunoadjuvants. Zhou et al166 designed and synthesized GNRs based hybrid nanomaterial’s mPEG-GNRs@BSA/R837 through functionalization of BSA-bioinspired GNRs with the imiquimod (R837, an FDA approved immunoadjuvant recognized by the toll-like receptor 7), to treat melanoma by PTT and immunotherapy. The mPEG-GNRs@BSA/R837 significantly increased the secretion levels of TNF-α, IL-6, and IL-12 and the percentages of mature DCs also increased a lot (65.1%). At the same time, after 7 days of treatment, mPEG-GNRs@BSA/R837 combined with laser irradiation effectively enhanced the infiltration of CD8+ T cells. More importantly, the nanocomposite combined with laser irradiation was found in animal tumor models to prevent lung metastasis of cancer cells, and to form long-term immune memories in response to tumor re-challenges. A drug delivery system based on the GNShs and an immunoadjuvant CpG oligodeoxynucleotides (CpG, an immunoadjuvant recognized by endosomal toll-like receptor 9) has been made by Zhang et al.167 PTT could destroy tumor cells at a macro level, and finally immunotherapy could track and kill cancer cells throughout the body. Yata et al168 synthesized a composite immunostimulatory hydrogel consisting of a hexapod-like structured DNA, CpG and gold nanoparticles. The hydrogels plus laser irradiation increased the tumor temperature and the expression of HSP70 in tumor tissue, as well as the amount of antitumor inflammatory mediators, such as TNF-α, IL-6 and IFN-γ. In addition, the therapy significantly slowed tumor growth and increased the survival rate of tumor-bearing mice.
Besides gold nanoparticles, carbon nanoparticles are another attractive photothermal agents. Taking advantage of the carrier nature of SWCNTs, Zhou et al169 modified SWCNTs with an immunoadjuvant glycated chitosan (GC, an immunoadjuvant that improves antigen transport between the epithelium and promotes antigen phagocytosis). After 980 nm laser irradiation, tumor cells were killed by the photothermal effect of SWCNT and the damaged cells produced antigens that were recognized by the immune system. Meanwhile, GC could induce a stronger anti-tumor immune response. Animal studies showed that the combination of PTT and SWCNT-GC not only effectively killed the local and metastatic tumors but also produced an effective long-term anti-tumor immune response. Tao et al170 synthesized a graphite oxide (GO) based complex GO-PEG-PEI-CpG through combination of GO, polyethyleneimine (PEI), polyethylene glycol (PEG) and immunoadjuvant CpG. Under laser irradiation, the nanocomposite generated local hyperthermia and accelerated intracellular migration of the nanoparticles. In vivo experiments also showed that the GO-PEG-PEI-CpG had a synergistic photothermal and immune effect on cancer treatment. Moreover, nanographene and graphene quantum dots also have been studied as photothermal agent for photothermal immunotherapy.
Sulfide metallic nanoparticles are also the popular choice as photothermal agents. Guo et al171 have developed chitosan-coated hollow CuS nanoparticles that assemble the immunoadjuvants CpG (HCuSNPs-CpG) to treat the primary and distant tumor. After 900 nm laser irradiation, the structure broke down into the chitosan-CpG nanocomplexes (Chi-CpG-NPs), and small CuS nanoparticles (SCuSNPs). The released Chi-CpG-NPs were uptake into plasmacytoid dendritic cells (pDCs). Under CpG stimulation, pDCs secreted IFN-α, promoting NK cell to activate innate immunity. Meanwhile, HCuSNPs-mediated photothermal ablation destroyed tumor cells and released TAAs to myeloid dendritic cells (mDCs). These mDCs subsequently migrated to lymph nodes, where they cross-prime tumor antigen-specific T cells. Then, the antigen-specific CD8+ T cells were recruited to primary tumor and distant site tumor, triggering adaptive immune response (Figure 6). Jang et al172 also functionalized CuS nanoparticles with lipopolysaccharide (LPS-CuS) for photothermal immunotherapy application. LPS is an immunoadjuvant that mediates immune activation by interacting with toll-like receptor 4. After laser irradiation, LPS-CuS induced ablation of CT26 tumor and released TAAs. Then, the LPS and TAAs promoted DCs activation and enhanced the tumor antigen-specific immune responses. Finally, LPS-CuS mediated PTT not only cured the primary tumors of the mice but also completely resisted secondary tumor injection in the spleen. Besides, CpG loaded MoS2 nanosheets also showed photothermal enhanced immunological activity, which dramatically reduced the viability of tumor cells.
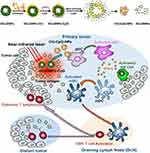 |
Figure 6 Schematics diagram of combined photothermal and immunotherapy using chitosan-coated hollow copper sulfide nanoparticles. Reproduced with permission from Guo L, Yan DD, Yang D, et al. Combinatorial photothermal andimmuno cancer therapy using chitosan-coated hollow coppersulfide nanoparticles. ACS Nano. 2014;8(6):5670–5681.171 Copyright 2014, American Chemical Society. |
Aside from sulfide metallic nanoparticles, organic dyes like indocyanine green (ICG) and its derivatives are another widely used photothermal agents. Chen et al173 first reported the use of ICG-loaded glycated chitosan gel for photothermal immunotherapy in 1997. After 805 nm laser irradiation, tumor cells were immediately destruction and concomitantly stimulated the immunological defense system against residual and metastatic tumor cells. Finally, eliminated both primary and metastatic tumor burden, and improved the survival rate of the rats. Li et al174 designed and synthesized a PTT agent IR-7-lipo/HA-CpG through surface functionalization of IR-7-lipo (fluorophore loaded liposomes) with a multivalent immunoadjuvant (HA-CpG), where IR-7 is a NIR active heptamethine cyanine dye. After 808 nm laser irradiation, IR-7-lipo induced tumor cell necrosis and released TAAs, while the multivalent immunoadjuvant improved the co-stimulatory molecules expression and promoted antigen presentation. Moreover, IR-7-lipo/HA-CpG could significantly reduce the amount of MDSCs to decrease immunosuppression and increase the number of CD8+ effector T cells to potentiate the anti-tumor immunity of the host. Most importantly, due to the enhanced anti-tumor immune response, combined photothermal immunotherapy could effectively eliminate tumors and inhibit tumor metastasis of the mice. Dong et al175 designed a composite hydrogel that contained CpG self-crosslinked nanoparticles and IR820 to achieve photothermal immunotherapy. IR820-hydrogel can effectively generate heat to eliminate the tumor and produced photothermal-induced tumor antigens. CpG self-crosslinked nanoparticles can boost immune response to melanoma. The combination of the two part got a synergistic effect. Further analysis found an increase in the number of CD8+ T cells and a decrease in the number of MDSCs in TME. In addition, the self-fluorescent IR820-hydrogel can be imaging to guide treatment and this visible photothermal immunotherapy offers the potential for precise cancer treatment. Moreover, Prussian blue nanoparticles (PBNPs) also have been used as a photothermal agent in photothermal immunotherapy. Other existing photothermal nanomaterials combined with immunoadjuvants for photothermal immunotherapy are listed in Table 1.
 |
Table 1 Photothermal Nanomaterials Combined with Immunoadjuvants for Photothermal Immunotherapy |
Photothermal Immunotherapy Combined with Immune Checkpoint Inhibitor
Immune response has checkpoints to maintain immune homeostasis and prevent its improper activation, immune checkpoint blockade refers to the use of antibodies that block negative regulatory receptors on T cells to enhance the endogenous natural immune responses. In a number of preclinical trials, immune checkpoint inhibitors have been shown to release T-cell-mediated immunosuppressive mechanisms and promote cytotoxic T lymphocytes (CTLs) responses.176 Two major immune checkpoints, cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed cell death protein 1 (PD-1)/programmed cell death-ligand 1 (PD-L1), are the hot topic of current research. Their inhibitors like Ipilimumab, Pembrolizumab and Atezolizumab have been approved by the FDA on the basis of striking clinical trial results in melanoma, renal cell carcinoma, and lung cancer, providing great potential in cancer immunotherapy.177–179 Therefore, blocking these negative signals is another strategy to elicit an immune response with PTT.
CTLA-4, as an immune checkpoint, is a critical negative regulator of T cell activation, which is constitutively expressed by regulatory T cells and up-regulated after T cell activation. Researchers have found that blocking CTLA-4 could inhibit the development of tumor by increasing activities of effector T cells and reducing the immunosuppressive activity of regulatory T cells in the TME. Combining anti-CTLA-4 antibody with PTT has been exploited in photothermal immunotherapy.
Wang et al180 found that SWCNT-based PTT together with anti-CTLA-4 antibody could regulate the adaptive immune responses, especially cellular immunity to treat metastatic cancer. As shown in Figure 7, upon thermal ablation by PEG-coated SWCNTs, TAAs were released along with the danger signals to induce DCs maturation, and then the antigens were presented, eventually recruiting tumor-specific CD8+ T cells. Meanwhile, anti-CTLA-4 could effectively reduce the immunosuppressive activity of regulatory T cells and increase the ratios of CD4+ and CD8+ T cells to regulatory T cells. As a result, combined with anti-CTLA-4 therapy, SWCNT-based PTT could inhibit the growth of cancer cells in subcutaneous tumor model and lung metastasis model. Mejia et al181 reported PBNPs-based PTT combined with anti-CTLA-4 to treat neuroblastoma. PBNPs-based PTT could reduce tumor burden and produce an immune response, and anti-CTLA-4 could promote the penetration of T cells in the tumor area. They observed that 55.5% of mice treated with photothermal immunotherapy had a survival rate of more than 100 days. Additionally, these long-term survived mice exhibited protection against neuroblastoma rechallenge.
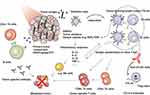 |
Figure 7 Schematic diagram of nanoparticle-based PTT together with anti-CTLA-4. Reproduced with permission from Wang C, Xu L, Liang C, et al. Immunological responses triggeredby photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancermetastasis. Adv Mater. 2014;26(48):8154–8162..180 Copyright 2014, John Wiley and Sons. |
Aside from CTLA-4 immune checkpoint, antibody against PD-1/PD-L1 have also been exploited in photothermal immunotherapy. PD-1, as an immune checkpoint, is highly expressed in activated T and B cells. As a ligand of PD-1, PD-L1 is a protein overexpressed on the surface of tumor cells, which contributes to the suppression of the immune system and cancer immune evasion. The PD-1/PD-L1 pathway mediates tumor immunosuppression by inhibiting T cell proliferation and inducing the activation of T cell apoptosis. To reverse tumor-mediated immunosuppression, anti-PD-1/PD-L1 antibodies have been designed to block the PD-L1/PD-1 interaction. Liu et al182 used GNS-mediated PTT combined with anti-PD-L1 to treat MB49 bladder cancer and achieved complete eradication of primary tumors and distant-untreated tumors (Figure 8). The cured mice received re-challenge of MB49 cells and had no new tumor formation after 60 days, suggesting that the photothermal immunotherapy effectively induced long-term immunity to MB49 cells. Peng et al183 used NIR dyes (IR820) chemotherapy drugs (docetaxel, DTX) to prepare nanoparticles with high drug-loading rates. In addition, a 27 amino acid polypeptide (named CF27) containing 12 units of amino acid PD-L1 agonist was introduced that could block the immune checkpoint PD-1/PD-L1 and self-crosslinking of drug-loading nanoparticles. Chemotherapy and PTT combined with PD-1/PD-L1 blockage led a much better therapeutic outcome, including more mice were cured and did not relapse for 90 days, indicating that the immune system could be stimulated during the treatment.
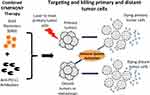 |
Figure 8 Schematic diagram of nanoparticle-based PTT together with anti-PD-L1. Reproduced from Liu Y, Maccarini P, Palmer GM, et al. Synergistic immuno photothermal nanotherapy (SYMPHONY) for the treatment of unre-sectable and metastatic cancers. Sci Rep. 2017;7(1):8606. Creative Commons license and disclaimer available from: (http://creativecommons.org/licenses/by/4.0/legalcode„http://creativecommons.org/licenses/by/4.0/legalcode).182 |
Moreover, combining the immunoadjuvant and immune checkpoint inhibitors for photothermal immunotherapy exhibited strong antitumor immune responses. Chen et al184 developed a poly (lactic-co-glycolic acid) (PLGA)-based nanoparticle loaded with ICG and the immunoadjuvant R837. It is worth noting that the PLGA-ICG-R837 nanoparticles are composed of three clinically approved components and therefore have high clinical application potential. After laser irradiation, photothermal effects caused by ICG could generate TAAs that acted as vaccine. With the assistance of R837, the released antigen would be treated by DCs and presented to T cells, resulting in strong anti-tumor immunity. Combined with anti-CTLA-4 antibody, the immunosuppressive activity of regulatory T cells could be inhibited. Animal experimental results showed that the combination therapy could not only effectively inhibit tumor metastasis in breast cancer (4T1) and colorectal cancer (CT26) murine models but also produced long-term immune memories (Figure 9). Li et al185 designed a SWCNT modified by immunoadjuvant glycated chitosan (GC) and anti-CTLA-4 antibody to treat metastatic mammary tumors in mice. After 1064 nm laser irradiation, the primary tumor was ablation by PTT. The treatment activated the body’s anti-tumor immunity, which inhibited lung metastasis and prolonged the animal’s survival time. Anti-CTLA-4 antibody could suppress the activity of immunosuppressive regulatory T cells and further enhance the immune response. These results showed that the combination of PTT, immunoadjuvant and immune checkpoint inhibitors could effectively suppress primary tumors, inhibit metastases, and provide a new treatment strategy for metastatic cancers. Other existing photothermal nanomaterials combined with immune checkpoint inhibitors for photothermal immunotherapy are listed in Table 2.
 |
Table 2 Photothermal Nanomaterials Combined with Immune Checkpoint Inhibitors for Photothermal Immunotherapy |
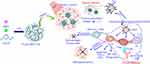 |
Figure 9 Schematic diagram of PTT with immunoadjuvant nanoparticles together with anti-CTLA-4. Reproduced with permissionfrom Chen Q, Xu L, Liang C, et al. Photothermal therapy withimmune-adjuvant nanoparticles together with checkpoint blockadefor effective cancer immunotherapy. Nat Commun. 2016;7(1):13193. Creative Commons license and disclaimer available from: (http://creativecommons.org/licenses/by/4.0/legalcode„http://creativecommons.org/licenses/by/4.0/legalcode).184 |
PTT-Based Combinatorial Treatments
Combining PTT with other therapies has great potential to improve cancer treatment. A combination of two or more therapeutic modalities could help overcome the deficiencies of individual therapy. Some recent studies have found that PTT combined with other treatments can induce anti-tumor immunity to metastatic cancers. Nam et al186 demonstrated that PTT combined with chemotherapy could trigger potent anti-tumor immunity against disseminated tumors. They developed a polydopamine-coated spiky gold nanoparticle (SGNP@PDA) combined with chemotherapy drug doxorubicin (DOX) for chemo-PTT. This stratagem could elicit robust anti-tumor responses and eliminate local as well as untreated distant tumors, leading to a remarkable survival rate of >85% in a bilateral murine tumor model of CT26 colon carcinoma. Moreover, treated animals exhibit long-term resistance against tumor re-challenge, indicating establishment of immunological memory against tumor recurrence. Jin et al187 designed a multifunctional PC10A/DOX/MoS2 hydrogel for chemo-photothermal-photodynamic therapy of 4T1 tumor. The MoS2 nanosheet in the hydrogel was simultaneously utilized as photothermal agent and photodynamic agent for the generation of hyperthermia and reactive oxygen species, respectively. After 808 nm laser irradiation, PC10A/DOX/MoS2 hydrogel could significantly increase the number of CD8+ and CD4+ T cells in lymph nodes to potentiate the anti-tumor immunity of the host. Most importantly, the activation of antitumor immune effects could suppress the growth of primary 4T1 breast tumors and distal lung metastatic nodules. These results demonstrated that the PC10A/DOX/MoS2 hydrogel was promising to be utilized in antitumor immunity therapy triggered by photothermal therapy and photodynamic therapy for malignant tumor.
Summary and Future Outlook
In summary, nanomaterial-based photothermal immunotherapy has displayed encouraging treatment effects. It provides an alternative to traditional cancer treatment, due to its effectiveness in killing tumor cells, reversing drug resistance, minimizing the side effects on healthy cells as well as boosting the immune response. This article summarized the mechanism of nanomaterial-based PTT against cancer and how nanomaterial-based PTT impacts the tumor microenvironment and induces an immune response and the most recent progress regarding nanomaterial-based photothermal immunotherapy. Changes in the laser type, nanomaterials location and photothermal dose play key roles in the cell death mechanism of PTT. Another important role of PTT is causing the ICD of the tumor cell. ICD is characterized by the release of TAAs, DAMPs, and pro-inflammatory cytokines to facilitate the presentation of TAAs to adaptive immune cells, activating the antigen-specific immune response. However, PTT-induced ICD requires an appropriate thermal dose. The nanomaterial-based photothermal immunotherapy cures the cancers through the following mechanisms. First, tumor cells are killed by PTT; then TAAs are released to stimulate the system immune effect, thereby activating the immune effector cells. With the aid of immunoadjuvants or immune checkpoint inhibitors, an enhanced antitumor immunity can be achieved for the treatment of primary and metastatic tumors.
However, there remain some crucial challenges and opportunities for further and higher demand for the nanomaterial-based PPT agent in photothermal immunotherapy. Firstly, the safety and targeting of nanoparticle-based PTT must be considered, and the feasibility in deep tumors and metastatic tumors should be considered. The standardization of the treatment is also a big challenge, as researches are still at the laboratory stage, it is difficult to compare the effectiveness of different nanoparticle platforms from different laboratories. If the conditions of the treatments like the type of nanoparticles, the type of cancer, the laser intensity can be standardized, it will help accelerate the clinical transformation of this treatment. Moreover, the immune response caused by PTT in vivo is complex and its mechanisms are not fully clear. The intensity and controllability of the immune response must be emphasized. Furthermore, more research is needed to monitor the dynamic immune response and to understand the specific effects of each combination on TME, with the aim of providing guidance for individual patients to choose the best combination. Therefore, it is necessary to further study on further studies photothermal immunotherapy strategy based on nanoparticles. This new model has great potential to be an important complement to traditional cancer treatment strategies.
Funding
The project was supported by the National Natural Science Foundation of China (21372183, 21807083) and the Program for Innovative Teams of Outstanding Young and Middle-aged Researchers in the Higher Education Institutions of Hubei Province (T201702).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168(4):670–691. doi:10.1016/j.cell.2016.11.037
3. Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904. doi:10.1038/nm1469
4. Pavelic SK, Sedic M, Bosnjak H, et al. Metastasis: new perspectives on an old problem. Mol Cancer. 2011;10(1):22. doi:10.1186/1476-4598-10-22
5. Schroeder A, Heller DA, Winslow MM, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2012;12(1):39–50.
6. Zhang P, Zhai Y, Cai Y, et al. Nanomedicine-based immunotherapy for the treatment of cancer metastasis. Adv Mater. 2019;31(49):1904156. doi:10.1002/adma.201904156
7. Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517(7534):293–301.
8. Jiang H, Hegde S, Knolhoff BL, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22(8):851–860. doi:10.1038/nm.4123
9. Medzhitov R, Shevach EM, Trinchieri G, et al. Highlights of 10 years of immunology in nature reviews immunology. Nat Rev Immunol. 2011;11(10):693–702.
10. Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305(5681):200–205. doi:10.1126/science.1100369
11. Rosenberg SA. Progress in human tumor immunology and immunotherapy. Nature. 2001;411(6835):380–384. doi:10.1038/35077246
12. Couzin-Frankel J. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. doi:10.1126/science.342.6165.1432
13. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi:10.1038/nature10673
14. Maggon K. Monoclonal antibody “gold rush. Curr Med Chem. 2007;14(18):1978–1987. doi:10.2174/092986707781368504
15. Zhang X, Gordon JR, Xiang J. Advances in dendritic cell-based vaccine of cancer. Cancer Biother Radiopharm. 2002;17(6):601–619. doi:10.1089/108497802320970217
16. Burgdorf SK, Fischer A, Myschetzky PS, et al. Clinical responses in patients with advanced colorectal cancer to a dendritic cell-based vaccine. Oncol Rep. 2008;20(6):1305–1311.
17. Curran KJ, Brentjens RJ. Chimeric antigen receptor T cells for cancer immunotherapy. J Clin Oncol. 2015;33(15):1703–1706. doi:10.1200/JCO.2014.60.3449
18. Yip A, Webster RM. The market for chimeric antigen receptor T cell therapies. Nat Rev Drug Discov. 2018;17(3):161–162. doi:10.1038/nrd.2017.266
19. Yadav DK, de Lu C, Yadav RK. Vaccine therapy for pancreatic cancer: a battle against deadly cancer. Cancer Sci Ther. 2014;6:268–277.
20. Aurisicchio L, Pallocca M, Ciliberto G, et al. The perfect personalized cancer therapy: cancer vaccines against neoantigens. J Exp Clin Cancer Res. 2018;37(1):86. doi:10.1186/s13046-018-0751-1
21. Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14(8):561–584.
22. Yuan J, Gnjatic S, Li H, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105(51):20410–20415. doi:10.1073/pnas.0810114105
23. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi:10.1056/NEJMoa1003466
24. Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from Phase II and Phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889–1894. doi:10.1200/JCO.2014.56.2736
25. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106. doi:10.1097/COC.0000000000000239
26. Gatalica Z, Vanderwalde AM, Rose I, et al. Distribution of PD-L1 expression in diverse cancer types: experience with over 10,000 cases. J Clin Oncol. 2016;34:4. doi:10.1200/JCO.2016.34.15_suppl.11548
27. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, Phase 2 trial. Lancet Oncol. 2016;17(10):1374–1385. doi:10.1016/S1470-2045(16)30364-3
28. Sade-Feldman M, Jiao YJ, Chen JH, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun. 2017;8(1):1136. doi:10.1038/s41467-017-01062-w
29. Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2018;29(1):250–255. doi:10.1093/annonc/mdx642
30. Costa R, Costa RB, Talamantes SM, et al. Analyses of selected safety endpoints in Phase 1 and late-phase clinical trials of anti-PD-1 and PD-L1 inhibitors: prediction of immune-related toxicities. Oncotarget. 2017;8(40):67782–67789. doi:10.18632/oncotarget.18847
31. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755. doi:10.1056/NEJMoa1609214
32. Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2(9):831–838. doi:10.1158/2326-6066.CIR-14-0069
33. Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256–265. doi:10.1093/jnci/djs629
34. Niu LZ, Li JL, Zeng JY, et al. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic hepatocellular cancer. World J Gastroenterol. 2013;19(22):3473–3480. doi:10.3748/wjg.v19.i22.3473
35. Niu L, Chen J, He L, et al. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas. 2013;42(7):1143–1149. doi:10.1097/MPA.0b013e3182965dde
36. Yuan YY, Niu LZ, Mu F, et al. Therapeutic outcomes of combining cryotherapy, chemotherapy and DC-CIK immunotherapy in the treatment of metastatic non-small cell lung cancer. Cryobiology. 2013;67(2):235–240. doi:10.1016/j.cryobiol.2013.08.001
37. Vankayala R, Hwang KC. Nea-infrared-light-activatable nanomaterial-mediated phototheranostic nanomedicines: an emerging paradigm for cancer treatment. Adv Mater. 2018;30(23):1706320.
38. Xu L, Mou F, Gong H, et al. Light-driven micro/nanomotors: from fundamentals to applications. Chem Soc Rev. 2017;46(22):6905–6926.
39. Luo L, Bian Y, Liu Y, et al. Combined near infrared photothermal therapy and chemotherapy using gold nanoshells coated liposomes to enhance antitumor effect. Small. 2016;12(30):4103–4112. doi:10.1002/smll.201503961
40. Song J, Yang X, Yang Z, et al. Rational design of branched nanoporous gold nanoshells with enhanced physico-optical properties for optical imaging and cancer therapy. ACS Nano. 2017;11(6):6102–6113. doi:10.1021/acsnano.7b02048
41. Manivasagan P, Jun SW, Hoang G, et al. Anti-EGFR antibody conjugated thiol chitosan-layered gold nanoshells for dual-modal imaging-guided cancer combination therapy. J Control Release. 2019;311:26–42. doi:10.1016/j.jconrel.2019.08.007
42. Lee C, Hwang HS, Lee S, et al. Rabies virus-inspired silica-coated gold nanorods as a photothermal therapeutic platform for treating brain tumors. Adv Mater. 2017;29(13):1605563. doi:10.1002/adma.201605563
43. Li Z, Huang H, Tang S, et al. Small gold nanorods laden macrophages for enhanced tumor coverage in photothermal therapy. Biomaterials. 2016;74:144–154. doi:10.1016/j.biomaterials.2015.09.038
44. An L, Wang Y, Lin J, et al. Macrophages-mediated delivery of small gold nanorods for tumor hypoxia photoacoustic imaging and enhanced photothermal therapy. ACS Appl Mater Interfaces. 2019;11(17):15251–15261. doi:10.1021/acsami.9b00495
45. Feng Y, Cheng Y, Chang Y, et al. Time-staggered delivery of erlotinib and doxorubicin by gold nanocages with two smart polymers for reprogrammable release and synergistic with photothermal therapy. Biomaterials. 2019;217:119327.
46. Wu S, Li A, Zhao X, et al. Silica-coated gold–silver nanocages as photothermal antibacterial agents for combined anti-infective therapy. ACS Appl Mater Interfaces. 2019;11(19):17177–17183. doi:10.1021/acsami.9b01149
47. Zhan C, Huang Y, Lin G, et al. A gold nanocage/cluster hybrid structure for whole-body multispectral optoacoustic tomography imaging, EGFR inhibitor delivery, and photothermal therapy. Small. 2019;15(33):1900309. doi:10.1002/smll.201900309
48. Xu P, Ning P, Wang JJ, et al. Precise control of apoptosis via gold nanostars for dose dependent photothermal therapy of melanoma. J Mater Chem B. 2019;7(44):6934–6944. doi:10.1039/C9TB01956A
49. Chen CC, Chang DY, Li JJ, et al. Investigation of biodistribution and tissue penetration of PEGylated gold nanostars and their application for photothermal cancer treatment in tumor-bearing mice. J Mater Chem B. 2020;8(1):65–77. doi:10.1039/C9TB02194A
50. Xu P, Feng QS, Yang XR, et al. Near infrared light triggered cucurbit [7] uril-stabilized gold nanostars as a supramolecular nanoplatform for combination treatment of cancer. Bioconjug Chem. 2018;29(8):2855–2866. doi:10.1021/acs.bioconjchem.8b00438
51. Yang J, Su H, Sun W, et al. Dual chemodrug-loaded single-walled carbon nanohorns for multimodal imaging-guided chemo-photothermal therapy of tumors and lung metastases. Theranostics. 2018;8(7):1966–1984. doi:10.7150/thno.23848
52. Lu GH, Shang WT, Deng H, et al. Targeting carbon nanotubes based on IGF-1R for photothermal therapy of orthotopic pancreatic cancer guided by optical imaging. Biomaterials. 2019;195:13–22. doi:10.1016/j.biomaterials.2018.12.025
53. Suo X, Eldridge BN, Zhang H, et al. P-glycoprotein-targeted photothermal therapy of drug-resistant cancer cells using antibody-conjugated carbon nanotubes. ACS Appl Mater Interfaces. 2018;10(39):33464–33473. doi:10.1021/acsami.8b11974
54. Wang H, Mu Q, Wang K, et al. Nitrogen and boron dual-doped graphene quantum dots for near-infrared second window imaging and photothermal therapy. Appl Mater Today. 2019;14:108–117. doi:10.1016/j.apmt.2018.11.011
55. Gu Z, Zhu S, Yan L, et al. Graphene-based smart platforms for combined cancer therapy. Adv Mater. 2019;31(9):1800662.
56. Jiang W, Mo F, Lin Y, et al. Tumor targeting dual stimuli responsive controllable release nanoplatform based on DNA-conjugated reduced graphene oxide for chemo-photothermal synergetic cancer therapy. J Mater Chem B. 2018;6(26):4360–4367. doi:10.1039/C8TB00670A
57. Li N, Sun Q, Yu Z, et al. Nuclear-targeted photothermal therapy prevents cancer recurrence with near-infrared triggered copper sulfide nanoparticles. ACS Nano. 2018;12(6):5197–5206. doi:10.1021/acsnano.7b06870
58. Wang D, Dong H, Li M, et al. Erythrocyte–cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. ACS Nano. 2018;12(6):5241–5252. doi:10.1021/acsnano.7b08355
59. Zhou B, Zhao J, Qiao Y, et al. Simultaneous multimodal imaging and photothermal therapy via renal-clearable manganese-doped copper sulfide nanodots. Appl Mater Today. 2018;13:285–297. doi:10.1016/j.apmt.2018.09.011
60. Maji SK, Yu S, Chung K, et al. Synergistic nanozymetic activity of hybrid gold bipyramid-molybdenum disulfide core@ shell nanostructures for two-photon imaging and anticancer therapy. ACS Appl Mater Interfaces. 2018;10(49):42068–42076. doi:10.1021/acsami.8b15443
61. Yu Y, Chi B, Lin L, et al. Microwave-assisted preparation of paramagnetic zwitterionic amphiphilic copolymer hybrid molybdenum disulfide for T 1-weighted magnetic resonance imaging-guided photothermal therapy. J Mater Chem B. 2018;6(40):6391–6398. doi:10.1039/C8TB01660G
62. Shin MH, Park EY, Han S, et al. Multimodal cancer theranosis using hyaluronate-conjugated molybdenum disulfide. Adv Healthc Mater. 2019;8(1):1801036. doi:10.1002/adhm.201801036
63. Pan H, Zhang C, Wang T, et al. In situ fabrication of intelligent photothermal indocyanine green-alginate hydrogel for localized tumor ablation. ACS Appl Mater Interfaces. 2018;11(3):2782–2789. doi:10.1021/acsami.8b16517
64. Shan W, Chen R, Zhang Q, et al. Improved stable indocyanine green (ICG)-mediated cancer optotheranostics with naturalized hepatitis B core particles. Adv Mater. 2018;30(28):1707567. doi:10.1002/adma.201707567
65. He Q, He X, Deng B, et al. Sorafenib and indocyanine green co-loaded in photothermally sensitive liposomes for diagnosis and treatment of advanced hepatocellular carcinoma. J Mater Chem B. 2018;6(36):5823–5834. doi:10.1039/C8TB01641K
66. Chen WR, Adams RL, Heaton S, et al. Chromophore-enhanced laser-tumor tissue photothermal interaction using an 808-nm diode laser. Cancer Lett. 1995;88(1):15–19. doi:10.1016/0304-3835(94)03609-M
67. Chen WR, Adams RL, Bartels KE, et al. Chromophore-enhanced in vivo tumor cell destruction using an 808-nm diode laser. Cancer Lett. 1995;94(2):125–131. doi:10.1016/0304-3835(95)03837-M
68. Chen WR, Adams RL, Higgins AK, et al. Photothermal effects on murine mammary tumors using indocyanine green and an 808-nm diode laser: an in vivo efficacy study. Cancer Lett. 1996;98(2):169–173. doi:10.1016/S0304-3835(06)80028-5
69. Song J, Zhang N, Zhang L, et al. IR780-loaded folate-targeted nanoparticles for near-infrared fluorescence image-guided surgery and photothermal therapy in ovarian cancer. Int J Nanomedicine. 2019;14:2757–2772. doi:10.2147/IJN.S203108
70. Shen Y, Lv W, Yang H, et al. FA-NBs-IR780: novel multifunctional nanobubbles as molecule-targeted ultrasound contrast agents for accurate diagnosis and photothermal therapy of cancer. Cancer Lett. 2019;455:14–25. doi:10.1016/j.canlet.2019.04.023
71. Chen Y, Li Z, Wang H, et al. IR-780 loaded phospholipid mimicking homopolymeric micelles for near-IR imaging and photothermal therapy of pancreatic cancer. ACS Appl Mater Interfaces. 2016;8(11):6852–6858. doi:10.1021/acsami.6b00251
72. Li WT, Peng JR, Tan LW, et al. Mild photothermal therapy/photodynamic therapy/chemotherapy of breast cancer by Lyp-1 modified Docetaxel/IR820 Co-loaded micelles. Biomaterials. 2016;106:119–133. doi:10.1016/j.biomaterials.2016.08.016
73. Zhang H, Li Q, Liu R, et al. A Versatile prodrug strategy to in situ encapsulate drugs in MOF nanocarriers: a case of cytarabine-IR820 prodrug encapsulated ZIF-8 toward chemo-photothermal therapy. Adv Funct Mater. 2018;28(35):1802830. doi:10.1002/adfm.201802830
74. Zhang D, Zhang J, Li Q, et al. pH- and enzyme-sensitive IR820-paclitaxel conjugate self-assembled nanovehicles for near-infrared fluorescence imaging-guided chemo-photothermal therapy. ACS Appl Mater Interfaces. 2018;10(36):30092–30102. doi:10.1021/acsami.8b09098
75. Zitvogel L, Kepp O, Senovilla L, et al. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16(12):3100–3104. doi:10.1158/1078-0432.CCR-09-2891
76. Dudek AM, Garg AD, Krysko DV, et al. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev. 2013;24(4):319–333. doi:10.1016/j.cytogfr.2013.01.005
77. Krysko DV, Garg AD, Kaczmarek A, et al. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–875.
78. Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72.
79. Inoue H, Tani K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ. 2014;21(1):39–49. doi:10.1038/cdd.2013.84
80. Showalter A, Limaye A, Oyer JL, et al. Cytokines in immunogenic cell death: applications for cancer immunotherapy. Cytokine. 2017;97:123–132. doi:10.1016/j.cyto.2017.05.024
81. Ma Y, Pitt JM, Li Q, et al. The renaissance of anti-neoplastic immunity from tumor cell demise. Immunol Rev. 2017;280(1):194–206.
82. Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43(1):33–56. doi:10.1016/S1040-8428(01)00179-2
83. Wust P, Hildebrandt B, Sreenivasa G, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3(8):487–497. doi:10.1016/S1470-2045(02)00818-5
84. Burlaka A, Lukin S, Prylutska S, et al. Hyperthermic effect of multi-walled carbon nanotubes stimulated with near infrared irradiation for anticancer therapy: in vitro studies. Exp Oncol. 2010;32(1):48–50.
85. Habash RWY, Bansal R, Krewski D, et al. Thermal therapy, part 2: hyperthermia techniques. Crit Rev Biomed Eng. 2006;34(6):491–542. doi:10.1615/CritRevBiomedEng.v34.i6.30
86. Oleson JR, Samulski TV, Leopold KA, et al. Sensitivity of hyperthermia trial outcomes to temperature and time: implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys. 1993;25(2):289–297. doi:10.1016/0360-3016(93)90351-U
87. Jaque D, Martínez Maestro L, Del Rosal B, et al. Nanoparticles for photothermal therapies. Nanoscale. 2014;6(16):9494–9530.
88. Riley RS, Day ES. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(4):1449
89. Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–284. doi:10.1016/S0168-3659(99)00248-5
90. Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151. doi:10.1016/j.addr.2010.04.009
91. Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65(1):71–79. doi:10.1016/j.addr.2012.10.002
92. Gai S, Yang G, Yang P, et al. Recent advances in functional nanomaterials for light-triggered cancer therapy. Nano Today. 2018;19:146–187. doi:10.1016/j.nantod.2018.02.010
93. Zhao J, Zhong D, Zhou S. NIR-I-to-NIR-II fluorescent nanomaterials for biomedical imaging and cancer therapy. J Mater Chem B. 2018;6(3):349–365.
94. Liu B, Li C, Cheng Z, et al. Functional nanomaterials for near-infrared-triggered cancer therapy. Biomater Sci. 2016;4(6):890–909.
95. Zhang P, Hu C, Ran W, et al. Recent progress in light-triggered nanotheranostics for cancer treatment. Theranostics. 2016;6(7):948–968. doi:10.7150/thno.15217
96. Kang H, Mintri S, Menon AV, et al. Pharmacokinetics, pharmacodynamics and toxicology of theranostic nanoparticles. Nanoscale. 2015;7(45):18848–18862.
97. Kim H, Beack S, Han S, et al. Multifunctional photonic nanomaterials for diagnostic, therapeutic, and theranostic applications. Adv Mater. 2018;30(10):1701460
98. Krishna V, Singh A, Sharma P, et al. Polyhydroxy fullerenes for non-invasive cancer imaging and therapy. Small. 2010;6(20):2236–2241.
99. Shi J, Wang L, Gao J, et al. A fullerene-based multi-functional nanoplatform for cancer theranostic applications. Biomaterials. 2014;35(22):5771–5784. doi:10.1016/j.biomaterials.2014.03.071
100. Shen Y, Skirtach AG, Seki T, et al. Assembly of fullerene-carbon nanotubes: temperature indicator for photothermal conversion. J Am Chem Soc. 2010;132(25):8566–8568. doi:10.1021/ja1026024
101. Cheng L, Liu J, Gu X, et al. PEGylated WS2 nanosheets as a multifunctional theranostic agent for in vivo dual-modal CT/photoacoustic imaging guided photothermal therapy. Adv Mater. 2014;26(12):1886–1893. doi:10.1002/adma.201304497
102. Zhang C, Yong Y, Song L, et al. Multifunctional WS2@ poly (ethylene imine) nanoplatforms for imaging guided gene-photothermal synergistic therapy of cancer. Adv Healthc Mater. 2016;5(21):2776–2787. doi:10.1002/adhm.201600633
103. Xie H, Li Z, Sun Z, et al. Metabolizable ultrathin Bi2Se3 nanosheets in imaging-guided photothermal therapy. Small. 2016;12(30):4136–4145.
104. Zhang XD, Chen J, Min Y, et al. Metabolizable Bi2Se3 nanoplates: biodistribution, toxicity, and uses for cancer radiation therapy and imaging. Adv Funct Mater. 2014;24(12):1718–1729. doi:10.1002/adfm.201302312
105. Stern JM, Kibanov Solomonov VV, Sazykina E, et al. Initial evaluation of the safety of nanoshell-directed photothermal therapy in the treatment of prostate disease. Int J Toxicol. 2016;35(1):38–46. doi:10.1177/1091581815600170
106. Ali MRK, Wu Y, El-Sayed MA. Gold-nanoparticle-assisted plasmonic photothermal therapy advances toward clinical application. J Phys Chem C. 2019;123(25):15375–15393. doi:10.1021/acs.jpcc.9b01961
107. Ayala-Orozco C, Urban C, Knight MW, et al. Au nanomatryoshkas as efficient near-infrared photothermal transducers for cancer treatment: benchmarking against nanoshells. ACS Nano. 2014;8(6):6372–6381. doi:10.1021/nn501871d
108. Movahedi MM, Alamzadeh Z, Hosseini-Nami S, et al. Investigating the mechanisms behind extensive death in human cancer cells following nanoparticle assisted photo-thermo-radiotherapy. Photodiagnosis Photodyn Ther. 2020;29:101600. doi:10.1016/j.pdpdt.2019.101600
109. Zeinizade E, Tabei M, Shakeri-Zadeh A, et al. Selective apoptosis induction in cancer cells using folate-conjugated gold nanoparticles and controlling the laser irradiation conditions. Artif Cells Nanomed Biotechnol. 2018;46:1026–1038. doi:10.1080/21691401.2018.1443116
110. Alamzadeh Z, Beik J, Pirhajati Mahabadi V, et al. Ultrastructural and optical characteristics of cancer cells treated by a nanotechnology based chemo-photothermal therapy method. J Photochem Photobiol B. 2019;192:19–25. doi:10.1016/j.jphotobiol.2019.01.005
111. Mirrahimi M, Abed Z, Beik J, et al. A thermo-responsive alginate nanogel platform co-loaded with gold nanoparticles and cisplatin for combined cancer chemo-photothermal therapy. Pharmacol Res. 2019;143:178–185. doi:10.1016/j.phrs.2019.01.005
112. Huang X, Kang B, Qian W, et al. Comparative study of photothermolysis of cancer cells with nuclear-targeted or cytoplasm-targeted gold nanospheres: continuous wave or pulsed lasers. J Biomed Opt. 2010;15(5):058002. doi:10.1117/1.3486538
113. Tong L, Zhao Y, Huff TB, et al. Gold nanorods mediate tumor cell death by compromising membrane integrity. Adv Mater. 2007;19(20):3136–3141. doi:10.1002/adma.200701974
114. Ali MRK, Ibrahim IM, Ali HR, et al. Treatment of natural mammary gland tumors in canines and felines using gold nanorods-assisted plasmonic photothermal therapy to induce tumor apoptosis. Int J Nanomedicine. 2016;11:4849–4863. doi:10.2147/IJN.S109470
115. Li JL, Gu M. Surface plasmonic gold nanorods for enhanced two-photon microscopic imaging and apoptosis induction of cancer cells. Biomaterials. 2010;31(36):9492–9498. doi:10.1016/j.biomaterials.2010.08.068
116. Pérez-Hernández M, Del Pino P, Mitchell SG, et al. Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms. ACS Nano. 2015;9(1):52–61. doi:10.1021/nn505468v
117. Mocan T, Matea CT, Cojocaru I, et al. Photothermal treatment of human pancreatic cancer using PEGylated multi-walled carbon nanotubes induces apoptosis by triggering mitochondrial membrane depolarization mechanism. J Cancer. 2014;5(8):679–688. doi:10.7150/jca.9481
118. Ali MRK, Wu Y, Han T, et al. Simultaneous time-dependent surface-enhanced Raman spectroscopy, metabolomics, and proteomics reveal cancer cell death mechanisms associated with gold nanorod photothermal therapy. J Am Chem Soc. 2016;138(47):15434–15442. doi:10.1021/jacs.6b08787
119. Ali MRK, Rahman MA, Wu Y, et al. Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proc Natl Acad Sci U S A. 2017;114(15):3110–3118. doi:10.1073/pnas.1619302114
120. Katschinski DM. On heat and cells and proteins. Physiology. 2004;19(1):11–15. doi:10.1152/nips.01403.2002
121. Fisher JW, Sarkar S, Buchanan CF, et al. Photothermal response of human and murine cancer cells to multiwalled carbon nanotubes after laser irradiation. Cancer Res. 2010;70(23):9855–9864. doi:10.1158/0008-5472.CAN-10-0250
122. van den Tempel N, Horsman MR, Kanaar R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int J Hyperthermia. 2016;32(4):446–454. doi:10.3109/02656736.2016.1157216
123. Jego G, Hazoumé A, Seigneuric R, et al. Targeting heat shock proteins in cancer. Cancer Lett. 2013;332(2):275–285. doi:10.1016/j.canlet.2010.10.014
124. Calderwood SK, Khaleque MA, Sawyer DB, et al. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31(3):164–172. doi:10.1016/j.tibs.2006.01.006
125. Wang S, Tian Y, Tian W, et al. Selectively sensitizing malignant cells to photothermal therapy using a CD44-targeting heat shock protein 72 depletion nanosystem. ACS Nano. 2016;10(9):8578–8590. doi:10.1021/acsnano.6b03874
126. Wang L, Gao C, Liu K, et al. Cypate-conjugated porous upconversion nanocomposites for programmed delivery of heat shock protein 70 small interfering RNA for gene silencing and photothermal ablation. Adv Funct Mater. 2016;26(20):3480–3489. doi:10.1002/adfm.201600035
127. Wang BK, Yu XF, Wang JH, et al. Gold-nanorods-siRNA nanoplex for improved photothermal therapy by gene silencing. Biomaterials. 2016;78:27–39. doi:10.1016/j.biomaterials.2015.11.025
128. Ali MRK, Ali HR, Rankin CR, et al. Targeting heat shock protein 70 using gold nanorods enhances cancer cell apoptosis in low dose plasmonic photothermal therapy. Biomaterials. 2016;102:1–8. doi:10.1016/j.biomaterials.2016.06.017
129. Wang Z, Li S, Zhang M, et al. Laser-triggered small interfering RNA releasing gold nanoshells against heat shock protein for sensitized photothermal therapy. Adv Sci. 2017;4(2):1600327. doi:10.1002/advs.201600327
130. Chen WH, Luo GF, Lei Q, et al. Overcoming the heat endurance of tumor cells by interfering with the anaerobic glycolysis metabolism for improved photothermal therapy. ACS Nano. 2017;11(2):1419–1431. doi:10.1021/acsnano.6b06658
131. Ariyasu S, Mu J, Zhang X, et al. Investigation of thermally induced cellular ablation and heat response triggered by planar MoS2-based nanocomposite. Bioconjug Chem. 2017;28(4):1059–1067. doi:10.1021/acs.bioconjchem.6b00741
132. Liu Y, Shu G, Li X, et al. Human HSP70 promoter-based prussian blue nanotheranostics for thermo-controlled gene therapy and synergistic photothermal ablation. Adv Funct Mater. 2018;28(32):1802026. doi:10.1002/adfm.201802026
133. Jiang A, Liu Y, Ma L, et al. Biocompatible heat-shock protein inhibitor-delivered flowerlike short-wave infrared nanoprobe for mild temperature-driven highly efficient tumor ablation. ACS Appl Mater Interfaces. 2019;11((7):):6820–6828. doi:10.1021/acsami.8b21483
134. Wang S, Li L, Ning X, et al. pH-activated heat shock protein inhibition and radical generation enhanced NIR luminescence imaging-guided photothermal tumour ablation. Int J Pharm. 2019;566:40–45. doi:10.1016/j.ijpharm.2019.05.056
135. Liu Y, Xu M, Zhao Y, et al. Flower-like gold nanoparticles for enhanced photothermal anticancer therapy by the delivery of pooled siRNA to inhibit heat shock stress response. J Mater Chem B. 2019;7(4):586–597. doi:10.1039/C8TB02418A
136. Tian H, Zhang J, Zhang H, et al. Low side-effect and heat-shock protein-inhibited chemo-phototherapy nanoplatform via co-assembling strategy of biotin-tailored IR780 and quercetin. Chem Eng J. 2020;382:123043. doi:10.1016/j.cej.2019.123043
137. Huang XF, Ren W, Rollins L, et al. A broadly applicable, personalized heat shock protein-mediated oncolytic tumor vaccine. Cancer Res. 2003;63(21):7321–7329.
138. Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101(4):937–949. doi:10.1002/jcb.21187
139. Junttila MR, de Sauvage FJ. Influence of tumor micro-environment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–354. doi:10.1038/nature12626
140. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
141. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–1437.
142. Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–5912. doi:10.1038/onc.2008.271
143. Mbeunkui F, Johann DJ. Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol. 2009;63(4):571–582. doi:10.1007/s00280-008-0881-9
144. Song G, Liang C, Gong H, et al. Core–shell MnSe@ Bi2Se3 fabricated via a cation exchange method as novel nanotheranostics for multimodal imaging and synergistic thermoradiotherapy. Adv Mater. 2015;27(40):6110–6117. doi:10.1002/adma.201503006
145. Shen S, Chao Y, Dong Z, et al. Bottom-up preparation of uniform ultrathin rhenium disulfide nanosheets for image-guided photothermal radiotherapy. Adv Funct Mater. 2017;27(28):1700250. doi:10.1002/adfm.201700250
146. Cheng L, Shen S, Shi S, et al. FeSe2-decorated Bi2Se3 nanosheets fabricated via cation exchange for chelator-free 64Cu-labeling and multimodal image-guided photothermal-radiation therapy. Adv Funct Mater. 2016;26(13):2185–2197. doi:10.1002/adfm.201504810
147. Wang S, Li X, Chen Y, et al. A facile one-pot synthesis of a two-dimensional MoS2/Bi2S3 composite theranostic nanosystem for multi-modality tumor imaging and therapy. Adv Mater. 2015;27(17):2775–2782. doi:10.1002/adma.201500870
148. Zhao P, Zheng M, Yue C, et al. Improving drug accumulation and photothermal efficacy in tumor depending on size of ICG loaded lipid-polymer nanoparticles. Biomaterials. 2014;35(23):6037–6046. doi:10.1016/j.biomaterials.2014.04.019
149. Zhao R, Han X, Li Y, et al. Photothermal effect enhanced cascade-targeting strategy for improved pancreatic cancer therapy by gold nanoshell@ mesoporous silica nanorod. ACS Nano. 2017;11(8):8103–8113. doi:10.1021/acsnano.7b02918
150. Saha S, Xiong X, Chakraborty PK, et al. Gold nanoparticle reprograms pancreatic tumor microenvironment and inhibits tumor growth. ACS Nano. 2016;10(12):10636–10651. doi:10.1021/acsnano.6b02231
151. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi:10.1016/j.immuni.2013.07.012
152. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi:10.1038/nature21349
153. Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi:10.1038/nm1523
154. Panaretakis T, Joza N, Modjtahedi N, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 2008;15(9):1499–1509. doi:10.1038/cdd.2008.67
155. Garg AD, Krysko DV, Verfaillie T, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31(5):1062–1079. doi:10.1038/emboj.2011.497
156. Martins I, Wang Y, Michaud M, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014;21(1):79–91. doi:10.1038/cdd.2013.75
157. Apetoh L, Ghiringhelli F, Tesniere A, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220(1):47–59. doi:10.1111/j.1600-065X.2007.00573.x
158. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi:10.1038/nature00858
159. Binder RJ. Functions of heat shock proteins in pathways of the innate and adaptive immune system. J Immunol. 2014;193(12):5765–5771. doi:10.4049/jimmunol.1401417
160. Tesniere A, Panaretakis T, Kepp O, et al. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15(1):3–12. doi:10.1038/sj.cdd.4402269
161. Obeid M, Panaretakis T, Joza N, et al. Calreticulin exposure is required for the immunogenicity of γ-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14(10):1848–1850. doi:10.1038/sj.cdd.4402201
162. Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi:10.1038/nm1622
163. Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed Engl. 2019;58(3):670–680. doi:10.1002/anie.201804882
164. Sweeney EE, Cano-Mejia J, Fernandes R. Photothermal therapy generates a thermal window of immunogenic cell death in neuroblastoma. Small. 2018;14(20):1800678. doi:10.1002/smll.201800678
165. Hou X, Tao Y, Pang Y, Li X, Jiang G, Liu Y. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int J Cancer. 2018;143(12):3050–3060.
166. Zhou B, Song J, Wang M, et al. BSA-bioinspired gold nanorods loaded with immunoadjuvant for the treatment of melanoma by combined photothermal therapy and immunotherapy. Nanoscale. 2018;10(46):21640–21647. doi:10.1039/C8NR05323E
167. Zhang J, Zhao T, Han F, et al. Photothermal and gene therapy combined with immunotherapy to gastric cancer by the gold nanoshell-based system. J Nanobiotechnology. 2019;17(1):80. doi:10.1186/s12951-019-0515-x
168. Yata T, Takahashi Y, Tan M, et al. DNA nanotechnology-based composite-type gold nanoparticle-immunostimulatory DNA hydrogel for tumor photothermal immunotherapy. Biomaterials. 2017;146:136–145. doi:10.1016/j.biomaterials.2017.09.014
169. Zhou F, Wu S, Song S, et al. Antitumor immunologically modified carbon nanotubes for photothermal therapy. Biomaterials. 2012;33(11):3235–3242. doi:10.1016/j.biomaterials.2011.12.029
170. Tao Y, Ju E, Ren J, et al. Immunostimulatory oligonucleotides-loaded cationic graphene oxide with photothermally enhanced immunogenicity for photothermal/immune cancer therapy. Biomaterials. 2014;35(37):9963–9971. doi:10.1016/j.biomaterials.2014.08.036
171. Guo L, Yan DD, Yang D, et al. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano. 2014;8(6):5670–5681. doi:10.1021/nn5002112
172. Jang B, Xu L, Moorthy MS, et al. Lipopolysaccharide-coated CuS nanoparticles promoted anti-cancer and anti-metastatic effect by immuno-photothermal therapy. Oncotarget. 2017;8(62):105584–105595. doi:10.18632/oncotarget.22331
173. Chen WR, Adams RL, Carubelli R, Nordquist RE. Laser-photosensitizer assisted immunotherapy: a novel modality for cancer treatment. Cancer Lett. 1997;115(1):25–30. doi:10.1016/S0304-3835(97)04707-1
174. Li L, Yang S, Song L, et al. An endogenous vaccine based on fluorophores and multivalent immunoadjuvants regulates tumor micro-environment for synergistic photothermal and immunotherapy. Theranostics. 2018;8(3):860–873. doi:10.7150/thno.19826
175. Dong X, Liang J, Yang A, et al. Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials. 2019;209:111–125. doi:10.1016/j.biomaterials.2019.04.024
176. Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1(1):32–42. doi:10.1158/2326-6066.CIR-13-0013
177. Peggs KS, Quezada SA, Korman AJ, et al. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18(2):206–213. doi:10.1016/j.coi.2006.01.011
178. Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi:10.3389/fphar.2017.00561
179. Iwai Y, Hamanishi J, Chamoto K, et al. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24(1):26. doi:10.1186/s12929-017-0329-9
180. Wang C, Xu L, Liang C, et al. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv Mater. 2014;26(48):8154–8162. doi:10.1002/adma.201402996
181. Cano-Mejia J, Burga RA, Sweeney EE, et al. Prussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma. Nanomedicine. 2017;13(2):771–781. doi:10.1016/j.nano.2016.10.015
182. Liu Y, Maccarini P, Palmer GM, et al. Synergistic immuno photothermal nanotherapy (SYMPHONY) for the treatment of unresectable and metastatic cancers. Sci Rep. 2017;7(1):8606. doi:10.1038/s41598-017-09116-1
183. Peng J, Yang Q, Xiao Y, et al. Tumor microenvironment responsive drug-dye-peptide nanoassembly for enhanced tumor-targeting, penetration, and photo-chemo-immunotherapy. Adv Funct Mater. 2019;29(19):1900004. doi:10.1002/adfm.201900004
184. Chen Q, Xu L, Liang C, et al. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7(1):13193. doi:10.1038/ncomms13193
185. Li Y, Li X, Doughty A, et al. Phototherapy using immunologically modified carbon nanotubes to potentiate checkpoint blockade for metastatic breast cancer. Nanomedicine. 2019;18:44–53. doi:10.1016/j.nano.2019.02.009
186. Nam J, Son S, Ochyl LJ, Kuai R, Schwendeman A, Moon JJ. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat Commun. 2018;9(1):1074. doi:10.1038/s41467-018-03473-9
187. Jin R, Yang J, Ding P, et al. Antitumor immunity triggered by photothermal therapy and photodynamic therapy of a 2D MoS2 nanosheet-incorporated injectable polypeptide-engineered hydrogel combinated with chemotherapy for 4T1 breast tumor therapy. Nanotechnology. 2020;31(20):205102. doi:10.1088/1361-6528/ab72b9
188. Tao Y, Ju E, Liu Z, et al. Engineered, self-assembled near-infrared photothermal agents for combined tumor immunotherapy and chemo-photothermal therapy. Biomaterials. 2014;35(24):6646–6656. doi:10.1016/j.biomaterials.2014.04.073
189. Li Y, He L, Dong H, et al. Fever-inspired immunotherapy based on photothermal CpG nanotherapeutics: the critical role of mild heat in regulating tumor microenvironment. Adv Sci. 2018;5(6):1700805. doi:10.1002/advs.201700805
190. Chen J, Lin L, Yan N, et al. Macrophages loaded CpG and GNR-PEI for combination of tumor photothermal therapy and immunotherapy. Sci China Mater. 2018;61(11):1484–1494. doi:10.1007/s40843-018-9238-6
191. Meng X, Wang K, Lv L, et al. Photothermal/photodynamic therapy with immune-adjuvant liposomal complexes for effective gastric cancer therapy. Part Part Syst Char. 2019;36(6):1900015. doi:10.1002/ppsc.201900015
192. Wu C, Wang L, Tian Y, et al. “Triple-punch” anticancer strategy mediated by near-infrared photosensitizer/CpG oligonucleotides dual-dressed and mitochondria-targeted nanographene. ACS Appl Mater Interfaces. 2018;10(8):6942–6955. doi:10.1021/acsami.7b18896
193. Wu C, Guan X, Xu J, et al. Highly efficient cascading synergy of cancer photo-immunotherapy enabled by engineered graphene quantum dots/photosensitizer/CpG oligonucleotides hybrid nanotheranostics. Biomaterials. 2019;205:106–119. doi:10.1016/j.biomaterials.2019.03.020
194. Han Q, Wang X, Jia X, et al. CpG loaded MoS2 nanosheets as multifunctional agents for photothermal enhanced cancer immunotherapy. Nanoscale. 2017;9(18):5927–5934. doi:10.1039/C7NR01460K
195. Chen W, Qin M, Chen X, et al. Combining photothermal therapy and immunotherapy against melanoma by polydopamine-coated Al2O3 nanoparticles. Theranostics. 2018;8(8):2229–2241. doi:10.7150/thno.24073
196. Chen R, Zhu C, Fan Y, et al. Polydopamine-based multifunctional platform for combined photothermal therapy, chemotherapy, and immunotherapy in malignant tumor treatment. ACS Appl Bio Mater. 2019;2(2):874–883. doi:10.1021/acsabm.8b00718
197. Cano-Mejia J, Bookstaver ML, Sweeney EE, et al. Prussian blue nanoparticle-based antigenicity and adjuvanticity trigger robust antitumor immune responses against neuroblastoma. Biomater Sci. 2019;7(5):1875–1887. doi:10.1039/C8BM01553H
198. Luo L, Zhu C, Yin H, et al. Laser immunotherapy in combination with perdurable PD-1 blocking for the treatment of metastatic tumors. ACS Nano. 2018;12(8):7647–7662. doi:10.1021/acsnano.8b00204
199. Yang Q, Peng J, Shi K, et al. Rationally designed peptide-conjugated gold/platinum nanosystem with active tumor-targeting for enhancing tumor photothermal-immunotherapy. J Control Release. 2019;308:29–43. doi:10.1016/j.jconrel.2019.06.031
200. Peng J, Xiao Y, Li W, et al. Photosensitizer micelles together with IDO inhibitor enhance cancer photothermal therapy and immunotherapy. Adv Sci. 2018;5(5):1700891. doi:10.1002/advs.201700891
201. Wang T, Wang D, Yu H, et al. A cancer vaccine-mediated postoperative immunotherapy for recurrent and metastatic tumors. Nat Commun. 2018;9(1):1532. doi:10.1038/s41467-018-03915-4
202. Sun W, Du Y, Liang X, et al. Synergistic triple-combination therapy with hyaluronic acid-shelled PPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomaterials. 2019;217:119264.
203. Ye X, Liang X, Chen Q, et al. Surgical tumor-derived personalized photothermal vaccine formulation for cancer immunotherapy. ACS Nano. 2019;13(3):2956–2968. doi:10.1021/acsnano.8b07371
204. Liang X, Ye X, Wang C, et al. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J Control Release. 2019;296:150–161. doi:10.1016/j.jconrel.2019.01.027
205. Luo L, Yang J, Zhu C, et al. Sustained release of anti-PD-1 peptide for perdurable immunotherapy together with photothermal ablation against primary and distant tumors. J Control Release. 2018;278:87–99. doi:10.1016/j.jconrel.2018.04.002
206. Yan M, Liu Y, Zhu X, et al. Nanoscale reduced graphene oxide-mediated photothermal therapy together with IDO inhibition and PD-L1 blockade synergistically promote antitumor immunity. ACS Appl Mater Interfaces. 2018;11(2):1876–1885. doi:10.1021/acsami.8b18751
207. Ge R, Liu C, Zhang X, et al. Photothermal-activatable Fe3O4 superparticle nanodrug carriers with PD-L1 immune checkpoint blockade for anti-metastatic cancer immunotherapy. ACS Appl Mater Interfaces. 2018;10(24):20342–20355.
208. Lu Q, Qi S, Li P, et al. Photothermally activatable PDA immune nanomedicine combined with PD-L1 checkpoint blockade for antimetastatic cancer photoimmunotherapy. J Mater Chem B. 2019;7(15):2499–2511. doi:10.1039/C9TB00089E
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
