Back to Archived Journals » Journal of Neurorestoratology » Volume 3
Nanofibrous scaffolds supporting optimal central nervous system regeneration: an evidence-based review
Authors Kamudzandu M, Roach P, Fricker R, Yang Y
Received 26 July 2015
Accepted for publication 9 October 2015
Published 2 December 2015 Volume 2015:3 Pages 123—131
DOI https://doi.org/10.2147/JN.S70337
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Hongyun Huang
Munyaradzi Kamudzandu, Paul Roach, Rosemary A Fricker, Ying Yang
Institute for Science and Technology in Medicine, School of Medicine, Keele University, Stoke-on-Trent, UK
Abstract: Restoration of function following damage to the central nervous system (CNS) is severely restricted by several factors. These include the hindrance of axonal regeneration imposed by glial scars resulting from inflammatory response to damage, and limited axonal outgrowth toward target tissue. Strategies for promoting CNS functional regeneration include the use of nanotechnology. Due to their structural similarity, synthetic nanofibers could play an important role in regeneration of CNS neural tissue toward restoration of function following injury. Two-dimensional nanofibrous scaffolds have been used to provide contact guidance for developing brain and spinal cord neurites, particularly from neurons cultured in vitro. Three-dimensional nanofibrous scaffolds have been used, both in vitro and in vivo, for creating cell adhesion permissive milieu, in addition to contact guidance or structural bridges for axons, to control reconnection in brain and spinal cord injury models. It is postulated that nanofibrous scaffolds made from biodegradable and biocompatible materials can become powerful structural bridges for both guiding the outgrowth of neurites and rebuilding glial circuitry over the “lesion gaps” resulting from injury in the CNS.
Keywords: scaffold, nanofibrous scaffold, CNS, regeneration, alignment
Introduction
Neurorestoration is concerned with restoring, promoting, or maintaining neurological function after injury, in order to improve patients’ quality of life. Neurorestoration often requires a combination of mechanisms that include neuroprotection, axonal regeneration/sprouting, neuroplasticity, neuromodulation, and neuroregeneration.1,2 The adult central nervous system (CNS) lacks the ability to reestablish appropriate axonal and dendritic connections after injury. Conversely, the developing CNS and peripheral nervous system (PNS) can regenerate to replace damaged axons.3 The PNS is capable of recovering from injury due to the presence of macrophages and also Schwann cells, which are responsible for myelination of axons.4 Transection of PNS axons resulting from injury is followed by Wallerian degeneration of distal axonal segments. Degeneration of axons, involving the breakdown of the cytoskeleton and cell membranes, causes demyelination. Schwann cells and macrophages clear axon and myelin debris resulting from degeneration or damage. Subsequent proliferation of Schwann cells leads to the formation of conduits used to provide guidance to sprouting axonal segments. In addition to providing guidance cues to axons, Schwann cells along with macrophages and monocytes, are also involved in secretion of factors for regeneration and remyelination of axons.5–7
The CNS however, responds differently to injury. Although mature CNS axons are capable of outgrowth or sprouting following injury, subsequent elongation or regeneration is very limited compared to the PNS. The embryonic or early postnatal CNS is more capable of axon outgrowth compared to the mature CNS.8
Consequent changes to the CNS microenvironment due to injury make it very difficult for lesioned neurons to reestablish connections with their neuronal targets. For example, a glial scar including reactive astrocytes is formed in response to injury, presenting a barrier for axon elongation and ultimately regeneration.9,10 Furthermore, expression of axon-inhibiting molecules is upregulated by reactive glia within the injured CNS.11 Axonal/glia debris remains around the injury site for longer periods of time compared to PNS sites, with this also contributing to inhibition of axon regeneration.12,13
Injury to the CNS results in permanent loss of function. For example, Huntington’s disease and Parkinson’s disease patients experience irreversible damage to neurons in the basal ganglia neuron circuit. The basal ganglia circuit, located at the base of the brain, is largely responsible for controlling movement.14 Current treatments for Huntington’s disease and Parkinson’s disease, or other CNS disorders, are only capable of temporarily alleviating symptoms.15 In previous decades, great effort has been made to regenerate damaged or degenerated CNS tissues. In fact, there are several factors to be considered for regeneration of the injured adult CNS. Damaged cells (neurons or glia) should be replaced, neurotrophic factors responsible for enhancing axon outgrowth should be delivered to the injury site, and outgrowth should be guided by topographical cues including lesion gap “bridging”. Furthermore, growth inhibition molecules should be removed from the damage site, and intracellular signaling and immune responses should be modulated.3
Thus, to regenerate the CNS, the following factors are crucial toward achieving recovery of neuron synaptic function or axon connectivity after injury: 1) the cell body needs to survive so that new axons will begin sprouting from the proximal part of the lesioned neuron; 2) sprouting axons need to be guided to reach their targets; 3) axons need to be myelinated to enable synaptic communication with targets; and 4) debris from degenerating axons needs to be cleared-up from the site of injury. Implementation of new techniques to achieve aforementioned targets will enable new axons to form connections with their intended targets and enable CNS regeneration.6 This review will focus on axonal regeneration/sprouting and neuroregeneration toward neurorestoration of CNS tissue.
The role of scaffolds in CNS regeneration
Tissue engineering has evolved into a highly promising technique to promote tissue regeneration under favorable conditions. This is through the smart use of a combination of cells (stem cells), materials (scaffolds), and engineering (culture environment) approaches. Substantial progress has been achieved in this area in the past few decades. Among the three key elements of tissue engineering, the scaffold plays a central role, in particular in CNS regeneration. The scaffolds can incorporate various artificial guidance in nanofiber, channel, groove or conduit format that mimic the ECM to bridge nerve lesions or gaps and guide axonal contact for both central and peripheral injuries.16 Hydrogel has been used as guidance channels, and has the capacity to include permissive agents for cell growth; eg, neurostimulatory ECM macromolecules such as laminin-1 or laminin-1 fragments; and neurotrophic factors, such as nerve growth factor and brain-derived neurotrophic factor. The use of artificial scaffolding biomaterials is crucial for CNS axon outgrowth guidance as well as for “bridging” the lesion. Conduits can provide artificial guidance to develop axons from the proximal part of the injured neuron to their synaptic target.6 Furthermore, these “contact guidance” scaffolds can be incorporated with neurotrophic factors, to promote outgrowth and guidance of axons17 or the ensheathing of glial cells and Schwann cells, for the enhancement of long distance axonal regeneration and guidance.18
The ability of neural tissue to function electrically requires the connectivity between neighboring neurons. Transmission of such electrical communication along a substantial length of tissue, therefore requires alignment of many cells in a particular direction, and cell patterning to mimic natural tissue is therefore commonplace within regenerative medicine. Nanofibrous scaffolds have been well used to present aligned structures to enable neural tissue development, both in vitro and in vivo. Natural or synthetic materials can be fabricated via phase-separation,19,20 self-assembly,21 or electrospinning22 techniques. Electrospinning is efficient, cost-effective, and versatile in comparison to the other two techniques. It is commonly used for manufacturing fiber meshes (eg, for tissue engineering applications),23 since it is less time-consuming and provides a simpler means of fabricating fiber scaffolds from a variety of polymers.24
Electrospinning: fabrication of contact guidance, nanofibrous scaffolds
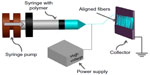
Biomaterials can be used to deliver drugs designed to digest scars that result from CNS injury, as well as provide topographical, biological, and chemical cues to promote and support regeneration.7 Electrospun nano- and micro-scale fibers can be used to provide topographical guidance required to reconnect axons regenerating from the proximal part of the lesioned neuron to its main target. The process of electrospinning uses electrostatic forces to generate polymer fibers. It evolved from electrospraying, which produces polymeric droplets as opposed to fibers.25,26 The electrospinning process involves the following elements: syringe, needle, pump, voltage power source, and a fiber collection device (Figure 1).
The polymer solution is loaded into the syringe. The pump drives a volume of polymer solution at a set flow rate. The dominant force at this point on the polymer is surface tension, resulting in formation of droplets at the end of the needle. Droplets break and fall down due to the force of gravity. When a high voltage is applied, an electrostatic force is generated in the polymer and increases up to a point where it becomes greater than the surface tension. A Taylor cone is formed at the end of the needle tip leading to an elongated fluid jet. The jet is driven toward the collector due to the potential difference between the positively charged polymer at the end of the needle and the negatively charged collector. The jet undergoes bending instability due to repulsive forces formed within the polymer. This instability increases the path length, set by the distance between the needle tip and the collector, and the time for the jet to reach the collector, resulting in evaporation of the solvent and consequently the formation of solid fiber meshes. Fibers are formed on a collector, either rotating or stationary. Aligned fibers can be fabricated via the use of a different collection method such as a rotating mandrel or parallel electrodes, instead of a stationary collector.24,27–30
Electrospinning parameters that define the structure and formation of the fibers include applied voltage, polymer flow rate, and needle-collector distance. For example, increasing polymer concentration generally results in an increase in diameter of electrospun fibers.31 The effect of applied voltage on fiber diameter is somewhat controversial,32 as Okutan et al found that increasing voltage resulted in larger fiber diameter.33 However, it has been reported that fiber diameter reduced with the increase in the applied voltage.28 These relationships differ depending on the polymer/solvent combination used, as well as the conditions (ie, humidity and temperature) under which the electrospinning is performed.28
Two-dimensional nanofibrous scaffolds for guiding CNS neurites
Type of materials and orientation of nanofibers
Synthetic polymers used to fabricate or develop nanofibrous scaffolds include polylactic acid (PLA),29,32–34 polycaprolactone (PCL),35–37 and poly(glycolide).35,38,39 Natural polymers include collagen,40–42 gelatin,43,44 chitosan,45,46 and silk fibroin.47,48 These fibers resemble the natural ECM, as they are thin continuous fibers with a high surface-to-volume ratio.28,36 Two-dimensional (2D) nanofiber meshes can be used to guide orientation of CNS neurites, not only to aid direct nerve tissue reconnection but also to permit the study of degeneration of the CNS neuron circuitry and high throughput screening.3,49 Neurites (axons and dendrites) respond to topography cues considerably.
Corey et al fabricated PLA aligned nanofibers using a rotating wheel collector.34 Primary rat spinal cord motor and sensory neurons were cultured on nanofiber substrates for 4 days in vitro. Prior to cell culture, the nanofibers were coated with polylysine and collagen I, for motor and sensory neurons, respectively. The processes for both types of neurons were elongated and aligned along the nanofibers. Wang et al cultured human embryonic stem cell-derived neural precursors (NPs) on tussah silk fibroin (TSF) scaffolds of both aligned and random orientation.47 On aligned TSF scaffolds, NPs differentiation and neurite outgrowth were significantly higher in comparison to random TSF scaffolds. The study also investigated the effect of TSF diameter by comparing diameters of 400 nm to 800 nm. The smaller diameter 400 nm scaffolds led to better differentiation and neurite outgrowth, compared to 800 nm scaffolds. Wen and Tresco also found that fiber diameter has an influence on how cells behave in vitro.50 An increase in dorsal root ganglia neurite outgrowth and Schwann cell migration was observed with decreasing fiber width from 500, 200, 100, and 30 nm to 5 μm.
The growth cone at the tip of the neuronal axon senses and responds to the extracellular environmental changes imposed by guidance molecules such as netrins, slits, semaphorins, and ephrins.51–53 The receptors on the surface of the growth cone are activated via interaction with guidance molecules. This leads to changes in cytoskeletal elements, particularly the actin cytoskeleton, which controls the morphology and motility of the growth cone. The flat sheet, fan-like structure at the end of an extending axon, the lamellipodia, consists of fine projections (filopodia) that sense the area around the axon. Filopodia normally appear when the growing axon changes direction, as noticed in vivo and in vitro in both the CNS as well as the PNS.54–56 It has been found that nanostructures, presenting curvature on the same length-scale as protein molecules, can be used to steer the protein adsorption process; the total quantity of protein can be controlled, having some degree of specificity over the final protein layer composition, as well as the conformational presentation of each protein interacting with the surface. This may explain why nanofibrous scaffolds induce and direct neural outgrowth effectively.
Primary neuron growth
In vitro models, most recently, have been fabricated to replicate the function and architecture of a brain neuron circuit. Simply, the circuit consists of neuronal cells that require aligned substrates to manipulate their attachment as well as the orientation of their neurites. Kamudzandu et al fabricated electrospun nanofibrous scaffolds for aligning primary striatal neurites.49 Striatal neurons were obtained from the developing rat brain. Fibers were precoated with polylysine and laminin to provide biochemical cues to neurons and promote attachment. Neurites protruding from neuronal cell bodies that were attached to the substrate responded to topography cues provided by fibers. Approximately 36% of neurites growing out from neurons on precoated nanofiber substrates, aligned to the nanofibers, while neurites on control, non-topography substrates had random orientation (Figure 2).
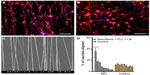  | Figure 2 CNS striatal neurite alignment on nanofiber scaffold. |
Multiple neuroglia growth
Glial cells, (oligodendrocytes, astrocytes, and microglial cells) support the development and function of neurons in the CNS. They constitute the majority of cells in the CNS (a ratio of 3:1 with neurons). Glial cells have an indirect control over how neurons communicate as they are involved in the electrical signaling and synapse formation between neurons. Oligodendrocytes provide the myelin sheath that wraps around some CNS axons to enhance the synaptic transmission by neurons. Astrocytes regulate the ionic or chemical milieu of neurons to aid neuron signaling. Glial cells also provide scaffolding to support certain aspects of neuron development. Following injury, microglial cells are involved in cleaning up cellular debris. Furthermore, glial cells aid regeneration after CNS injury by initiating and supporting remyelination of developing axons as well as formation of synapses.3,57
Weightman et al fabricated a nanofibrous scaffold for supporting and controlling the formation of glial cell circuitry that could be transplanted on to CNS injury sites to promote axonal regeneration.58 Aligned electrospun PLA fibers were placed on collagen gel for support. Astrocytes were seeded on the scaffolds and they became elongated and aligned, as they were guided by the nanofibers. It was found that the aligned astrocytes promoted adherence, survival, and elongation of oligodendrocyte precursor cells (OPCs). On the contrary, OPCs cultured in the absence of aligned astrocytes (Figure 3) remained rounded, had limited elongation and proliferation, and no alignment was observed.
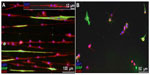  | Figure 3 Micrographs showing response of OPCs cultured in vitro. |
Embryonic stem cell differentiation
Mahairaki et al used human NPs derived from embryonic stem cell line BGO1 to investigate directed differentiation and axonal growth.59 Cells were cultured on micro and nano-scale PCL scaffolds precoated with poly-l-ornithine/laminin to promote cellular attachment, viability, and differentiation. Two sets of PCL scaffolds were fabricated, aligned, and randomly orientated. NPs cultured on aligned nanofibers showed a higher percentage of differentiation, 86% (indicated by TUJ1, a class III β-tubulin protein that identifies early stage neurons) compared to other scaffolds, ie, aligned fibers of micro-scale (62%), random nanofibers (27%), and random microfibers (32%). Only 40% of the NPs cultured on the tissue culture plate (with no substrate) were differentiated. Hoffman-Kim et al provide guidelines of materials and parameters for defining optimal conditions to manipulate differentiation of human embryonic stem cells toward neuronal regeneration.60
Three-dimensional nanofiber scaffolds for guiding CNS neurites
CNS nerve regeneration can benefit from three-dimensional (3D) scaffolds as a strategy to promote neurite guidance. Thus incorporating a nanofiber mesh into 3D nanofibrous scaffolds can potentially be used to bridge the gap resulting from injury toward restoration of normal function in the CNS.60,61 Ellis-Behnke et al developed an in vivo mammalian visual system model using nanofibers.62 Self-assembling peptide nanofiber scaffolds (SAPNS) were used to bridge a brain tissue lesion as well as to promote axonal regeneration. Following transection of a 1.5 mm deep and 2 mm wide lesion to the optical tract in the hamster midbrain, the SAPNS solution injected into the lesion site yielded a nanofibrous scaffold that permitted axonal regrowth and reconnection to the target tissue. Complete tissue reconnection was observed in lesioned animals injected with SAPNS compared to the controls, which were injected with a saline instead. The scaffold was biodegradable and yielded nontoxic products; it did not cause inflammation and promoted restoration of vision.
Liu et al used poly(lactic-co-glycolic acid) (PLGA) and PLGA modified with polyethylene glycol (PEG) to fabricate scaffolds for studying rat spinal cord injury.63 PLGA or PLGA-PEG electrospun nanofibers were incorporated with a gelatin sponge to form 3D scaffolds used for an in vivo transplantation designed to bridge a completely transected injury. Scaffolds were seeded with induced neural stem cells (iNSCs) reprogrammed and derived from mouse embryonic fibroblasts. PLGA-PEG scaffolds promoted iNSC proliferation as well as differentiation into neurons and glial cells; the PLGA-PEG scaffold yielded better iNSC adhesion and proliferation compared to PLGA scaffolds. The patch clamp technique was used to confirm generation of action potentials by iNSC-derived neurons. Functional recovery was observed in both PLGA-PEG and PLGA transplants from 2 weeks post-operation. This study demonstrates the use of electrospun nanofibers for supporting cell adhesion and growth in vivo to aid CNS recovery.
Yang et al developed a 3D construct consisting of portable electrospun nanofiber meshes combined with hydrogel, to replicate natural ECM.64 Aligned electrospun PLA nanofibers collected on parallel electrodes which had an average diameter of 450 nm. A hydrogel base, 200 μm thick, was prepared using rat-tail collagen type 1 solution. A bottom-up approach was used for fabricating the 3D scaffold. Multiple layers of nanofibers were placed on top of the hydrogel base and these layers were separated by filter papers (spacers). Cells (and hydrogel) were seeded onto the assembled nanofibers and frames holding excessive nanofibers were trimmed off (Figure 4). A few hours later, the cells in different layers aligned along nanofibers individually, and at different angles relative to each other. This new technique enables us to regulate an individual cell’s orientation and maintain their orientation within 3D constructs.
  | Figure 4 Schematic drawing showing the assembly of 3D nanofibrous scaffolds via layer-by-layer technique. |
A 3D organotypic slice in vitro model was developed by Weightman et al to study spinal cord injury.65 A double-scalpel tool was used to form a reproducible lesion site (complete transection) on mouse organotypic slices to mimic spinal cord injury. The models presented responses similar to in vivo injury such as activated microglia, reactive gliosis, and randomly oriented and limited outgrowth. Portable meshes of aligned electrospun nanofibers64 were laid across the lesion site to provide “bridging” material to guide the reconnection of developing axons. Incorporation of the biochemical cues, polylysine and laminin, promoted outgrowth of axons. Developing axons were guided onto the injury site by nanofibers. The in vitro model fabricated in the study could potentially be used as a model for high throughput screening of spinal cord injury therapies.
Dissociated cell cultures have also been used, as models mainly to study CNS neuron network establishment and connectivity. For example, Puschmann et al developed a CNS 3D cell culture system for studying the formation of neuronal networks including synaptic connectivity.66 The scaffold used to support hippocampal dissociated neuron cultures was built from electrospun polyurethane fibers. The group found that neurite extension on fibers occurred not only in the x- and y-directions but also in the z-direction. Furthermore, neurite alignment depended on fiber width, that is, more neurite outgrowth was observed on fibers with 450 nm diameters compared to 1,350 nm and 2,500 nm. Also, reduced astrocyte proliferation (less astrocyte reactivity) was observed on 3D cultures in comparison to 2D cultures. The 3D arrangement of the cultures did not affect the formation of synapses. This demonstrates that nanofibers, in this case made of polyurethane, can be employed to fabricate 3D dissociated cell cultures to build systems that can somewhat mimic in vivo CNS neuron circuitry.
Summary/future perspectives
The CNS lacks capacity to regenerate after injury; this contrasts with the PNS, which is capable of regeneration, providing the injury is not too severe. Spinal cord and traumatic brain (CNS) injuries encompass primary and secondary pathophysiological mechanisms. Primary injury involves initial mechanical damage, which results in disruption, compression, or transection of the spinal cord or brain structure. This is followed by secondary injury including neurogenic shock, apoptosis, demyelination, and inflammation.67–69 Current strategies toward the restoration of damaged tissue focus on addressing or controlling secondary injury.70,71 The main factors contributing to the difficulty of CNS regeneration (one neurorestoration mechanism) include formation of “glial scars” and expression of growth-inhibiting molecules. Transection of axons leading to “glial scars” obstructs axons sprouting from the proximal part of the damaged CNS neuron to reach their synaptic targets. Additionally, absence of Schwann cells means some axon outgrowth permissive molecules, such as neurotrophins, are not produced. Furthermore, debris resulting from axons and the “demyelination” process remain in the microenvironment for a long period of time, contributing to the limitations of axon CNS regeneration.3,10,11 Interestingly, biomaterials such as electrospun nanofibers provide a means of addressing the challenges concerning CNS recovery. Electrospun fibers can be used to provide a “bridge” for contact guidance for Schwann and ensheathing glial cells, as for example they can be incorporated as a feeder layer. Moreover, 3D biocompatible nanofibrous scaffolds can be used with stem cells for in vivo spinal cord injury transplantation studies.7,63 Yang et al developed an electrospinning fiber collection technique, involving the collection of portable fiber meshes,64 which has been used for developing spinal cord models.65 The portable and handleable nanofiber meshes enable a convenient 3D nanofibrous scaffold to be generated, with the capacity to guide neurites and glial cells individually through the thickness of the scaffolds. This can be used as a platform for developing a model that will incorporate axon growth permissive factors and intercellular signaling molecules, and digestion of growth-inhibiting molecules, among other things. Electrospun fibers can also be used for developing in vitro circuit models for studying CNS disease processes. CNS neurite alignment49 has already provided a means of controlling orientation and arrangement of neuron circuitry as a starting point. The next steps will involve coculture of neuron subtypes, or neurons and glia toward the formation of circuits that can be used for studying the CNS degeneration and regeneration process. More importantly, transferring the laboratory based outcomes of nanofibrous scaffold strategies into clinical treatment of primary and secondary injury cascades is urgently needed.
Disclosure
The authors report no conflicts of interest in this work.
References
Huang H, Chen L, Sanberg P. Cell therapy from bench to bedside translation in CNS neurorestoratology era. Cell Med. 2010;1(1):15–46. | |
Huang H, Chen L. Neurorestorative process, law, and mechanisms. Journal of Neurorestoratology. 2015;3:23–30. | |
Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407(6807):963–970. | |
Taylor JSH, Bampton ETW. Factors secreted by Schwann cells stimulate the regeneration of neonatal retinal ganglion cells. J Anat. 2004;204(1):25–31. | |
Stoll G, Griffin JW, Li CY, Trapp BD. Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J Neurocytol. 1989;18(5):671–683. | |
Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. | |
Tian L, Prabhakaran MP, Ramakrishna S. Strategies for regeneration of components of nervous system: scaffolds, cells and biomolecules. Regenerative Biomaterials. 2015;2(1):31–45. | |
Kalil K, Reh T. A light and electron microscopic study of regrowing pyramidal tract fibers. J Comp Neurol. 1982;211(3):265–275. | |
Pasterkamp RJ, Giger RJ, Ruitenberg MJ, et al. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci. 1999;13(2):143–166. | |
Rhodes KE, Fawcett JW. Chondroitin sulphate proteoglycans: preventing plasticity or protecting the CNS? J Anat. 2004;204(1):33–48. | |
McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11(11):3398–3411. | |
Perry VH, Brown MC, Gordon S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J Exp Med. 1987;165(4):1218–1223. | |
Stoll G, Trapp BD, Griffin JW. Macrophage function during Wallerian degeneration of rat optic nerve: clearance of degenerating myelin and Ia expression. J Neurosci. 1989;9(7):2327–2335. | |
Obeso JA, Rodriguez-Oroz MC, Stamelou M, Bhatia KP, Burn DJ. The expanding universe of disorders of the basal ganglia. Lancet. 2014;384(9942):523–531. | |
Utter AA, Basso MA. The basal ganglia: an overview of circuits and function. Neurosci Biobehav Rev. 2008;32(3):333–342. | |
Han D, Cheung KC. Biodegradable cell-seeded nanofiber scaffolds for neural repair. Polymers. 2011;3(4):1684–1733. | |
Houweling DA, Lankhorst AJ, Gispen WH, Bär PR, Joosten EA. Collagen containing neurotrophin-3 (NT-3) attracts regrowing injured corticospinal axons in the adult rat spinal cord and promotes partial functional recovery. Exp Neurol. 1998;153(1):49–59. | |
Ramón-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;18(10):3803–3815. | |
Guan J, Fujimoto KL, Sacks MS, Wagner WR. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. 2005;26(18):3961–3971. | |
Martínez-Pérez C, Olivas-Armendariz I, Castro-Carmona JS, García-Casillas PE. Scaffolds for tissue engineering via thermally induced phase separation. In: Wislet-Gendebien S, editor. Advances in Regenerative Medicine. InTech; 2011:275–294. Available from: http://orbi.ulg.ac.be/bitstream/2268/101891/1/Advances_in_Regenerative_Medicine.pdf#page=287. Accessed January 11, 2015. | |
Zhong C, Cooper A, Kapetanovic A, Fang Z, Zhang M, Rolandi M. A facile bottom-up route to self-assembled biogenic chitin nanofibers. Soft Matter. 2010;6(21):5298–5301. | |
Kim SW, Han SO, Sim IN, Cheon JY, Park WH. Fabrication and characterization of cellulose acetate/montmorillonite composite nanofibers by electrospinning. Journal of Nanomaterials. 2015;doi 10.1155/2015/275230. | |
Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12(5):1197–1211. | |
Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Science and Technology. 2003;63(15):2223–2253. | |
Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28(3):325–347. | |
Garg K, Bowlin GL. Electrospinning jets and nanofibrous structures. Biomicrofluidics. 2011;5(1):1–19. | |
Li D, Xia Y. Electrospinning of canofibers: reinventing the wheel? Advanced Materials. 2004;16(14):1151–1170. | |
Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989–2006. | |
Lee Y-S, Livingston Arinzeh T. Electrospun nanofibrous materials for neural tissue engineering. Polymers. 2011;3(1):413–426. | |
Liu H, Ding X, Zhou G, Li P, Wei X, Fan Y. Electrospinning of nanofibers for tissue engineering applications. Journal of Nanomaterials. 2013;2013:1–11. | |
Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26(15):2603–2610. | |
Li Z, Wang C. Effects of working parameters on electrospinning. In: One-Dimensional nanostructures: Electrospinning Technique and Unique Nanofibers. SpringerBriefs in Materials. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013:15–29. | |
Okutan N, Terzi P, Altay F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocolloids. 2014;39:19–26. | |
Corey JM, Gertz CC, Johnson SL, et al. The design of electrospun PLLA nanofiber scaffolds compatible with serum-free growth of primary motor and sensory neurons. Acta Biomater. 2008;4(4):863–875. | |
Boland ED, Wnek GE, Simpson DG, Pawlowski KJ, Bowlin GL. Tailoring tissue engineering scaffolds using electrostatic processing techniques: A study of poly(glycolic acid) electrospinning. Journal of Macromolecular Science, Part A. 2001;38(12):1231–1243. | |
Schnell E, Klinkhammer K, Balzer S, et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28(19):3012–3025. | |
Chen H, Fan X, Xia J, et al. Electrospun chitosan-graft-poly (ε-caprolactone)/poly (ε-caprolactone) nanofibrous scaffolds for retinal tissue engineering. Int J Nanomedicine. 2011;6:453–461. | |
Boland ED, Telemeco TA, Simpson DG, Wnek GE, Bowlin GL. Utilizing acid pretreatment and electrospinning to improve biocompatibility of poly(glycolic acid) for tissue engineering. J Biomed Mater Res B Appl Biomater. 2004;71(1):144–152. | |
Carnell LS, Siochi EJ, Holloway NM, et al. Aligned mats from electrospun single fibers. Macromolecules. 2008;41(14):5345–5349. | |
Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3(2):232–238. | |
Zhong S, Teo WE, Zhu X, Beuerman RW, Ramakrishna S, Yung LYL. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J Biomed Mater Res A. 2006;79(3):456–463. | |
Punnoose AM, Elamparithi A, Kuruvilla S. Electrospun type 1 collagen matrices using a novel benign solvent for cardiac tissue engineering. Journal of Cellular Physiology. 2015;(August 2014). | |
Panzavolta S, Gioffrè M, Focarete ML, Gualandi C, Foroni L, Bigi A. Electrospun gelatin nanofibers: optimization of genipin cross-linking to preserve fiber morphology after exposure to water. Acta Biomaterialia. 2011;7(4):1702–1709. | |
Maleknia L, Majdi ZR. Electrospinning of Gelatin Nanofiber for Biomedical Application. Orient J Chem. 2014;30(4). | |
Haider S, Al-Zeghayer Y, Ahmed Ali FA, et al. Highly aligned narrow diameter chitosan electrospun nanofibers. J Polym Res. 2013;20(105), doi 10.1007/s10965-013-0105-9. | |
Lee SJ, Heo DN, Moon JH, et al. Electrospun chitosan nanofibers with controlled levels of silver nanoparticles. Preparation, characterization and antibacterial activity. Carbohydrate Polymers. 2014;111:530–537. | |
Wang J, Ye R, Wei Y, et al. The effects of electrospun TSF nanofiber diameter and alignment on neuronal differentiation of human embryonic stem cells. J Biomed Mater Res A. 2012;100(3):632–645. | |
Liu Z, Zhang F, Ming J, Bie S, Li J, Zuo B. Preparation of electrospun silk fibroin nanofibers from solutions containing native silk fibrils. Journal of Applied Polymer Science. 2014;132(1). | |
Kamudzandu M, Yang Y, Roach P, Fricker RA. Efficient alignment of primary CNS neurites using structurally engineered surfaces and biochemical cues. RSC Adv. 2015;5(28):22053–22059. | |
Wen X, Tresco PA. Effect of filament diameter and extracellular matrix molecule precoating on neurite outgrowth and Schwann cell behavior on multifilament entubulation bridging device in vitro. Journal of Biomedical Materials Research. Part A. 2006;76A(3):626–637. | |
Kalil K, Dent EW. Touch and go: guidance cues signal to the growth cone cytoskeleton. Current Opinion in Neurobiology. 2005;15(5):521–526. | |
Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol. 2011;3(6):pii: a001727. | |
Gomez TM, Letourneau PC. Actin dynamics in growth cone motility and navigation. Journal of Neurochemistry. 2014;129(2):221–234. | |
Purves D, Augustine GJ, Fitzpatrick D. Neuroscience. 2nd ed. Sunderland (MA): Sinauer Associates, Inc.; 2001. | |
Curinga G, Smith GM. Molecular/genetic manipulation of extrinsic axon guidance factors for CNS repair and regeneration. Exp Neurol. 2008;209(2):333–342. | |
Polleux F, Snider W. Initiating and growing an axon. Cold Spring Harb Perspect Biol. 2010;2(4):a001925. | |
Purves D, Augustine GJ, Fitzpatrick D, et al. Neuroscience. 3rd ed. Sunderland, MA: Sinauer Associates, Inc.; 2004. | |
Weightman A, Jenkins S, Pickard M, Chari D, Yang Y. Alignment of multiple glial cell populations in 3D nanofiber scaffolds: toward the development of multicellular implantable scaffolds for repair of neural injury. Nanomedicine. 2014;10(2):291–295. | |
Mahairaki V, Lim SH, Christopherson GT, et al. Nanofiber matrices promote the neuronal differentiation of human embryonic stem cell-derived neural precursors in vitro. Tissue Eng Part A. 2011;17(5–6):855–863. | |
Hoffman-Kim D, Mitchel JA, Bellamkonda RV. Topography, cell Response, and nerve regeneration. Annu Rev Biomed Eng. 2010;12:203–231. | |
Lai B-Q, Wang J-M, Ling E-A, Wu J-L, Zeng Y-S. Graft of a tissue-engineered neural scaffold serves as a promising strategy to restore myelination after rat spinal cord transection. Stem Cells Dev. 2014; 23(8):910–921. | |
Ellis-Behnke RG, Liang Y-X, You S-W, et al. Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(13):5054–5059. | |
Liu C, Huang Y, Pang M, et al. Tissue-engineered regeneration of completely transected spinal cord using induced neural stem cells and gelatin-electrospun poly (lactide-co-glycolide)/polyethylene glycol scaffolds. PLoS One. 2015;10(3):e0117709. | |
Yang Y, Wimpenny I, Ahearne M. Portable nanofiber meshes dictate cell orientation throughout three-dimensional hydrogels. Nanomedicine. 2011;7(2):131–136. | |
Weightman AP, Pickard MR, Yang Y, Chari DM. An in vitro spinal cord injury model to screen neuroregenerative materials. Biomaterials. 2014;35(12):3756–3765. | |
Puschmann TB, Pablo Y De, Zande C, et al. A novel method for three-dimensional culture of central nervous system neurons. Tissue Eng Part C Methods. 2014;20(6):485–493. | |
Dumont RJ, Okonkwo DO, Verma S, et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clinical Neuropharmacol. 2001;24(5):254–264. | |
Thuret S, Moon LDF, Gage FH. Therapeutic interventions after spinal cord injury. Nature Reviews Neuroscience. 2006;7(8):628–643. | |
Ray SK, Dixon CE, Banik NL. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol Histopathol. 2002;17(4):1137–1152. | |
Onifer SM, Rabchevsky AG, Scheff SW. Rat models of traumatic spinal cord injury to assess motor recovery. ILAR J. 2007;48(4):385–395. | |
Shibuya S, Yamamoto T, Itano T. Glial and axonal regeneration following spinal cord injury. Cell Adh Migr. 2009;3(1):99–106. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.