Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Nanoencapsulation of pomegranate bioactive compounds for breast cancer chemoprevention
Authors Shirode A, Bharali D, Nallanthighal S, Coon J, Mousa S , Reliene R
Received 29 March 2014
Accepted for publication 14 September 2014
Published 9 January 2015 Volume 2015:10(1) Pages 475—484
DOI https://doi.org/10.2147/IJN.S65145
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Carlos Rinaldi
Amit B Shirode,1,2,* Dhruba J Bharali,3,* Sameera Nallanthighal,1,2 Justin K Coon,1,2 Shaker A Mousa,3 Ramune Reliene1,2
1Department of Environmental Health Sciences, University at Albany, State University of New York, Albany, NY, USA; 2Cancer Research Center, University at Albany, Rensselaer, NY, USA; 3Pharmaceutical Research Institute, Albany College of Pharmacy and Health Sciences, Albany, NY, USA
*These authors contributed equally to this work
Abstract: Pomegranate polyphenols are potent antioxidants and chemopreventive agents but have low bioavailability and a short half-life. For example, punicalagin (PU), the major polyphenol in pomegranates, is not absorbed in its intact form but is hydrolyzed to ellagic acid (EA) moieties and rapidly metabolized into short-lived metabolites of EA. We hypothesized that encapsulation of pomegranate polyphenols into biodegradable sustained release nanoparticles (NPs) may circumvent these limitations. We describe here the development, characterization, and bioactivity assessment of novel formulations of poly(D,L-lactic-co-glycolic acid)–poly(ethylene glycol) (PLGA–PEG) NPs loaded with pomegranate extract (PE) or individual polyphenols such as PU or EA. Monodispersed, spherical 150–200 nm average diameter NPs were prepared by the double emulsion–solvent evaporation method. Uptake of Alexa Fluor-488-labeled NPs was evaluated in MCF-7 breast cancer cells over a 24-hour time course. Confocal fluorescent microscopy revealed that PLGA–PEG NPs were efficiently taken up, and the uptake reached the maximum at 24 hours. In addition, we examined the antiproliferative effects of PE-, PU-, and/or EA-loaded NPs in MCF-7 and Hs578T breast cancer cells. We found that PE, PU, and EA nanoprototypes had a 2- to 12-fold enhanced effect on cell growth inhibition compared to their free counterparts, while void NPs did not affect cell growth. PU-NPs were the most potent nanoprototype of pomegranates. Thus, PU may be the polyphenol of choice for further chemoprevention studies with pomegranate nanoprototypes. These data demonstrate that nanotechnology-enabled delivery of pomegranate polyphenols enhances their anticancer effects in breast cancer cells. Thus, pomegranate polyphenols are promising agents for nanochemoprevention of breast cancer.
Keywords: PLGA–PEG nanoparticles, pomegranate extract, punicalagin, ellagic acid, MCF-7 cells, Hs578T cells
Introduction
For centuries, pomegranates (Punica granatum L.) have been used for medicinal purposes, in particular, against diarrheal, gum, parasitic, and inflammatory disorders.1 In addition, antioxidants present in pomegranate fruit have been implicated in protection against oxidative-stress-related diseases including diabetes, cardiovascular disorders, and cancer.1,2
Pomegranates contain many polyphenolic compounds with high antioxidant and free-radical-scavenging activity, including flavonoids, condensed tannins, and hydrolyzable tannins (ellagitannins [ETs] and gallotannins).3,4 ETs are considered to be the most bioactive polyphenols of pomegranates.4 The most abundant ET in pomegranates is punicalagin (PU). PU is found at high quantities (>2 g/L) in commercial pomegranate juice, which is obtained by pressing whole fruit, and is responsible for more than half of the total antioxidant capacity of the juice.5 However, ETs are inherently unstable compounds and are susceptible to spontaneous and enzymatic hydrolysis.4 For example, PU, the largest ET with a molecular weight (MW) of over 1,000 Da, is hydrolyzed to structurally related compounds such as punicalin (MW 782 Da), gallagic acid (MW 638 Da), gallic acid (MW 170 Da), and ellagic acid (EA) (MW 302 Da).6,7 EA and EA glycosides are also found in pomegranate juice but at about ten times lower concentrations than PU.5 PU is unique to pomegranate, while EA is also present in berries, including raspberries, blackberries, and strawberries, and nuts, including walnuts, pistachio, cashew nuts, and pecans.8
Pomegranate extract (PE), pomegranate juice, and/or individual pomegranate polyphenols exhibit anticancer effects in vitro and in vivo. PE, containing primarily ETs, exhibits antiproliferative, pro-apoptotic, anti-invasive, and/or anti-inflammatory properties in vitro in cancer cell lines.9–15 In addition, PE reduced the growth of human prostate and lung cancer xenografts in immunodeficient mice and suppressed prostate tumorigenesis in the TRAMP mouse model.16–18 Phase II clinical trials in prostate cancer patients with rising prostate-specific antigen (PSA) showed that a daily intake of pomegranate juice or PE (POMx; POM Wonderful, Los Angeles, CA, USA) prolongs PSA doubling time, which is used as a predictor of clinical outcomes and survival in patients with prostate cancer.19,20 PU inhibited the growth of human lung, breast, colon, and cervical cancer cells in vitro.14,21 EA decreased the incidence of chemically induced lung, mammary, and oral tumors, reduced the volume and multiplicity of estrogen-induced mammary tumors, and induced apoptosis in cancer cells in vitro.22–29 These data demonstrated that pomegranate phytochemicals provide protection against various cancer-related processes.
Despite the documented beneficial effects, poor absorption, low systemic bioavailability, and short retention time of ETs and their metabolites may undermine their full chemopreventive potential. For example, ETs such as PU do not enter the human body intact but are hydrolyzed in the intestinal tract to EA moieties and converted to urolithins by colonic microbiota prior to absorption.30–32 EA mostly accumulates in intestinal epithelial cells with limited absorption into systemic circulation.33,34 As a result, low nanomolar range concentrations of free EA and/or urolithins have been detected in human blood after consumption of pomegranate juice.30,35 In addition, absorbed EA and urolithins have short half-life due to rapid metabolism in the liver and excretion through urine.
Encapsulation of ETs into biocompatible and biodegradable nanoparticles (NPs) may overcome their susceptibility to gastrointestinal hydrolysis, poor absorption, low systemic bioavailability, and short half-life. Nanotechnology approaches were initially applied to cancer therapeutics to decrease toxicity, increase stability and bioavailability, and promote selective tumor uptake.36 More recently, these approaches are being exploited in cancer prevention with dietary phytochemicals.37–39 As a result, a new area of investigation, nanochemoprevention, was born which holds promise to enhance the efficacy of bioactive food compounds through nanoencapsulation. In fact, recent studies showed that nanoprototypes of epigallocatechin-3-gallate (EGCG) from green tea, curcumin from turmeric, and resveratrol from table grapes are more efficacious than their free counterparts.37,40–42
Poly(lactic-co-glycolic acid) (PLGA) NPs are biocompatible, biodegradable, and stable in biological fluids and have been shown to protect the loaded compounds from degradation, resulting in sustained release.43,44 PLGA NPs are taken up by cells via fluid-phase pinocytosis and/or clathrin-mediated endocytosis.44,45 PLGA NPs rapidly exit the endo-lysosomes and enter the cytoplasm.44 PLGA undergoes spontaneous and enzymatic hydrolysis of their ester linkages to produce lactic acid and glycolic acid.43 Because both lactic acid and glycolic acid are endogenous molecules, they are easily metabolized to carbon dioxide and water via the Krebs cycle, and PLGA polymer is considered to be a safe agent in humans.43 The US Food and Drug Administration and European Medicine Agency have approved the use of PLGA NPs via parenteral route and the use of PLGA microparticles as implants.44 In addition, PLGA NPs are being extensively investigated as oral drug carriers.41,46–48 A major disadvantage of PLGA NPs is that they are rapidly opsonized by immunoglobulins and complement proteins and cleared by the reticulo-endothelial system and thus may not reach target tissues.43,44 Modifying their surface with biocompatible polymers such as poly(ethylene glycol) (PEG) reduces opsonization and prolongs their circulation time in the blood by several orders of magnitude.43,44
We hypothesized that encapsulation of pomegranate bioactive compounds in PLGA–PEG NPs would increase their anticancer activity through increased cellular uptake, attenuated hydrolysis, and sustained release in the cytoplasm. Thus, we designed, synthesized, and characterized PLGA–PEG NPs loaded with PE, PU, or EA (hereafter designated as PE-NP, PU-NP, and EA-NP, respectively) and examined their effects in MCF-7 and Hs578T breast cancer cells. We found that all pomegranate nanoformulations exhibited superior antiproliferative effects compared to their free counterparts.
Materials and methods
Reagents
PE is derived from pomegranate fruit grown in California (Wonderful variety; Paramount Farms, Lost Hills, CA, USA) and is commercially available for human consumption (POMx). PE consists of 95% glycone ETs (mono and oligomeric) standardized to 37% PU and 3.4% free EA.13,30 EA was obtained from Selleckchem (Houston, TX, USA). PU, PLGA–PEG, polyvinyl alcohol (PVA), and dichloromethane were obtained from Sigma-Aldrich (St Louis, MO, USA). Alexa Fluor-488 dye was purchased from Invitrogen (Carlsbad, CA, USA).
NP synthesis
NPs were synthesized by the double emulsion–solvent evaporation method. Figure 1 depicts the synthesis of PE-NPs. Briefly, a stock solution of PLGA–PEG polymer was prepared by dispersing 80 mg/mL of PLGA–PEG in dichloromethane. A stock solution of PE (10 mg/mL) was prepared in dichloromethane. Five hundred microliters of each stock solution were mixed together by vortexing. Then, 1 mL of this solution, containing 40 mg/mL PLGA–PEG and 5 mg/mL PE, was mixed with 200 μL of phosphate-buffered saline (PBS) by probe sonication three times for 30 seconds each time (probe power level 6: power density of 0.55 W/mL) at room temperature to obtain the primary emulsion. The primary emulsion was then intermittently emulsified by sonication for 30 seconds in 2 mL of 1% (w/v) PVA solution. This water-in-oil-in-water emulsion was then added to 40 mL of 1% PVA solution and stirred for 30 minutes under constant magnetic stirring. Immediately afterward, dichloromethane was evaporated at low pressure at 37°C using a rotatory evaporator. NPs were dialyzed using a 10–12 kDa dialysis membrane against water for 8 hours to remove impurities and then lyophilized. The lyophilized powder was redispersed for further use. EA-NPs and void NPs were synthesized using the same method. Alexa Fluor-488-labeled NPs were synthesized by conjugating Alexa Fluor-488 dye to void PLGA–PEG NPs functionalized with carboxyl groups. PU-NPs were synthesized using the same method as PE-NPs and EA-NPs with the exception that the PU stock solution (10 mg/mL) was prepared in PBS and the primary emulsion was obtained by emulsifying 200 μL of this solution with 1 mL of 40 mg/mL PLGA–PEG.
NP characterization
NPs were characterized by transmission electron microscopy (TEM) and dynamic light scattering (DLS). The size distribution and morphology of NPs were examined using a JEOL JEM-100CX transmission electron microscope (JEOL Inc, Peabody, MA, USA). One drop of NPs dispersed in deionized water was mounted on a thin film of amorphous carbon deposited on a copper grid (300 meshes), air dried, and examined. The size distribution and surface charge (zeta potential) of NPs was determined by DLS using a Malvern Zetasizer (Malvern Instrumentation Co, Westborough, MA, USA). After the redispersion of the lyophilized powder in deionized water, 1 mL of the NP solution was transferred in a four-sided, clear plastic cuvette and a capillary zeta potential cell for size distribution and zeta potential measurements, respectively, and measured directly at 25°C.
Cell culture
MCF-7 cells (a gift from Dr Welsh, Cancer Research Center, University at Albany, State University of New York) were maintained in Minimum Essential Eagle’s Medium (Sigma-Aldrich) supplemented with 25 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; Fisher Scientific, Fair Lawn, NJ, USA), 20 mM D-(+)glucose, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5% fetal bovine serum (Sigma-Aldrich). Hs578T cells (a gift from Dr Welsh) were maintained in Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich) supplemented with 0.01 mg/mL bovine insulin (Sigma-Aldrich) and 10% fetal bovine serum (Sigma-Aldrich). Cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. PE or PU stock solutions (1 mg/mL) were prepared in deionized water. EA stock solution (2 mg/mL) was prepared in 1 N NaOH (note, EA is insoluble in water). NP stock solutions (1 mg/mL PE- or EA-NPs and 0.5 mg/mL of PU-NPs) were prepared in deionized water.
Determination of cell growth
Cell growth was determined by acid phosphatase assay.49 The assay is based on the hydrolysis of p-nitrophenyl phosphate by intracellular acid phosphatases in viable cells to produce p-nitrophenol, which absorbs light at a wavelength of 405 nm. At the end of treatment period, the culture medium was removed and each well was washed once with 200 μL of PBS. Buffer, containing 0.1 M sodium acetate (pH 5.0), 0.1% Triton X-100, and 5 mM p-nitrophenyl phosphate (Sigma-Aldrich), was added and the plates were incubated at 37°C for 2 hours. The reaction was stopped with the addition of 100 μL of 1 N NaOH. The absorbance was read with a VICTOR3 V 1420 Multi-Label Counter (PerkinElmer Inc, Waltham, MA, USA) at 405 nm. NP preparations and their respective controls were examined in at least three independent experiments in triplicate.
Confocal imaging
Forty microliters of Alexa Fluor-488-conjugated PLGA–PEG NPs was added to each well of a four-well Lab-Tek®II Chamber slides™ (NUNC A/S, Roskilde, Denmark) containing MCF-7 cells in 2 mL of medium and mixed into the medium. Cells were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2 for 15 minutes, 2 hours, 6 hours, and 24 hours. After incubation, the cells were rinsed with sterile PBS and fixed in 1% formaldehyde. Cells were imaged using a TCS SP5 confocal microscope (Leica, Exton, PA, USA) equipped with a 63× (numerical aperture: 1.3 glycerol immersion) objective lens at an excitation wavelength of 488 nm. Emission was detected between 500 nm and 540 nm.
Statistical analysis
Comparisons between groups were made using a two-tailed Student’s t-test. P-values <0.05 were considered statistically significant.
Results
NP characterization
NPs were characterized by TEM and DLS. TEM analysis showed that NPs were sphere-shaped monomers and their diameter was approximately 150 nm (Figure 1). DLS analysis showed that the average NP size (Z-average diameter) ranged from ~150 nm to 200 nm and the polydispersity index was 0.1–0.2, indicating the formation of nearly monodispersed NPs (Figure 2 and Table 1). Consistent with the negative charge of PLGA–PEG, the surface charge of NPs (zeta potential) was negative (Table 1).
In vitro uptake of NPs
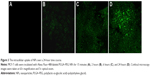
The intracellular uptake and distribution of NPs were analyzed over a 24-hour time course. MCF-7 cells were incubated with Alexa Fluor-488-labeled PLGA–PEG NPs for 15 minutes, 2 hours, 6 hours, and 24 hours and examined by confocal microscopy. Fluorescent intensity was observed starting at 2 hours post incubation, which significantly increased at 6 hours. The highest intensity was at 24 hours, indicating that NP uptake reached the maximum at about 24 hours of incubation (Figure 3). NPs accumulated predominantly in the cell membrane and in the cytoplasm.
Effect of NPs on cell growth
To determine whether nanoencapsulation enhances biological activity of pomegranate phytochemicals, we compared the effect of free PE, PU, and EA versus their nanoprototypes in MCF-7 and Hs578T breast cancer cells. We found that all pomegranate nanoprototypes inhibited cancer cell growth to a significantly greater extent than their respective free counterparts in both cell lines (Figure 4). Void NPs had no significant effect on cell viability at the same concentrations of polymer as in pomegranate phytochemical-loaded NPs, indicating that PLGA–PEG nanocarriers are relatively nontoxic.
We determined the half maximal inhibitory concentration (IC50) for growth inhibition for PE, PU, and EA nanoprototypes and their respective free counterparts (Table 2). We found that nanoencapsulation reduced the IC50 values by more than two-fold for PE and EA and by more than five-fold for PU in MCF-7 cells. In addition, in Hs578T cells, nanoencapsulation reduced the IC50 values by two-fold for PE and EA and by 12-fold for PU. These data imply that PLGA–PEG NP delivery enhances the efficacy of pomegranate bioactive compounds. We also compared the IC50 values of individual pomegranate phytochemicals such as PU and EA in terms of molarity (note that PE is a mixture of polyphenols, hence molarity is not applicable). We confirmed that PU-NPs were more potent than EA-NPs. For example, in MCF-7 cells, the IC50 of PU-NPs was about seven-fold lower than that of EA-NPs (7.5 μM versus 50.5 μM), while IC50 of free PU was only three-fold lower than that of free EA (40.9 μM versus 119.9 μM). In Hs578T cells, the IC50 of PU-NPs was 20-fold lower than that of EA-NPs (4.1 μM versus 83.5 μM), while IC50 of free PU was only four-fold lower than the IC50 of free EA (48.4 μM versus 190.1 μM). Thus, we concluded that PU-NPs are the most potent pomegranate nanoprototype in inhibiting breast cancer growth in vitro.
Discussion
In this study, we prepared three novel nanoformulations of pomegranate polyphenols, namely PE-, PU-, and EA-NPs, and examined their effects in MCF-7 and Hs578T breast cancer cells. We found that all pomegranate nanoprototypes were superior in enhancing cell growth inhibition compared to their respective free counterparts in both cell lines. PU-NPs were the most potent of the three nanoformulations. Void NPs did not affect cell growth at the same concentrations of polymer as in pomegranate phytochemical loaded NPs. These results are significant in chemoprevention research. First, these data demonstrate that PLGA–PEG NPs provide a safe delivery system to enhance the bioactivity of chemopreventive phytochemicals from pomegranates. Second, we have identified a highly effective pomegranate nanoprototype, namely PU-NPs, for further characterization in vivo.
Although PU is a predominant polyphenol in pomegranates, it has extremely low bioavailability. Studies have failed to detect PU in human plasma after ingestion of pomegranate juice.30,32 It was concluded that, in humans, intact PU is not absorbed but is hydrolyzed to EA moieties. In rats, low levels of intact PU were detected in plasma and urine after a 37-day dietary administration of PU.6,50 In contrast, no detectable PU was measured in plasma of rats in another study, where PU was administered via diet or subcutaneous polymeric implants for 10 days.51 The authors of that study suggested that the low extraction efficiency of PU from plasma and/or limit of detection by UPLC-UV (ultra performance liquid chromatography with UV detection) explained the failure to detect PU even when delivered by subcutaneous implants. However, the study demonstrated that PU delivered via subcutaneous implants inhibited benzo[a]pyrene-induced DNA adducts at 38-fold lower dose as compared to PU delivered via diet. In addition, PU delivered via implants increased plasma EA levels by two orders of magnitude. PU remained stable in vivo in implants grafted in the animals, implying that PU hydrolysis to EA occurred in bodily fluids rather than in implants. Similarly, other studies demonstrated that the PU content remained constant under shelf-life conditions and/or in aqueous solutions at pH 3–7.3 However, a significant portion of PU was metabolized to EA and EA-derived metabolites in human colon adenocarcinoma Caco-2 cells within 48 hours and some spontaneous hydrolysis occurred in growth medium without Caco-2 cells.52 These studies demonstrated that PU is an elusive and difficult-to-detect polyphenol that is rapidly hydrolyzed in biological systems to generate EA and EA metabolites.
Thus, it is currently unclear whether PU is bioactive per se. Most importantly, our data demonstrating that PU-NPs were significantly more potent in inhibiting cancer cell growth than other NPs suggests that PU-NPs may be prime candidates for further chemoprevention studies with pomegranate nanoprototypes. We postulate that NP-enabled intracellular delivery of PU would provide us with a significant number of bioactive compounds. Punicalin, gallagic acid, gallic acid, and EA would be produced upon hydrolysis of PU (Figure 5).6,7 Consequently, PU hydrolysis products would be processed by Phase II enzymes to produce other metabolites. Each of these compounds may have a complementary, additive, and/or synergistic effect on the cells. In addition, PU may be bioactive per se before hydrolysis occurs. The PLGA–PEG core would protect PU from rapid hydrolysis, providing slow and sustained release of PU and other bioactive compounds into the cell.
  | Figure 5 Hydrolysis of PU. |
Our study shows for the first time that nanotechnology-enabled delivery of pomegranate phytochemicals provides an advantage over their free counterparts. In particular, these results are important in the light of previous negative results reported by Li et al.53 Their study used partially purified pomegranate ellagitannins (PPE) and gelatin to prepare NPs and tested their efficacy in HL-60 leukemia cell line.53 However, PPE loaded into gelatin NPs were less effective than free PPE in inducing the early stage of apoptosis and no difference was observed in late stage of apoptosis. Insufficient cellular uptake of the gelatin NPs was suggested as a plausible reason for the lack of improved efficacy. In contrast to gelatin NPs, PLGA-based NPs have been shown to have excellent cellular uptake both in vitro and in vivo.54
At present, our study success is limited to cultured cancer cells. However, studies have demonstrated efficient delivery of bioactive food compounds (ie, curcumin and EGCG) through PLGA-based NPs in vivo in mice and rats.37,41,55 We propose therefore to confirm our results in preclinical studies in whole animals, where NP delivery of pomegranate ETs is expected to protect against intestinal hydrolysis, increase absorption and systemic bioavailability, and prolong their half-life through sustained release, collectively leading to enhanced protection against cancer. ETs can reduce tumor development and progression due at least in part to their capability to inhibit cell growth.9–15 We found that PE reduces growth of proliferating cells in both tumorigenic and nontumorigenic mammary epithelial cell lines (unpublished data). It is, however, unlikely that ETs affect the viability of normal quiescent and postmitotic cells. Thus, pomegranate ETs may retard proliferation of rapidly growing cells including preneoplastic cells in the early stages of cancer development and neoplastic cells in advanced cancers and have only minor effects on normal cells.
Conclusion
In summary, this proof-of-principle study demonstrated that encapsulation of pomegranate polyphenols in PLGA–PEG NPs enhances their bioefficacy. Furthermore, we identified PU-NPs as the most potent of the three pomegranate nanoformulations, suggesting that PU represents a polyphenol of choice for further investigations of pomegranate nanoprototypes. This study serves as the first step toward establishing pomegranate nanoformulations as promising cancer chemopreventive agents.
Acknowledgment
The authors would like to thank Dr JoEllen Welsh, University at Albany, State University of New York, for a critical reading of the manuscript.
Disclosure
The authors report no conflicts of interests in this work.
References
Jurenka JS. Therapeutic applications of pomegranate (Punica granatum L.): a review. Altern Med Rev. 2008;13(2):128–144. | ||
Johanningsmeier SD, Harris GK. Pomegranate as a functional food and nutraceutical source. Annu Rev Food Sci Technol. 2011;2: 181–201. | ||
Heber D. Pomegranates: Ancient Roots to Modern Medicine. Boca Raton, FL: CRC Press; 2006. | ||
Heber D. Pomegranate ellagitannins. In: Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects. Boca Raton, FL: CRC Press; 2011. | ||
Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000; 48(10):4581–4589. | ||
Cerda B, Llorach R, Ceron JJ, Espin JC, Tomas-Barberan FA. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr. 2003; 42(1):18–28. | ||
Seeram N, Lee R, Hardy M, Heber D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep Purif Technol. 2005;41(1):49–55. | ||
Larrosa M, Garcia-Conesa MT, Espin JC, Tomas-Barberan FA. Ellagitannins, ellagic acid and vascular health. Mol Aspects Med. 2010; 31(6):513–539. | ||
Syed DN, Afaq F, Mukhtar H. Pomegranate derived products for cancer chemoprevention. Semin Cancer Biol. 2007;17(5):377–385. | ||
Kasimsetty SG, Bialonska D, Reddy MK, Ma G, Khan SI, Ferreira D. Colon cancer chemopreventive activities of pomegranate ellagitannins and urolithins. J Agric Food Chem. 2010;58(4):2180–2187. | ||
Wang L, Alcon A, Yuan H, Ho J, Li QJ, Martins-Green M. Cellular and molecular mechanisms of pomegranate juice-induced anti-metastatic effect on prostate cancer cells. Integr Biol (Camb). 2011; 3(7):742–754. | ||
Adhami VM, Khan N, Mukhtar H. Cancer chemoprevention by pomegranate: laboratory and clinical evidence. Nutr Cancer. 2009;61(6): 811–815. | ||
Shirode AB, Kovvuru P, Chittur SV, Henning SM, Heber D, Reliene R. Antiproliferative effects of pomegranate extract in MCF-7 breast cancer cells are associated with reduced DNA repair gene expression and induction of double strand breaks. Mol Carcinog. 2014;53(6):458–470. | ||
Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54(3):980–985. | ||
Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113(3):423–433. | ||
Albrecht M, Jiang W, Kumi-Diaka J, et al. Pomegranate extracts potently suppress proliferation, xenograft growth, and invasion of human prostate cancer cells. J Med Food. 2004;7(3):274–283. | ||
Khan N, Hadi N, Afaq F, Syed DN, Kweon MH, Mukhtar H. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis. 2007;28(1):163–173. | ||
Adhami VM, Siddiqui IA, Syed DN, Lall RK, Mukhtar H. Oral infusion of pomegranate fruit extract inhibits prostate carcinogenesis in the TRAMP model. Carcinogenesis. 2012;33(3):644–651. | ||
Pantuck AJ, Leppert JT, Zomorodian N, et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12(13):4018–4026. | ||
Paller CJ, Ye X, Wozniak PJ, et al. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis. 2013;16(1):50–55. | ||
Aqil F, Munagala R, Vadhanam MV, et al. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res Int. 2012;49(1):345–353. | ||
Khanduja KL, Gandhi RK, Pathania V, Syal N. Prevention of N-nitrosodiethylamine-induced lung tumorigenesis by ellagic acid and quercetin in mice. Food Chem Toxicol. 1999;37(4):313–318. | ||
Berni A, Grossi MR, Pepe G, et al. Protective effect of ellagic acid (EA) on micronucleus formation induced by N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) in mammalian cells, in in vitro assays and in vivo. Mutat Res. 2012;746(1):60–65. | ||
Anitha P, Priyadarsini RV, Kavitha K, Thiyagarajan P, Nagini S. Ellagic acid coordinately attenuates Wnt/beta-catenin and NF-kappaB signaling pathways to induce intrinsic apoptosis in an animal model of oral oncogenesis. Eur J Nutr. 2013;52(1):75–84. | ||
Aiyer HS, Srinivasan C, Gupta RC. Dietary berries and ellagic acid diminish estrogen-mediated mammary tumorigenesis in ACI rats. Nutr Cancer. 2008;60(2):227–234. | ||
Edderkaoui M, Odinokova I, Ohno I, et al. Ellagic acid induces apoptosis through inhibition of nuclear factor kappa B in pancreatic cancer cells. World J Gastroenterol. 2008;14(23):3672–3680. | ||
Ho CC, Huang AC, Yu CS, et al. Ellagic acid induces apoptosis in tsgh8301 human bladder cancer cells through the endoplasmic reticulum stress- and mitochondria-dependent signaling pathways. Environ Toxicol. 2014;29(11):1262–1274. | ||
Pitchakarn P, Chewonarin T, Ogawa K, et al. Ellagic acid inhibits migration and invasion by prostate cancer cell lines. Asian Pac J Cancer Prev. 2013;14(5):2859–2863. | ||
Qiu Z, Zhou B, Jin L, et al. In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food Chem Toxicol. 2013;59:428–437. | ||
Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. 2006;136(10):2481–2485. | ||
Cerda B, Tomas-Barberan FA, Espin JC. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. J Agric Food Chem. 2005;53(2):227–235. | ||
Cerda B, Espin JC, Parra S, Martinez P, Tomas-Barberan FA. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr. 2004;43(4):205–220. | ||
Smart RC, Huang MT, Chang RL, Sayer JM, Jerina DM, Conney AH. Disposition of the naturally occurring antimutagenic plant phenol, ellagic acid, and its synthetic derivatives, 3-O-decylellagic acid and 3,3′-di-O-methylellagic acid in mice. Carcinogenesis. 1986;7(10):1663–1667. | ||
Whitley AC, Stoner GD, Darby MV, Walle T. Intestinal epithelial cell accumulation of the cancer preventive polyphenol ellagic acid – extensive binding to protein and DNA. Biochem Pharmacol. 2003;66(6):907–915. | ||
Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin Chim Acta. 2004;348(1–2):63–68. | ||
Bharali DJ, Mousa SA. Emerging nanomedicines for early cancer detection and improved treatment: current perspective and future promise. Pharmacol Ther. 2010;128(2):324–335. | ||
Siddiqui IA, Adhami VM, Bharali DJ, et al. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69(5):1712–1716. | ||
Bharali DJ, Siddiqui IA, Adhami VM, et al. Nanoparticle delivery of natural products in the prevention and treatment of cancers: current status and future prospects. Cancers. 2011;2011(3):4024–4045. | ||
Sanna V, Siddiqui IA, Sechi M, Mukhtar H. Nanoformulation of natural products for prevention and therapy of prostate cancer. Cancer Lett. 2013;334(1):142–151. | ||
Srivastava AK, Bhatnagar P, Singh M, et al. Synthesis of PLGA nanoparticles of tea polyphenols and their strong in vivo protective effect against chemically induced DNA damage. Int J Nanomedicine. 2013; 8:1451–1462. | ||
Xie X, Tao Q, Zou Y, et al. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J Agric Food Chem. 2011;59(17):9280–9289. | ||
Sanna V, Siddiqui IA, Sechi M, Mukhtar H. Resveratrol-loaded nanoparticles based on poly(epsilon-caprolactone) and poly(d,l-lactic-co-glycolic acid)-poly(ethylene glycol) blend for prostate cancer treatment. Mol Pharm. 2013;10(10):3871–3881. | ||
Lü JM, Wang X, Marin-Muller C, et al. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 2009;9(4):325–341. | ||
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–522. | ||
Sah H, Thoma LA, Desu HR, Sah E, Wood GC. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. Int J Nanomed. 2013;8:747–765. | ||
Kalaria DR, Sharma G, Beniwal V, Ravi Kumar MN. Design of biodegradable nanoparticles for oral delivery of doxorubicin: in vivo pharmacokinetics and toxicity studies in rats. Pharm Res. 2009; 26(3):492–501. | ||
Kumar G, Sharma S, Shafiq N, Pandhi P, Khuller GK, Malhotra S. Pharmacokinetics and tissue distribution studies of orally administered nanoparticles encapsulated ethionamide used as potential drug delivery system in management of multi-drug resistant tuberculosis. Drug Deliv. 2011;18(1):65–73. | ||
He W, Horn SW, Hussain MD. Improved bioavailability of orally administered mifepristone from PLGA nanoparticles. Int J Pharm. 2007;334(1–2):173–178. | ||
Yang TT, Sinai P, Kain SR. An acid phosphatase assay for quantifying the growth of adherent and nonadherent cells. Anal Biochem. 1996; 241(1):103–108. | ||
Cerda B, Ceron JJ, Tomas-Barberan FA, Espin JC. Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic. J Agric Food Chem. 2003;51(11): 3493–3501. | ||
Aqil F, Vadhanam MV, Gupta RC. Enhanced activity of punicalagin delivered via polymeric implants against benzo[a]pyrene-induced DNA adducts. Mutat Res. 2012;743(1–2):59–66. | ||
Larrosa M, Tomas-Barberan FA, Espin JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem. 2006;17(9):611–625. | ||
Li Z, Percival SS, Bonard S, Gu L. Fabrication of nanoparticles using partially purified pomegranate ellagitannins and gelatin and their apoptotic effects. Mol Nutr Food Res. 2011;55(7):1096–1103. | ||
Bharali DJ, Yalcin M, Davis PJ, Mousa SA. Tetraiodothyroacetic acid-conjugated PLGA nanoparticles: a nanomedicine approach to treat drug-resistant breast cancer. Nanomedicine (Lond). 2013;8(12): 1943–1954. | ||
Khalil NM, do Nascimento TC, Casa DM, et al. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf B Biointerfaces. 2013; 101:353–360. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.