Back to Journals » International Journal of Nanomedicine » Volume 19
Nano-Drug Delivery Systems Targeting CAFs: A Promising Treatment for Pancreatic Cancer
Authors Wang M, Xue W, Yuan H, Wang Z, Yu L
Received 4 December 2023
Accepted for publication 6 March 2024
Published 18 March 2024 Volume 2024:19 Pages 2823—2849
DOI https://doi.org/10.2147/IJN.S451151
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Mingjie Wang,1,* Wenxiang Xue,2,* Hanghang Yuan,2 Zhicheng Wang,2 Lei Yu1
1Department of Radiotherapy, Second Hospital of Jilin University, Changchun, Jilin, People’s Republic of China; 2NHC Key Laboratory of Radiobiology, School of Public Health, Jilin University, Changchun, Jilin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhicheng Wang; Lei Yu, Tel +86-431-85619443, Email [email protected]; [email protected]
Abstract: Currently, pancreatic cancer (PC) is one of the most lethal malignant tumors. PC is typically diagnosed at a late stage, exhibits a poor response to conventional treatment, and has a bleak prognosis. Unfortunately, PC’s survival rate has not significantly improved since the 1960s. Cancer-associated fibroblasts (CAFs) are a key component of the pancreatic tumor microenvironment (TME). They play a vital role in maintaining the extracellular matrix and facilitating the intricate communication between cancer cells and infiltrated immune cells. Exploring therapeutic approaches targeting CAFs may reverse the current landscape of PC therapy. In recent years, nano-drug delivery systems have evolved rapidly and have been able to accurately target and precisely release drugs with little or no toxicity to the whole body. In this review, we will comprehensively discuss the origin, heterogeneity, potential targets, and recent advances in the nano-drug delivery system of CAFs in PC. We will also propose a novel integrated treatment regimen that utilizes a nano-drug delivery system to target CAFs in PC, combined with radiotherapy and immunotherapy. Additionally, we will address the challenges that this regimen currently faces.
Keywords: nanoparticle, nano-drug delivery system, cancer-associated fibroblasts, pancreatic cancer, tumor microenvironment
Introduction
Pancreatic cancer (PC) is a highly aggressive disease that has been causing a gradual increase in morbidity and mortality. In recent decades, only about 4% of patients have been able to survive 5 years after diagnosis. The survival rate of PC has stagnated due to its resistance to current conventional therapies, including surgery, chemotherapy, radiotherapy, and immunotherapy. Additionally, the fact that most patients are detected at an advanced stage contributes to the challenge.1 The resistance of PC to current therapeutic modalities is primarily attributed to the structural characteristics of PC itself, as it is surrounded by a rich tumor microenvironment (TME). The rich TME not only produces various pro-tumor growth and metastatic active factors, but also serves as a physical barrier preventing drug penetration from reaching the tumor.2 Cancer-associated fibroblasts (CAFs) play a crucial role in the composition of the TME, which is a major component in the composition and regulation of the tumor’s physical barrier. CAFs can be differentiated from normal fibroblasts and PC astrocytes and they can be classified into numerous subtypes.3 Different subtypes of CAFs secrete a variety of regulatory factors that play crucial roles in PC proliferation, invasion, and metastasis. However, recent studies have shown that excessive depletion of CAFs accelerates tumor progression.4 CAFs, with their pro-tumorigenic and anti-tumorigenic functional heterogeneity, are promising targets for the treatment of PC. Currently, there are no effective therapeutic modality available to improve the prognosis of PC. Novel regimens targeting the CAFs that comprise the TME may revolutionize the current landscape of PC treatment. The ability to remodel the TME to reverse the physical barrier that protects tumor cells and creates a rapid pathway for drug penetration is crucial. Currently, there is no effective treatment to improve the prognosis of PC. However, there has been limited progress in efficiently targeting CAFs and appropriately converting tumor-promoting CAFs into anti-tumor CAFs. The use of nano-drug delivery systems has made significant advancements in recent years. These systems not only enable precise targeting of the affected area but also allow for precise temporal, spatial, and dosimetric release of drugs through suitable stimulus-responsive systems.5 Nanosystems can also target specific organelles and signaling pathways after entering cells to interfere with energy metabolism and signaling in tumor cells. This interference affects the function of tumor cells and has the potential to target CAFs in the treatment of PC.6 Furthermore, these nanosystems are virtually non-toxic to normal tissues, making them highly promising for targeted therapy. Dual targeting of CAFs and their internal organelles by nanosystems may help alleviate the issue of accelerated tumor progression caused by excessive depletion of CAFs. This review summarizes recent research advances on CAFs in PC and nano-drug delivery system. It explores potential strategies for nanosystem-targeted CAFs in PC as well.
CAFs in PC
PC is highly lethal and mesenchymal-rich, with a significant proportion of CAFs in the TME. CAFs are a class of cell types that are critical for the generation and development of PC, and they play important roles in the formation, proliferation, and invasion of PC.1 In TME, CAFs secrete growth factors, inflammatory ligands, and extracellular matrix (ECM) to promote cancer cell proliferation, resistance to treatment, and immune evasion.7 However, recent studies have revealed that, under certain circumstances, CAFs may also inhibit tumor progression.4 It has been shown that CAFs are heterogeneous in origin and species.8 Therefore, it is essential to have a comprehensive understanding of the complex role that CAFs plays in the cancer progression.
Landscape of CAFs
Origin of CAFs
A significant reason for the phenotypic difference between CAFs and quiescent fibroblasts in normal tissues is their distinct origins. Fibroblasts in normal and damaged fibrotic tissues originate from mesodermal cells during embryonic development. Although fibroblasts can come from peripheral tissues, bone marrow stem cells, endothelial cells, and bone marrow-derived fibroblasts,9–12 the origins of CAFs are still a subject of controversy. Furthermore, there is limited information regarding the specific cell types that give rise to different subtypes of CAFs. It has been demonstrated in pancreatic ductal adenocarcinoma (PDAC) that in vitro, pancreatic stellate cells (PSCs) can differentiate into inflammatory fibroblasts (iCAFs) and myofibroblasts (myCAFs).13 Antigen-presenting CAF (apCAF) isoforms14 and mesothelial cells were recently identified in PDAC through scRNA-seq and immunohistochemistry analysis.8 This observation suggests that apCAF may originate from mesothelial cells.15 Thus, in PDAC, CAFs are primarily derived from PSCs and potentially from mesothelial cells. The different origins create heterogeneity in CAF subpopulations, and these studies provide a foundation for our understanding of the heterogeneity of CAFs in TME and promote further research on the origin and function of CAF subtypes.
Subtypes of CAFs
The subtypes of CAFs correlate with specific types of cancer. The most consistently observed subtypes across different cancer types are likely the myCAFs and non-myCAFs.16–18 In addition, the presence of myCAFs and iCAFs has also been confirmed in PC.19
With the help of a 3D co-culture system, study found that a small subset of PDAC fibroblasts expresses fibroblast activation protein (FAP) and high levels of alpha smooth muscle actin (α-SMA), which were defined as myCAF. Additionally, a CAF subtype with low α-SMA, a high interleukin-6 (IL-6) inflammatory profile, and a loss of myofibroblast characteristics was also defined as iCAF.13 Elyada et al identified a subtype of CAF that expresses the major histocompatibility complex (MHC) class II family of genes and named it apCAF. MyCAF requires direct contact with cancer cells in the PDAC to be activated and is in the periglandular region. In addition, experimental studies have shown that PDAC depleted of myCAFs exhibits inhibition of angiogenesis and enhancement of tumor hypoxia.4 Furthermore, myCAFs have been found to be associated with metastasis and immunosuppression in PDAC.20 iCAF, which is located far away from the tumor cells and myCAF, is able to be activated by paracrine factors secreted by the tumor cells. iCAF can secrete various immunosuppressive factors, such as IL-6 and prostaglandin E2 (PGE2), to inhibit the activity of immune cells. It also releases CXCL12 in an NF-κB-mediated manner, leading to immunosuppression and promoting tumor growth in PDAC. iCAF is closely associated with inflammation related to the TME.21–23 ApCAF can express professional antigen-presenting cells (APCs) and other co-stimulatory factors, but their levels are significantly reduced, which suggests that the role of apCAF in PDAC is different from that of professional APCs. apCAF is regulated by IFN-γ signaling in vivo and exhibits an antioxidant response, making it inextricably linked to the immunity of the pancreatic TME. 14 The TGF-β/SMAD2/3 pathway can induce myCAF activation in PDAC and it is believed that myCAF can be converted to iCAF.24 However, there is a lack of direct experimental evidence to confirm whether these two cell states can be interconverted in vivo.
The Role of CAFs in the Progression of PC
The Interaction Between CAFs and PC
CAFs participate in a complex signaling network that encompasses tumor cells, immune infiltrates, and other cellular constituents within the intricate TME. Within this context, cancer cells are recognized as the instigators of malignancy due to their ability to reprogram normal fibroblasts and quiescent PSCs into CAFs through diverse mechanisms.25 Biffi et al, utilizing organoids and murine models, demonstrated that gradients of tumor-secreted ligands, primarily TGF-β and IL-1, diffuse outward from epithelial lesions, influencing intracellular signaling in fibroblasts and driving the differentiation of distinct CAF subtypes at an epigenetic level.24 This underscores the potential of TGF-β as a crucial target for pancreatic cancer therapy through CAFs. Several therapies targeting ligands or receptors involved in TGF-β signaling, such as Trabedersen (targeting TGF-β2 with antisense oligonucleotides), LY3022859 (anti-TβRII mAb), LY2157299 (TβRI inhibitor), and TEW-7197 (TGF-β receptor ALK4/ALK5 inhibitor), have progressed to clinical trials.26
The reciprocal communication between cancer cells and CAFs is intricate and intimate. Upon activation by transformed cancer cells, CAFs engage in reciprocal signaling, promoting cancer cell proliferation, metabolism, stemness, and drug resistance.27 Through systematic proteomic studies of secretory mediators, Shi and colleagues identified leukemia inhibitory factor (LIF) as a critical paracrine factor released by activated PSCs, driving tumor progression and chemoresistance in cancer cells.28 Clinically, trials targeting LIF as a therapeutic candidate are undergoing Phase I trials.26 CAFs enhance PDAC cell proliferation and survival by secreting CXCL12 and stimulating the corresponding receptor CXCR4 in cancer cells. Various signaling pathways within cancer cells are activated by the CXCL12-CXCR4 axis, including the MAPK, PI3K, Wnt and Shh pathways, collectively contributing to pancreatic cancer progression.29 This highlights the potential of the CXCL12-CXCR4 axis as a therapeutic target for pancreatic cancer therapy through CAF modulation. Drugs targeting this axis, such as Olaptesed (anti-CXCL12 oligonucleotide), BL-8040 (CXCR4 peptide antagonist), and Plerixafor/AMD3100 (CXCR4 inhibitor), are currently undergoing clinical trials.26
Functional Heterogeneity of CAFs
CAFs present a multifaceted array of functions within the realm of tumor biology, profoundly shaped by their distinct phenotypic traits. Broadly, these functions bifurcate into two principal categories: the facilitation and the restraint of tumor progression.4 Different types of CAFs have distinct pro-tumorigenic and anti-tumorigenic roles (Table 1).
 |
Table 1 Functional Heterogeneity of CAFs in PC: Tumor Promoting and Tumor Suppressive Effects |
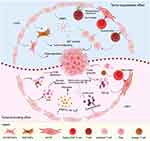
CAFs significantly contribute to the onset and progression of cancers. They enhance tumorigenesis, proliferation, invasion, and metastasis by secreting a myriad of cytokines, chemokines, growth factors, and enzymes that degrade the stromal matrix. These fibroblasts also modulate the TME, establishing direct interactions with cancer cells. Furthermore, they influence the immune response to tumors by releasing inflammatory cytokines, fostering angiogenesis, and providing crucial support for tumor metastasis and invasion. For example, CAFs can secrete a variety of growth factors, cytokines, and soluble factors, such as TGF-β, IL-6, HGF, heat shock factor 1 (HSP1), various angiogenic factors, stromal cell-derived factor 1 (SDF-1, also known as CXC motif chemokine ligand 12, CXCL12), and members of the matrix metalloproteinase (MMP) family (including MMP-1, -2, and -3). These factors can promote the growth, proliferation, and metastasis of neighboring cancer cells.34–38 Expression of associated transcription factors and iconic proteins in CAFs can also promote tumor growth, invasion, and metastasis. For example, overexpression of the transcription factor ETV1 increased tumor volume by promoting stromal expansion, altering stromal morphology, enhancing invasive capacity, and up-regulating regulators of EMT and MMP, including SLUG, SNAIL, TWIST, Vimentin (VIM), zinc finger E-box binding homeobox 1 (ZEB1), ZEB2, and MMP9.39 Another key player in tumor promotion is FAP, which has been shown to play a crucial role in extracellular matrix (ECM) formation, angiogenesis, cell motility, immunosuppression, and final clinical outcome.40 Chiwaki et al demonstrated the role of FAP in cancer cell invasion and EMT.41 A study by McAndrews et al found that specific depletion of FAP+ CAFs could significantly inhibit PDAC tumor progression and significantly increase overall survival in mice (Figure 1).42 CAFs also act as promoters of tumor growth and invasion by influencing tumor metabolism. Increased expression of monocarboxylate transporters 4 (MCT4) and hypoxia-inducible factor 1 alpha (HIF-1α) in CAFs, compared with healthy fibroblasts, increases glycolytic activity, resulting in the secretion of excess lactic acid. This excess lactic acid can stimulate cancer cells and promote tumor progression.43 CAFs-derived exosomes carry amino acids, TCA cycle intermediates, and lipids. Once ingested by cancer cells, these exosomes will inhibit mitochondrial respiration while promoting cancer cell proliferation through increased glycolysis and glutamine-dependent reductive carboxylation.44 CAFs can also exchange lipid-derived metabolites with tumor cells. For example, PSC-derived CAFs secrete large amounts of lysophosphatidylcholine (LPC) compared to their healthy counterparts. The extracellular enzyme autocrine motility factor, secreted by cancer cells and CAFs, subsequently converts LPC into the wound-healing mediator which is called lysophosphatidic acid (LPA). LPA promotes the proliferation, migration, and activation of AKT in PC cells.45 CAFs can significantly reduce the killing ability of various immune cells against tumor cells, thereby promoting the proliferation and progression of tumor cells. In the case of PC, which is typically characterized by a high degree of connective tissue proliferation, reduced vascularity, and extreme hypoxia, these factors have led to the limited effectiveness of immunotherapy for treating this type of cancer.46 In addition, even if immune cells enter the TME, their activity is inhibited by immunosuppressive factors and the hypoxic environment.47
Contrasting with their tumor-promoting activities, select CAF contingents are known to impede tumor growth and progression.4,48,49 This is accomplished through the activation of specific signaling pathways, such as the hedgehog (Hh) pathway, which has the capacity to alter the rigidity of the tumor microenvironment and decelerate tumor progression. Emerging research, including pivotal studies by Özdemir et al, underscores the tumor-suppressive capabilities of certain CAF subsets, particularly myCAFs in PC.4 Experimental depletion of myCAFs has been observed to intensify tumor infiltration and diminish survival rates, indicating a potential defensive role in both the early and advanced stages of PDAC. Additionally, a correlation has been identified between lower α-SMA scores in patients and an increased prevalence of poorly differentiated cancers, suggesting a role for myCAFs in sustaining immune surveillance.
The paradoxical roles of CAFs in tumor biology sketch a complex therapeutic landscape, pointing to the feasibility of a “stromal switch” strategy to transform tumor-promoting CAFs into tumor-suppressing entities. Nonetheless, the diversity and intricacy of CAF phenotypes demand extensive research to fully decipher their specific functions across different tumor types and to formulate precise therapeutic approaches. The pronounced heterogeneity of CAFs highlights their critical status as therapeutic targets, and a deeper comprehension of this heterogeneity is crucial for enhancing treatment modalities and prognoses for cancer patients, particularly those battling PC.
Relationship Between CAFs and PC Treatment
Recently, CAFs, as a major component of TME, have been identified as another key factor in promoting tumor progression.3 Although more specific roles and detailed mechanisms of CAFs in cancer pathogenesis and progression still need to be further explored, many studies have found that CAFs are important markers of PC nowadays. CAFs not only correlate with prognosis and resistance to chemotherapy, radiotherapy and immunosuppression, but also serve as key targets that have the potential to improve the current status of PC treatment. The specific mechanisms of action of CAFs in chemotherapeutic resistance, radiotherapeutic resistance, and immunosuppression are shown in Figure 2.
CAF Markers for PC Prognosis
Specific markers or certain types of CAFs play a more significant role in the diagnosis and prognosis determination of PDAC. Histologic studies have shown that a higher expression of a-SMA or a high percentage of stroma predicts a poor clinical outcome in patients with PDAC.50 Related studies have already discovered that LIF can activate CAFs.20 Furthermore, alterations in circulating LIF levels have been closely linked to the tumor’s response to therapy.28 Therefore, LIF would be an attractive therapeutic target and circulating marker. Sun et al found a negative correlation between IL-33 and CXCL3 levels and the survival of PDAC patients. It can be inferred that IL-33 and CXCL3 can serve as prognostic markers for PDAC.20 Meanwhile, leucine-rich repeat-containing protein 15 (LRRC15) myCAF surrounds tumor islets and it is absent in normal tissues of PDAC. An immunotherapy clinical trial involving more than 600 patients found that elevated levels of LRRC15 myCAF signaling were associated with poor outcomes after anti-PD-L1 therapy. This suggests that LRRC15 myCAF may serve as a prognostic marker for immune checkpoint blockade therapy in PDAC.8 Mizutani et al found that PSCs can be activated to become CAFs in PDAC and Meflin expression was detected in CAFs in the tumor stroma of both human PDAC and a PDAC mouse model. Moreover, Meflin CAFs could be used as a marker of a good prognosis in PDAC patients.51 In a meta-analysis of 29 patients from 4000 studies, the combination of gibberellins and CAFs was found to result in a decrease in overall survival (OS) and disease-free survival (DFS) in patients with solid tumors. This suggests that gibberellins could serve as a prognostic marker with therapeutic potential.52 In addition to the aforementioned studies, the staging of PDAC disease can be assessed by integrating multiple markers to actively monitor the status and its interconversion of CAFs. This approach is preferable to relying on a single marker as a prognostic criterion, as it provides more robust and dependable evidence for treatment.
CAFs and Chemotherapeutic Resistance
The modifications in the extracellular environment induced by CAFs constitute a significant contributory element to the emergence of resistance to chemotherapy. Multiple mechanisms have been identified and verified, with the principal mechanism involving the creation of physical barriers that impede the infiltration of chemotherapy agents. The extracellular matrix barrier constructed by CAFs poses a physical obstacle to the transportation of drugs, thereby limiting drug delivery. The excessive expression of polysaccharides, particularly hyaluronic acid, by CAFs activated in PDAC,53 leads to the development of densely stromal compartments characterized by elevated interstitial pressure. This heightened interstitial pressure, predominantly mediated by hyaluronic acid, is linked to vascular collapse and inadequate perfusion, resulting in alterations to the physiological functions within tumors and consequentially influencing drug delivery. Furthermore, the overexpression of hyaluronic acid is correlated with unfavorable prognoses in PDAC patients.54
In addition to external environmental alterations precipitating resistance to chemotherapy, intrinsic mechanisms of chemoresistance inherent to tumor cells can also be directly or indirectly modified by CAFs, thereby facilitating the survival of cancer cells under the influence of chemotherapy. Intrinsic mechanisms such as anti-apoptosis and enhanced survival capabilities in cancer cells are orchestrated through various signaling pathways, including RAS-RAF-MEK-ERK and PI3K-AKT. This interplay between cancer cells and CAFs is facilitated by the expression of diverse molecules (such as laminin, fibronectin, IGF, SDF-1α, and basement membrane proteoglycans), triggering multiple downstream signaling pathways that bolster the survival of cancer cells.55–60
Numerous experiments have illustrated that chemotherapy can induce stromal reactions to promote tumor progression. These acquired resistance mechanisms, mediated through different pathways, furnish a theoretical underpinning for the failure of chemotherapy following prolonged clinical treatment. In vitro experiments have delineated molecular alterations and functional modifications associated with PDAC CAFs, following gemcitabine treatment and corroborated that CAFs subjected to chemotherapy assume a more supportive role in tumor promotion compared to their untreated counterparts.61 Moreover, gemcitabine chemotherapy can prompt CAFs to release exosomes containing various tumor-promoting molecules.62 Research has revealed that the generation and secretion of exosomal miRNA-106b and SNAIL1 can augment the viability and proliferation of recipient tumor cells.61,63 Furthermore, miRNA-451a is abundantly present in the exosomal profile of PDAC CAFs.64 This miRNA can modulate the drug transport protein P-glycoprotein,65 potentially fostering chemotherapeutic resistance by exploiting this transport protein.
CAFs and Radiotherapeutic Resistance
Another significant characteristic of CAFs is their ability to induce radio-resistance, which reduces the efficacy of radiotherapy in tumor treatment and ultimately promotes tumor growth.
Both in vitro and in vivo studies have shown that CAFs can hinder the response of cancer cells to radiation therapy through direct or paracrine interactions. For example, radiation therapy leads to increased secretion of HGF, CXCL12, and elevated c-Met phosphorylation of the HGF receptor in CAFs. This promotes proliferation, metastasis, and stimulates EMT-associated drug resistance in PC cells.66,67 In addition, radiotherapy induces CAFs to produce TGF-β. This not only promotes the migration and potential metastatic escape of cancer cells but also enhances radio-resistance, ultimately leading to poorer survival outcomes in patients.68,69 The proliferation of connective tissues in TME can also be mediated through integrin β1 in cancer cells, as well as downstream FAK and MAPK-AKT signaling pathways, which are involved in radio-resistance.70 The hypoxic tumor microenvironment generated by the pro-fibroproliferative response will further exacerbate radio-resistance in cancer cells.71
CAFs and Immunosuppression
CAF assumes a central role in modulating the anti-tumor efficacy of immune cells within the tumor immune microenvironment (TIME).72,73 Furthermore, they facilitate the expression of immune checkpoint molecules and ECM remodeling, thereby indirectly influencing the recruitment and function of immune cells.72 Through the secretion of cytokines, chemokines, and other effector molecules, CAFs foster the initiation and progression of cancer while promoting ECM degradation and remodeling.74,75 Numerous investigations have substantiated that the interplay between CAFs and immune cells, alongside other immune constituents, can govern the TIME, consequently impeding anti-tumor immune responses.76,77
CAFs Inhibit Innate Immunity
The innate immune system of the human body is a sophisticated entity comprising macrophages, neutrophils, natural killer (NK) cells, antigen-presenting cells, mast cells, and their respective secreted molecules. CAFs, functioning as cells exerting immunosuppressive effects in tumors, can interact with various cellular molecules in the innate immune system, diminishing their anti-tumor effects and thereby promoting tumor progression.
Infiltrating macrophages within tumors, known as tumor-associated macrophages (TAMs), are divided into two distinct subgroups activated by different polarizing cytokines, termed M1 (induced by lipopolysaccharide alone or in conjunction with Th1 cytokines) and M2 (Th2 cytokines).78 As a critical component of the TIME, TAMs play a crucial role in its regulation, particularly in tumor immune suppression.79,80 TAMs are the most prominent immune cells near areas of CAF aggregation, indicating close interaction between these two cell types.81 Increasing evidence suggests that tumor-associated neutrophils (TANs), as an important component of the TIME, also exhibit phenotypic heterogeneity and functional diversity.82,83 Notably, CAFs may regulate neutrophil polarization. As recently reported in a hepatocellular carcinoma study, CAFs-derived cardiotrophin-like cytokine factor 1 (CLCF1) induces polarization of N2 phenotype neutrophils by upregulating CXCL6 and TGF-β expression in tumor cells, thereby promoting tumor progression.84 Furthermore, CAFs may be involved in all stages of malignant progression of TANs, ultimately inhibiting anti-tumor immune responses in the TME. By secreting SDF-1α, CAFs can recruit peripheral neutrophils to the tumor.85 In recent decades, increasing attention has been focused on the role of mast cells (MC) in cancer, rather than their role in allergic diseases.86,87 Excessive presence of CAFs and MCs in the tumor stroma is closely associated with cancer invasiveness, and their interaction directly leads to tumor progression.88 Natural killer (NK) cells are members of the innate immune system with natural responses to tumor cells.89 Increasing evidence suggests that CAFs inhibit NK cells through various processes, including direct or indirect activation of NK cell receptors, cytotoxic activity, and cytokine production.90,91 In recent years, several studies have shown that CAFs can drive immune escape of tumor cells by blocking dendritic cell (DC) maturation, antigen presentation, and related adaptive immune responses.92
CAFs Inhibit Acquired Immunity
The adaptive immune system primarily comprises T lymphocytes, B lymphocytes, their respective activated types, and a myriad of cytokines, exhibiting extensive interactions with the innate immune system. It constitutes a pivotal component in tumor immunity, serving as the vanguard in tumor immune responses. CAFs employ various pathways to diminish the functionality of the acquired immune system, thereby effecting immune suppression.
T lymphocytes play a pivotal role in regulating acquired immune responses, encompassing diverse subgroups such as Treg cells, helper T (Th) cells, and cytotoxic T lymphocytes (CTLs).93 Extensive research has elucidated the role of CAFs in modulating T cell activity and function. Treg cells characterized by high Foxp3 expression are known to play a crucial role in curtailing anti-tumor immune responses.94 Kinoshita et al employed immunohistochemical staining to confirm the proximity of Treg cells to CAFs. Furthermore, clinical data indicate an association between the infiltration of Foxp3+ Tregs and CAFs in the tumor stroma and adverse prognosis.95 These findings suggest potential crosstalk between CAFs and Treg cells. Some reports suggest significant effects of CAF-related activity on Th cell polarization, although their specific role remains unclear. For instance, when DNA vaccines target CAF-activating proteins, polarization of the Th2 subset is concurrently inhibited, suggesting that activated CAFs may promote the aforementioned differentiation.96 Extensive research has reported interactions between CAFs and CD8+ T cells, detailing CAFs’ inhibitory effects on infiltration, proliferation, and anti-tumor immunity of CD8+ T cells.97
Interactions Between CAFs and Other Immune Components
In addition to directly influencing the innate and acquired immune systems, CAFs can achieve immune suppression by interacting with other immune components. Specific mechanisms involve upregulating the expression of cell surface immune checkpoint molecules on CAFs, inducing immune tolerance, and reshaping the ECM to promote immune suppression.
The heightened expression of immune checkpoint molecules on the surface of T cells and tumor cells has been identified as the primary cause of T cell dysfunction in the TME.98 CAF itself can express various immune checkpoint molecule ligands on its cell surface, including PD-L1, PD-L2, B7-H3/H4, galectins, and IDO enzymes.99 In addition to upregulating molecules on their own surface, CAFs also produce various types of cytokines and exosomes to upregulate checkpoint molecules on other cells, such as tumor cells and immune cells in the TME, indirectly inhibiting T cell function and anti-tumor immune responses. For example, it has been reported that CAFs in pancreatic cancer upregulate the expression of certain immune checkpoint molecules, including PD-1, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), T cell immunoglobulin, mucin domain-containing-3 (TIM-3), and lymphocyte activation gene-3 (LAG-3), on the surface of CD4+ and CD8+ T cells, thereby inhibiting the proliferation of T cells and their specific recognition of tumor cells.100
Changes in ECM in the TME are a common phenomenon in tumor tissues, often associated with cancer progression.101 Several studies have demonstrated the crucial role of CAFs in reshaping the ECM. 102 CAFs can promote the degradation of normal ECM structure by secreting various matrix proteins (such as fibronectin and type I collagen) and producing various MMPs, while increasing matrix stiffness.103 The modified ECM, in turn, promotes CAF activation and tumor function. Increasing evidence suggests that CAF-induced ECM modifications are associated with cancer cell migration and invasion.104 Moreover, this modified matrix is involved in inducing immune suppression in the TME. The ECM protein network reshaped by CAFs can act as a physical barrier to immune cells (especially T lymphocytes), thereby inhibiting their recruitment to cancer sites and reducing their opportunities to participate in TME immune responses.105 Additionally, CAF-modified ECM can also regulate the activity of other immune cell populations. Aberrant carcinogenic collagen matrices are involved in the recruitment and function of TAMs.106
Potential Targets of CAFs in PC
The abundant stromal components in pancreatic cancer tissues can not only promote pancreatic cancer invasion and metastasis but also limit drug infiltration and reduce the killing effect of drugs on cancer cells. CAFs are associated with PC development, metastasis, immunosuppression, and drug resistance in many aspects, so targeting CAFs is an increasingly important direction for the treatment of PC. With the advancement of nanotechnology, various nanocarriers with different sizes, structures, and surface properties are now available, allowing systemic or localized drug delivery. Besides, nanosystems have the advantage of directly targeting CAF-related targets, which has great potential in the treatment of pancreatic cancer. The potential targets of CAFs and their advantages and disadvantages are shown in Table 2.
 |
Table 2 Potential Targets and Advantages of CAF Treatment |
FAP
FAP is a fibroblast-activated protein produced by CAFs and an oncogenic factor. FAP is highly expressed in CAFs. It has long been customary to consider FAP as a better target for CAFs.117 Yu et al achieved this goal by encapsulating paclitaxel-albumin nanoparticles (HSA-PTX) in CAP-modified heat-sensitive liposomes (CAP-TSL) and then adding the photothermal agent IR-TSL to form HSA-PTX@CAP-ITSL, resulting in the development of novel dual-responsive nanoparticles. HSA-PTX@CAP-ITSL first accumulates at the tumor site and releases HSA-PTX through the cleavable CAPs that are responsive to FAP-α. Upon irradiation with near-infrared (NIR) laser light, the nanoparticle not only kills tumor cells through the thermal effect but also enhances the release of HSA-PTX and facilitates deep penetration into tumor tissues. The anti-tumor effects were verified by in vivo and in vitro experiments in this study.118 Several studies have combined targeted therapy for CAFs with photodynamic therapy (PDT). Since FAP occupies an important position in CAFs-targeted therapy, an anti-FAP antibody and its humanized version, sibrotuzumab, have been used in clinical studies for various experiments. However, after conducting preliminary studies, researchers discovered that the expression of FAP in normal tissues cannot be ignored.108 Additionally, treatment with sibrotuzumab resulted in severe side effects, highlighting the need for a more targeted approach to specifically target CAFs. Equally disappointing, however, is the variety of FAP-targeting agents, including bispecific antibodies, antibody-drug couplings, vaccines, and chimeric antigen receptor (CAR) T-cells, all of which are designed to eliminate FAP fibroblasts. But anti-FAP therapy leads to muscle loss, osteotoxicity, cachexia, and even death, which may be related to the tumor suppressor function of CAFs. Excessive depletion of CAFs leads to loss of tumor suppressor function, which leads to malignant outcomes.107
α-SMA
α-SMA is a classical CAF membrane surface marker, MMF precursor (MMF-LA) by chemically derivatizing MMF with linoleic acid (LA) was constructed to develop MMF-LA@DSPE-PEG nanoparticles by encapsulating MMF-LA using DSPE-PEG2000. It was observed by immunofluorescence that MMF-LA@DSPE-PEG could aggregate in the α-SMA-positive region. The results showed that MMF-LA@DSPE-PEG significantly reduced the density of CAFs and exhibited higher anti-tumor activity.109 Unfortunately, ablation of α-SMA CAFs resulted in reduced survival in a PDAC animal model. This outcome was closely associated with an increase in Treg cells and a suppression of immune surveillance.4
IL-6 and Its Receptor
iCAFs are characterized by low α-SMA and high IL-6. IL-6 has the ability to inhibit NK cells and promote PDAC metastasis. In order to promote the development of PDAC by increasing mitogen-activated protein kinase (MAPK) signaling activation and to reduce the therapeutic response by inhibiting anticancer immunotherapy for PDAC in multiple ways, targeting IL-6 is a wise choice. Relevant clinical trial studies have demonstrated that IL-6 activates STAT3 and leads to chemo-resistance of PDAC in mouse models. Furthermore, the efficacy of chemotherapy in PDAC has been shown to improve after blocking IL-6 signaling.110 As the most important marker of iCAF, IL-6 plays a very important role, and the inhibition of IL-6 or its receptor is significant in arresting tumor progression.
Sonic Hedgehog (SHH) Signaling
A study found that the SHH signaling pathway is specifically activated in myCAFs. The antagonist LDE225 effectively inhibited SHH signaling, leading to a reduction in the number of myCAF and an increase in the number of iCAF in mice. This led to the accumulation of fibroblasts and immune infiltration in the PDAC microenvironment.111 In another experiment, a combination of gemcitabine and the SHH inhibitor IPI-926 was used in a mouse model of PC. The results showed that the concentration of gemcitabine in the tumors increased and the treatment stabilized the disease process.119
Other Relevant Targets
The IL-1/LIF/JAK/STAT3 pathway, TGF-β and integrins, and the CXCL-associated axis are among the other targets. Biffi et al demonstrated that the IL-1/LIF/JAK/STAT 3 pathway promotes tumor progression and activates iCAF in the PDAC, thereby promoting tumor growth. Inhibiting this pathway can slow down tumor progression. Current experiments using Anakinra to inhibit IL-1R have shown prolonged overall survival in pancreatic cancer. This study also found that TGF-β promotes the transformation from fibroblasts to myCAF. It listed TGF-β as a research target for PDAC treatment as well.112 Additionally, FAP+CAF secretes CXCL12, which leads to immunosuppression in PDAC. Inhibition of CXCR4, a receptor for CXCL12, using the inhibitor AMD3100 resulted in the accumulation of T cells in cancerous tissues, along with PD-L1 blockade, leading to a reduction in cancer cells in KPC mice.114 Several studies have confirmed the effectiveness of these two inhibitory PDAC pathways.20,120 CAFs also have many potential targets, such as hyaluronidase, ECM and so on. The multiple targets of CAFs offer various alternative pathways for treating pancreatic cancer by targeting CAFs. With the continuous advancement of biotechnology and chemical synthesis technology, an increasing number of suitable targets have been discovered and characterized. The discovery of these markers provides more options for nanomedicine research that targets CAFs. The study of specifically targeting certain types of CAFs, rather than all CAFs, is likely to address the drawback of over-depletion of CAFs that worsens the deterioration of PC. It can also preserve the tumor suppressor function of CAFs to some extent. This approach may provide a new direction for the treatment of PDAC and suggest a new perspective for future experimental studies.
Nanomaterials for Targeting CAFs
Efficient Drug Delivery Systems
As one of the most lethal and devastating human cancers, conventional treatments for PC are extremely ineffective. Therefore, nano-drug delivery systems are ideal for transporting anticancer drugs deep into cancerous tissues as well as reducing both damage and side effects against their healthy cells. Many nanomaterials have been used as delivery carriers, including inorganic particles such as silica particles, metallic nanoparticles (eg, gold, silver, and titanium), carbon nanostructures (eg, nanotubes, nanodiamonds, and graphene), organic materials such as liposomes, polymeric carriers (eg, micelles, hydrogels, polymersomes, dendrimers, and nanofibers), and hybridized nanomaterials.120 Various nanomaterials are suitable for carrying different drugs because of their distinct properties. The specific categorization is shown in Table 3.
 |
Table 3 Classification of Nanotherapeutic Drugs and Their Effects |
Various nanomedicine delivery systems possess distinct advantages and drawbacks. As listed in Table 3, within the realm of inorganic nanoparticles, exemplified by TGNS, their application as imaging agents or for molecular targeting imaging and photothermal therapy can achieve efficacy at concentrations over two orders of magnitude lower than conventional gold nanoparticles. Nonetheless, they are linked to numerous adverse biological outcomes including inhibiting bacterial iron uptake, renal injury, and mediating inflammatory activity.121 Another illustration of inorganic nanoparticles is phosphate, which when employed as a drug delivery medium, can effectively integrate photodynamic therapy and magnetic hyperthermia, thereby inducing Ca2+ homeostasis imbalance to augment tumor cytotoxicity. However, the substantial size of these particles and the intricate synthesis process pose significant impediments to their prospective clinical utility.124 Among orous structure materials, Mesoporous silica nanoparticles emerge as a quintessential example. They provide meticulous control over drug release, sustained maintenance of optimal drug concentrations, prevention of potential drug degradation, and the capacity to accommodate multiple drugs to surmount tumor multidrug resistance. Nevertheless, this type of nano-carrier may harbor inherent toxicity and may not be completely eliminated from the body via hepatic and renal routes.125 Dendritic nanomicelles, a pivotal class of diminutive organic nanoparticles, showcase notable characteristics including high drug loading, diminutive size, stable formulations, low toxicity, and acid-triggered drug release. These attributes render them auspicious candidates for novel drugs that can efficaciously and continuously enhance cellular uptake. Preparations concomitantly composed of dendritic nanomicelles and gemcitabine nanoagents exhibit a substantial drug loading capacity of 33%. Comparative to free gemcitabine, they evince enhanced anticancer efficacy both in vitro and in vivo. Nevertheless, these materials manifest cytotoxicity towards normal cells, thereby impeding their clinical application.129 Lastly, within the domain of Hybridized nanoparticles, MLs are prominently discussed materials. Their utilization as carriers significantly prolongs circulation time and achieves substantial tumor deposition comparable to liposomes of analogous composition. They function as superb MRI T2 contrast agents, persisting in tumors for up to one week. However, the clinical application of this substance entails certain limitations. Relative to traditional hyperthermia, the technology for controlled release utilizing low-frequency magnetic fields remains nascent. Consequently, further elucidation of its release mechanism and optimization of magnetic field conditions are imperative.132,135
Among the common nanomaterials used for PC treatment and detection, liposomes are the first nanoparticles approved as carriers for cancer therapy. The lipid bilayer of liposomes is very similar to that of mammalian cell membranes in terms of both composition and structure. This property contributes to better targeting the PC cells and being uptaken by them.136 Liposome nanoparticles carrying gemcitabine have been confirmed to be more effective than the drug alone in the treatment of PC in mice.134 Meanwhile, Liposome-encapsulated paclitaxel for the treatment of PC is under study.137 Kim et al found that coupling liposomes with polymeric compounds, such as polyethylene glycol, enhanced the cytotoxicity of liposomes and improved their delivery to target sites.138 A new type of liposomal nanoparticles loaded with 1, 3-disubstituted hydrofuran-2yl-5FU (MFU), which is called Zhubech liposomal nanoparticles, is currently under investigation. They have enhanced anticancer effects under normal physiological conditions. However, methods to enhance their stability need to be explored in the future due to drawbacks such as poor retention and a short half-life.139 Hybridized nanoparticles that combine liposomes with magnetic nanomaterials have been shown to exhibit improved therapeutic efficacy. These nanoparticles not only serve as a traceable alternative to MRI, but also increase TME permeability and drug perfusion, and enhance the deposition of liposomal drug carriers at the organ and tissue levels.78 Recent studies have found that cationic polymer nanoparticles (cNPs) can be excreted in the form of extracellular vehicles (EVs), encapsulated within cNP@EVs. This suggests that cNP@EVs are an effective form of nanoparticles for intercellular transport and have the potential to be used as an efficient biomimetic drug delivery system.140
Response Mode of Nano-Drug Delivery System
The high efficiency of a nano-drug delivery system is demonstrated not only by its ability to efficiently target and deliver drugs to the tumor site, but also by the design of an appropriate stimulus response system that can achieve the goal of time, space, and dose control of the nano-drug delivery system, resulting in a more efficient therapeutic effect. The nanosystem response can be categorized into endogenous stimulus response and exogenous stimulus response.5
Endogenous stimulation of nanosystem responses includes pH response and enzyme stimulation response. The pH response of nanosystems is achieved through the acidic nature of TME in PC. This property allows the nanosystems to release the drug controllably at specific pH conditions, which not only protects the drug from the harsh conditions in the gastric lumen but also improves its absorption in the intestine.140 The stimulation of nanosystems by enzymes refers to the ability of nanoparticles to be recognized and broken down by specific enzymes. For example, pancreatic elastase IIIA and its variants, as well as amyloidogenic proteases, are highly expressed in the pancreas. By utilizing a sensitive nanoparticle drug-carrying system, when these nanoparticles enter pancreatic cancer tissues, proteolytic enzymes hydrolyze the nanoparticles and release the drug. This process increases the concentration of the drug at the tumor site, thereby enhancing the therapeutic effect.141,142
Exogenous stimuli activate the nanosystem response through temperature, magnetic, ultrasound, light, and electrical responses. Temperature-responsive nanosystems maintain their payload under body temperature (approximately 37°C) and deliver drugs rapidly within a locally heated tumor (40–42°C) to counteract the rapid blood passage time and tumor washout. Thermoresponsive systems typically consist of liposomes, polymeric micelles, or nanoparticles. Among these, thermosensitive liposomes (TSLs) are probably the most advanced thermoresponsive nanosystems, currently being investigated for their potential use in treating breast cancer, liver metastases from colorectal cancer, and further experimental studies are needed for their application in PC.143 Modification of magnetic materials on the surface of nanoparticles can be directed to the target region in vivo through the application of an external magnetic field, enabling localized and controlled drug release. For example, Indocyanine Green (ICG) carrying iron oxide and NIR dye can specifically target PC cells expressing the early disease marker NGAL. When coupled with NGAL, TGNS can enable molecularly targeted imaging and targeted photothermal therapy through in vitro NIR photothermal therapy.144 Other external stimuli, such as ultrasound, light, and electricity, can also stimulate the response of nanosystems.5 The choice of these nanosystem response modalities depends on the specific therapeutic needs and goals. Different response modalities can achieve precise treatment for pancreatic cancer with varying characteristics.
Nano-Drug Delivery System Acting on PC-Associated CAFs
CAFs are densely distributed in pancreatic tumor sites, and their presence can also be detected in some benign tissues and ducts.145–147 Emerging studies have emphasized the role of CAFs in PC, revealing their mechanism of action in the development of drug resistance. Specifically, CAFs contribute to the development of drug resistance and the hindering effect in drug delivery by upregulating the expression of α-SMA, increasing the secretion of vascular endothelial growth factor (VEGF), and releasing pro-angiogenic molecules.148 Considering these findings, therapeutic strategies that target CAFs have been considered to have potential therapeutic benefits, especially in the treatment of pancreatic cancer. Targeting CAFs via nano-drug delivery systems may offer a potential solution to overcome drug resistance and improve the effectiveness of pancreatic cancer treatment. The implementation of this strategy is expected to yield more positive outcomes for patients with pancreatic cancer.
Proliferation and activation of CAFs are prerequisites for driving tumor progression and drug resistance. Therefore, intervening in this process may become an important strategy to achieve tumor suppression. A novel approach was developed to establish biodegradable polymer nanoparticles targeted towards CAFs. These nanoparticles were loaded with α-Mangostin and coated with the CREKA peptide. α-Mangostin is known to regulate TME by interfering with the TGF-β/SMAD signaling pathway and blocking the activation of CAFs. On the other hand, the CREKA peptide exhibits a specific affinity for fibronectin, which is overexpressed on the membrane surface of CAFs. Thus, the peptide coating of nanoparticles enhances the uptake of nanoparticles by CAFs. Additionally, the combined application of α-Mangostin strengthens the inhibitory effect on CAFs, thereby remodeling the TME by compromising the matrix barrier.149
The formation of CAFs is associated with two types of cells: fibroblasts, which are important components of CAFs, and pancreatic stellate cells, which serve as precursor cells to tumor-associated fibroblasts. Fibroblast formation is influenced by TGF-β1, which increases levels of reactive oxygen species (ROS) and α-SMA, and is inhibited by antioxidants.150 Ariely et al found that cerium oxide nanoparticles were able to regulate fibroblast formation and reduce α-SMA levels, thereby inhibiting tumor cell infiltration. These results suggest that cerium oxide nanoparticles may be an effective and safe therapeutic strategy.151 Mardhian et al developed a superparamagnetic iron oxide nanomaterial by modifying relaxin-2. This nanomaterial inhibits the differentiation of pancreatic stellate cells by suppressing SMAD2 signaling. It reveals that relaxin-2 nanoparticles could facilitate targeted drug delivery and inhibit collagen deposition.152 In addition, other studies have designed an injectable peptide hydrogel nanoparticles with achieving self-assembly and losartan encapsulation. These nanoparticles were successfully retained for several days in a mouse tumor in situ model and inhibited the levels of collagen and CAFs.153 A study reported a co-delivery and pH-sensitive nanoparticle system consisting of p-GEM and paclitaxel nanoparticles. This system could not only deliver drugs but also target deeper layers of the matrix. It was confirmed that these nanoparticles were able to specifically reduce α-SMA levels in tumor tissues while destroying cancer cells without affecting the extracellular matrix.154 Thus, these nanoparticles exhibit higher efficiency in drug delivery and specific targeting characteristics.
Despite evidence that CAFs act as a barrier to antitumor therapy, recent studies have shown that specific subsets of CAFs can have antitumor effects under certain circumstances. It was found that quiescent fibroblasts may differentiate into cancer-inhibiting (F1 subtype) and cancer-promoting (F2 subtype) CAFs, depending on the stage of tumorigenesis. If CAFs are directly eliminated, it may disrupt their homeostasis in the body and lead to tumor progression.7 Consequently, placing CAFs in an inactivated state or converting them to oncogenic phenotypes may be a safe and effective treatment. Several studies have attempted to reprogram CAFs at the transcriptional level. For example, Kim et al determined in a study that the small molecule Scriptaid, an HDAC 1/3/8 inhibitor, reduced the TGF-β-induced differentiation of CAFs. This also resulted in the reduction in ECM secretion, cell invasiveness and stiffness in preclinical animal models.155 Albrengues et al demonstrated the preclinical efficacy of restoring CAFs to a wild-type phenotype by targeting the pro-inflammatory cytokine LIF. This was achieved using a DNA methyltransferase (DNMT) inhibitor and a JAK inhibitor.156 Another study identified the methyltransferase nicotinamide N-methyltransferase (NNMT) as a central regulator of CAF activation in the TME. Treatment with NNMT inhibitors alone reduced the tumor burden in a mouse model. In addition, kinases involved in the complex signaling network of CAFs may also act as potential targets for inhibiting the function of CAFs.157 A study found that the multikinase receptor inhibitor Nintedanib downregulated the induction of collagen and α-SMA in TGF-β1-stimulated fibroblasts.158 Experiments investigated the effects of inhibiting the JAK2/STAT3 and MEK/ERK/1/2 pathways using ruxolitinib and trametinib to reverse the activation of a subset of pro-tumor CAFs. They observed an increase in tumor tissue response to etoposide after inhibiting JAK2/STAT3 and MEK/ERK1/2 via using ruxolitinib and trametinib. In the meantime, there was an increase in overall survival in hormone-treated mice.159 The study by Ford et al focused on the inhibition of NOX4, a ROS-generating enzyme that is a downstream target of TGF-β1 and regulates the CAF phenotype. siRNA knockdown or pharmacological inhibition GKT137831 (Setanaxib) of NOX4 “normalized” CAFs to a quiescent phenotype and promoted intratumoral infiltration of CD8+ T cells, thereby overcoming the immunosuppressive effect. These findings suggest that inhibiting NOX4 can effectively overcome CAF-mediated immunotherapy resistance and potentially improve outcomes in various types of cancers.160 Miao et al discovered that nanoparticles loaded with a plasmid encoding secreted tumor necrosis factor (TNF)-related apoptosis-inducing ligand (sTRAIL) triggered apoptosis in tumor cells located near CAFs in a mouse xenograft model. Interestingly, it restored the remaining CAFs to a quiescent state, remodeled the TME, and further inhibited tumor growth, thus facilitating a second wave of nanotherapeutics.161 A TME-responsive nano-system based on PEGylated polyethylenimine-coated gold nanoparticles was developed. This system was used to simultaneously deliver all-trans retinoic acid (ATRA), which induces quiescence in PSCs, and siRNA targeting heat shock protein 47 (HSP47), a collagen-specific molecular chaperone, for remodeling of PSCs. This nanosystem concurrently induces quiescence in PSC and inhibits ECM proliferation, which facilitated drug delivery to PC and significantly enhancing the antitumor efficacy of chemotherapeutic agents.162
In summary, various strategies have been utilized in preclinical and clinical research to target the tumor-promoting effects of CAFs. These approaches include efficient depletion of TME and inhibition of signaling pathways, with the aim of making significant advancements in the therapy of CAFs tumors. However, the heterogeneity of the CAFs population in the TME poses a significant challenge in terms of the necessity to “eliminate the tumor-promoting population without completely abolishing their anti-tumor properties”. As nano-drug delivery systems and methods for reprogramming CAFs continue to be advanced, we expect greater success in related therapeutic areas in the coming years.
Feasibility of Using Nanosystems to Target CAFs for the Treatment of PC and Proposal of New Strategies
Comparison with Conventional Treatments
The therapy of PC, with high degree of malignancy, poor prognosis and high mortality rate of PC, has shown little improvement in recent decades.163 Nowadays, the treatment of PC mainly includes surgery, chemotherapy, radiotherapy, targeted therapy and combined treatment options.164 Most patients with pancreatic cancer are typically treated with chemotherapy. However, due to the presence of vascular infiltration and distant metastasis, only a small percentage (less than 15%) of these patients are eligible for surgical removal.165 Despite ongoing efforts to improve prognosis, the 5-year survival rate for pancreatic cancer remains extremely low.166 Therefore, it is urgent to explore new therapeutic approaches. Nanoparticle-targeted therapy for pancreatic cancer has been rapidly developing in recent years and offers unique advantages over traditional treatment options. The following aspects are compared to introduce a novel treatment option using nanosystems that target CAFs to treat PC.
Targeting and biosafety: We can achieve highly specific recognition and targeted delivery of drugs to PC tissues or even specific subcellular organelles by modifying the targeting ligands on the surface of the particles.6,167 The utilization of nanosystems to specifically target CAFs can either damage PC cells by delivering PC-sensitive drugs or remodeling the TME to diminish its protective effects on PC. Nano-drug delivery systems, such as liposomes, have been widely used in the treatment of PC. Nanoparticle-targeted therapy can achieve localized release of drugs and reduce damage to normal tissues, leading to minimizing toxic side effects.164 In contrast, conventional surgery, chemotherapy, and radiotherapy are poorly targeted and often result in systemic side effects, such as infection, nausea, vomiting, and hair loss. Immunotherapy may cause side effects related to the immune response, such as inflammatory reactions and autoimmune diseases.168
Drug delivery efficiency and control of recurrent metastasis: Nanoparticles can cross the vessel wall and enter the TME and metastatic foci by altering their shape, size and surface properties. They can also enhance the solubility and stability of the drug, thereby improving the efficiency of drug delivery in tumor tissue. Many nanoparticle delivery systems for PC CAFs have been reported, such as the utilization of nanoparticles for targeted drug delivery.109 Nanosystems can also be designed to activate nanoparticles by designing response systems that enable precise release of drugs in time, space, and dose.5 It is due to these features that nanosystems have more efficient and accurate drug delivery, as well as greater control over the recurrence and metastasis of PC, compared to traditional therapeutic methods.
The specific clinical trials of nanoparticle targeted CAFs for the treatment of pancreatic cancer are shown in Table 4. In these experiments, the delivery system targeting CAFs mediated by nanoparticles has exhibited various effective anti-tumor effects. These effects include reducing the percentage of poorly to moderately differentiated tumor phenotypes, enhancing apoptosis as well as decreasing the expression of collagen, α-SMA, and Glioma-associated oncogene-1 (GLI-1) in tumor tissues. Jonas Schnittert et al devised novel peptide-based nanocomplexes (NCs) for delivering anti-miRNA oligonucleotides to human-derived pancreatic stellate cells (hPSCs) and inhibiting their differentiation into a tumor-promoting CAFs phenotype. This protocol may show significant and long-term anti-tumor effects in stromal-rich PC models.169
 |
Table 4 Clinical Trial Proves Feasibility of Nanoparticles Targeting CAFs in Pancreatic Cancer |
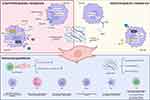
Nanoparticle Targeting of CAFs and Organelles with a View to Obtaining Enhanced Anti-Tumor Efficacy
Conventional treatments do not significantly contribute to the survival of patients with PC due to drug resistance and toxicity to normal tissues. In order to achieve better results, nanoparticles are dual-modified so that they can accurately deliver the drug to CAFs and target the nanoparticles internalized by the CAFs to specific organelles. As a result, the appropriate concentration of the drug is delivered directly to the specific site of action (Figure 3). Nanoparticles can impact the function of different organelles. For instance, biological selenium nanoparticles (SeNPs) can safeguard against intestinal barrier dysfunction by influencing endoplasmic reticulum stress (ERS) and mitochondrial autophagy-related AMPK signaling pathways.174 Nano-drug delivery systems targeting mitochondria, nucleus, lysosomes, and Golgi have been investigated.6 For example, Yu et al constructed a pillar arene-based rotaxane (R1) with tetraphenylene (TPE) and triphenylphosphine (TPP) groups as plugs. The nanoparticles loaded with DOX formed by this construction are able to release significant amounts of DOX after being internalized by HeLa cells and the DOX will enrich in the mitochondria to kill cancer cells.6 The strategy of using lipid nanoparticles modified with peptides and biofilm encapsulation to target the endoplasmic reticulum and mitochondria has been employed for targeted therapy in breast, cervical, and lung cancers. This strategy not only holds the promise of overcoming multidrug resistance (MDR) but also of amplifying the therapeutic effect by sharing drugs and/or signaling molecules to achieve crosstalk between endoplasmic reticulum and mitochondria.173 Nanoparticle-targeted CAFs in combination with chemotherapy, can enhance anti-tumor efficacy by specifically targeting CAFs or even subcellular organelles in the TME. This approach disrupts the interactions between tumor cells and their interactions, ultimately increasing the sensitivity of tumor cells to chemotherapy.
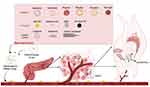
Nanoparticle-Targeted CAFs Combined with Radiotherapy and Immunotherapy
Conventional chemotherapy has significant side effects and is being used more frequently as an adjuvant therapy. Radiotherapy is a commonly used adjuvant therapy for PC. However, its efficacy is often limited by tumor hypoxia-induced radiation resistance. Additionally, radiotherapy is associated with severe side effects and carries a high risk of recurrence and metastasis.175,176 Nanomedicines synergize effectively with standard chemotherapeutic treatments. Recently, the combination of liposomal irinotecan (Onivyde®) with 5-fluorouracil (5-FU) and calcium folinate (LV) has received approval for systemic combination therapy in PC.177 Currently, nanoparticles (NPs) are being increasingly used for radiosensitization. Chen Q made poly (lactic-co-glycolic acid) (PLGA)-based core-shell nanoparticles by encapsulating water-soluble catalase (Cat) in the core and loading hydrophobic imiquimod (R837) in the PLGA shell. The PLGA-R837@Cat nanoparticle that could alleviate tumor hypoxia and significantly improve the efficacy of radiotherapy.178 In addition to metal nanoparticles such as Cu, Bi, and Gd, there has been rapid development of nano-delivery carriers capable of delivering tumor-specific radiosensitizing drugs in recent years. These carriers have the potential to enhance immune checkpoint blockade and improve radiosensitization.179 Gao et al developed a new mitochondria-targeted nanoplatform called hydrogel-based plasmonic nanosensors. This nanoplatform can be utilized for the combined radiotherapy of tumors by simultaneously inhibiting dual-energy metabolism. The study demonstrated that the nanosensors have excellent radiosensitization effects, enhancing the effectiveness of radiotherapy.180 Proton therapy has unique dose deposition properties which can protect normal tissues and improve patient prognosis.181 An important reason for the high lethality of PC is its acquired immunosuppressive privilege. Therefore, immunotherapy, which can disrupt this immunosuppressive privilege, holds promise as an approach to treating PC. This includes the utilization of immune checkpoint inhibitors, therapeutic vaccines, engineered T-cells and the advancement of prophylactic vaccines.182 However, there are certain challenges associated with immunotherapy, such as autoimmune responses, cytokine syndromes, and vascular leakage syndrome.182 The combination of immunotherapy with other treatments can modulate the immune response of tumor cells and produce synergistic therapeutic effects. A significant portion of nanomedicines can be targeted to modulate the adaptive immune system.183 CAFs can promote the presence of immunosuppressive cells in the TME by secreting pro-inflammatory cytokines and chemokines.184,185 Nanoparticles can convert immunosuppression in TME to an immunosupportive state by targeting CAFs, which play an important role in immunotherapy for PC. The main principle behind nanosystems in conjunction with immunotherapy is to deliver checkpoint inhibitors that can suppress immune checkpoints. Therapeutic peptide-assembled nanoparticles, which are antagonists of D-peptide programmed cell death ligand 1 (DPPA-1), co-assembled with NLG919, an inhibitor of indoleamine 2, 3-dioxygenase 1 (IDO-1), inhibited both immune checkpoints and tryptophan metabolism. Additionally, they increased cytotoxic T-lymphocyte (CTL) activation and survival, resulting in effective anti-tumor immunity.186 It has been shown that targeting hyaluronan synthesis by incorporating inhibitors into nanocarriers can result in ECM remodeling and enhance infiltration of γδ-T cells.187 pH-responsive nanomicelles (P/A/B@NM) containing paclitaxel (PTX), the CXCR1 antagonist AMD1, and the PD-4/PD-L1 inhibitor BMS-1 are used to activate the T-cell-mediated anti-tumor immune response. These nanomicelles can remodel the tumor stroma mediated by CAFs and the immunosuppressive microenvironment. As a result, CD8+ T-cell infiltration in breast cancer is enhanced, leading to the reactivation of anti-tumor immunity in unresponsive triple-negative breast cancer (TNBC) cases.188 Lipid poly-γ-glutamic acid (PGA)/PolyMet-pRLN nanoparticles (LPPR) can rapidly penetrate tumors and weaken the proliferation of CAFs, remodeling the TME, and decreasing the infiltration of immunosuppressive cells to achieve efficient breast cancer immunotherapy.189
Tumor immunotherapy and radiotherapy are the best partners based on nanosystems governance. Radiotherapy has certain immunostimulatory effects, and the synergistic effect of the two is beneficial for tumor control.190 Guan et al developed a novel modality combining radiotherapy and immunotherapy with a unique nanosystem based on the IPI549@HMP. They used subcutaneous melanoma in mice as a model and achieved a highly effective synergistic effect. This approach not only induced systemic anti-tumor immune memory but also demonstrated minimal toxicity.191 Chen Q fabricated multifunctional PLGA-R837@Cat nanoparticles that could be used for combination radioimmunotherapy of cancer. Cat is able to reduce the degree of tumor hypoxia and modulate the tumor’s immune-suppressing microenvironment to enhance the efficacy of radiotherapy. Additionally, R837 can further stimulate strong immune responses in residual tumors after radiotherapy-induced ICD.178 There is also an increasing number of nanoparticles that have been designed to be more conducive to combining radiotherapy and immunotherapy to achieve better therapeutic effects. For example, bacterial membrane-encapsulated nanoparticles (BNPs), natural herb Astragalus polysaccharide NPs (ANPs), and multifunctional core-shell PLGA nanoparticles have been shown to overcome tumor hypoxia-related radio- resistance.175
Clinical Application and Prospects of Nano-Drug Delivery Systems
In recent years, significant efforts in scientific research and clinical trials have aimed to refine therapeutic approaches to enhance the quality of life and prolong the survival of pancreatic cancer patients. The treatment strategies for PDAC can be broadly classified into three categories based on their specific targets. Firstly, interventions directly addressing CAFs. For instance, certain compounds such as ATRA and vitamin D analogs have demonstrated the ability to modulate various cellular pathways within CAFs, leading to a partial reversal of their activated state to a more quiescent state. Secondly, strategies involve inhibiting soluble proteins in the TME that contribute to CAF-mediated signaling. This includes blocking the production and secretion of tumor-promoting or immunosuppressive factors by CAFs, as well as inhibiting the activation of PSCs and normal fibroblast growth factors. Thirdly, approaches focus on suppressing cell surface receptors through which CAFs communicate with other cell types.26 In castration-resistant prostate cancer (CRPC), communication between TAMs and the bone microenvironment (BME) plays a significant role in disease development. Ongoing research targeting the overexpression of CAF-related proteins such as A Disintegrin and Metalloproteinase Domain 9 (ADAM9) and CAFs Monoamine Oxidase A (MAO-A) has shown promising progress in CRPC therapy. Immunotherapies directed against CAFs within the TME or BME hold crucial potential in the treatment of metastatic CRPC patients.192
Several chemotherapy formulations delivered through nanocarrier systems have progressed through various stages of clinical trials, with some reaching Phase III and nearing approval. These formulations encompass a range of nanoparticles, including albumin-bound paclitaxel nanoparticles, pathogenic nanoparticle-based gene delivery systems, micelle nanoparticles, and liposome nanoparticles.193 For instance, liposomes, polymer nanoparticles, and inorganic nanoparticles exhibit precise targeting of the melanoma tumor microenvironment, offering potential in targeted drug delivery for melanoma therapy while enhancing the efficacy of conventional drugs and reducing toxicity.194 Rexin-G, comprising nanoparticles/gene delivery vectors based on a pathogenic retrovirus, was evaluated by Gordon et al in pancreatic cancer treatment in the Philippines. The study demonstrated tumor stability and growth inhibition in two-thirds of patients without dose-limiting toxicity.195
Circular RNAs (circRNAs) transmitted via exosomes have been identified as significant contributors to chemotherapy resistance in cancer by influencing the tumor or immune microenvironment.196 Additionally, interference-based RNA (iRNA) therapy has emerged as a promising alternative in pancreatic cancer treatment, exhibiting progress in specificity, toxicity, and overcoming resistance to existing drugs. iRNA therapy, an endogenous process involving RNA sequences that complement genes in the body acting through corresponding mRNA, faces challenges in efficient siRNA delivery into target cells due to stability and degradation issues.197–199 Nanoparticle-based delivery systems, including liposomes, lipid polymers, and dendritic polymer-based nanoparticles, offer potential solutions by encapsulating siRNA, protecting it from degradation, and facilitating targeted delivery to specific cells.200–202
Furthermore, nanoparticle-based systems present opportunities for both direct and indirect immune modulation in pancreatic cancer. Direct modulation involves interactions between nanodrug delivery systems and immune cells, influencing their activation and function. These nanoparticles serve as carriers for immune stimulants, enhancing immune responses against pancreatic cancer cells. Indirectly, nanoparticle delivery systems target the tumor microenvironment, delivering therapeutic molecules to reprogram immunosuppressive cells and enhance anti-tumor immune responses. Leveraging the potential of nanodrug delivery systems, combined with emerging molecular biology approaches and innovative immune modulation strategies, holds promise in fundamentally altering the therapeutic landscape of pancreatic cancer.203,204
Prospects or Questions Raised
Conventional therapies offer limited benefits for PC. However, a potential solution lies in the use of nanosystem-targeted CAFs to deliver drugs in combination with radiotherapy and immunotherapy. This integrated treatment strategy shows promise in breaking the resistance of PC. Modified lipid nanoparticles, which are more biocompatible and like exosomes secreted by CAFs, enter the TME to interfere with the original exosome-related signaling pathways and deliver drugs to target CAFs for TME remodeling. However, due to the functional heterogeneity of CAFs, indiscriminate killing of CAFs may have counterproductive effects. The specific molecular mechanisms underlying the functional heterogeneity of CAFs are not yet clear. Therefore, designing nano-drug delivery systems that target specific tumor-promoting CAFs or release appropriate concentrations of cytotoxic substances into the organelles through nanoparticles internalized by CAFs could be the key to making breakthroughs in nano-targeting technology for CAFs. This would affect the function of CAFs and their specific regulatory mechanisms. However, achieving a balance in the quantity and ratio of various types of CAFs to maximize the anti-tumor effect necessitates a comprehensive understanding of the specific mechanisms underlying CAFs’ tumor-promoting or anti-tumor properties. This, in turn, calls for further in-depth research and exploration. Reversing the tumor-promoting TME of PC to an anti-tumor TME by targeting CAFs through a nano-drug delivery system, followed by a combination of radiotherapy and immunotherapy, could potentially offer new survival opportunities for patients with difficult-to-treat PC cases. This combination therapy strategy may become an important direction for future tumor therapy.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
This work was supported in part by the Science and Technology Development Plan of Jilin (20210402026GH), and Research plan by Health Commission of Jilin Province (2020SCZT066).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature Reviews Cancer. 2006;6(5):392–401. doi:10.1038/nrc1877
2. Giraldo NA, Sanchez-Salas R, Peske JD, et al. The clinical role of the TME in solid cancer. British Journal of Cancer. 2019;120(1):45–53. doi:10.1038/s41416-018-0327-z
3. Mao X, Xu J, Wang W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Molecular Cancer. 2021;20(1):131. doi:10.1186/s12943-021-01428-1
4. Ozdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25(6):719–734. doi:10.1016/j.ccr.2014.04.005
5. Mura S, Nicolas J, Couvreur P Stimuli-responsive nanocarriers for drug delivery. Nature Materials. 2013;12(11):991–1003. doi:10.1038/nmat3776
6. Wei G, Wang Y, Yang G, Wang Y, Ju R Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics. 2021;11(13):6370–6392. doi:10.7150/thno.57828
7. Kalluri R The biology and function of fibroblasts in cancer. Nature Reviews Cancer. 2016;16(9):582–598. doi:10.1038/nrc.2016.73
8. Dominguez CX, Müller S, Keerthivasan S, et al. Single-Cell RNA sequencing reveals stromal evolution into LRRC15(+) myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discovery. 2020;10(2):232–253. doi:10.1158/2159-8290.Cd-19-0644
9. Driskell RR, Lichtenberger BM, Hoste E, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi:10.1038/nature12783
10. Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nature Medicine. 2012;18(8):1262–1270. doi:10.1038/nm.2848
11. Rinkevich Y, Walmsley GG, Hu MS, et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science (New York, NY). 2015;348(
12. Shook BA, Wasko RR, Rivera-Gonzalez GC, et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science. 2018;362(6417)doi:10.1126/science.aar2971
13. Ohlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214(3):579–596. doi:10.1084/jem.20162024
14. Elyada E, Bolisetty M, Laise P, et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019;9(8):1102–1123. doi:10.1158/2159-8290.CD-19-0094
15. Li Y, Wang J, Asahina K Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proceed Nat Acad Sci Unit Stat Am. 2013;110(6):2324–2329. doi:10.1073/pnas.1214136110
16. Bartoschek M, Oskolkov N, Bocci M, et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nature Communic. 2018;9(1):5150. doi:10.1038/s41467-018-07582-3
17. Costa A, Kieffer Y, Scholer-Dahirel A, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33(3):463–479.e10. doi:10.1016/j.ccell.2018.01.011
18. Peng J, Sun BF, Chen CY, et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 2019;29(9):725–738. doi:10.1038/s41422-019-0195-y
19. Bernard V, Semaan A, Huang J, et al. Single-cell transcriptomics of pancreatic cancer precursors demonstrates epithelial and microenvironmental heterogeneity as an early event in neoplastic progression. Clin Can Res. 2019;25(7):2194–2205. doi:10.1158/1078-0432.Ccr-18-1955
20. Sun X, He X, Zhang Y, et al. Inflammatory cell-derived CXCL3 promotes pancreatic cancer metastasis through a novel myofibroblast-hijacked cancer escape mechanism. Gut. 2022;71(1):129–147. doi:10.1136/gutjnl-2020-322744
21. Garg B, Giri B, Modi S, et al. NFκB in pancreatic stellate cells reduces infiltration of tumors by cytotoxic T cells and killing of cancer cells, via up-regulation of CXCL12. Gastroenterology. 2018;155(3):880–891.e8. doi:10.1053/j.gastro.2018.05.051
22. Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10(8):554–567. doi:10.1038/nri2808
23. Flint TR, Janowitz T, Connell CM, et al. Tumor-Induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metabolism. 2016;24(5):672–684. doi:10.1016/j.cmet.2016.10.010
24. Biffi G, Oni TE, Spielman B, et al. IL1-Induced JAK/STAT signaling is antagonized by TGFβ to Shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discovery. 2019;9(2):282–301. doi:10.1158/2159-8290.Cd-18-0710
25. Bailey JM, Swanson BJ, Hamada T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Can Res. 2008;14(19):5995–6004. doi:10.1158/1078-0432.Ccr-08-0291
26. Liu H, Shi Y, Qian F Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts. Advan Dru Deliv Rev. 2021;172:37–51. doi:10.1016/j.addr.2021.02.012
27. Apte MV, Wilson JS, Lugea A, Pandol SJ A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144(6):1210–1219. doi:10.1053/j.gastro.2012.11.037
28. Shi Y, Gao W, Lytle NK, et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature. 2019;569(7754):131–135. doi:10.1038/s41586-019-1130-6
29. Sleightholm RL, Neilsen BK, Li J, et al. Emerging roles of the CXCL12/CXCR4 axis in pancreatic cancer progression and therapy. Pharmacol Therap. 2017;179:158–170. doi:10.1016/j.pharmthera.2017.05.012
30. Fearon DT The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res. 2014;2(3):187–193. doi:10.1158/2326-6066.CIR-14-0002
31. Looi CK, Chung FF, Leong CO, Wong SF, Rosli R, Mai CW Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J Exp Clin Cancer Res. 2019;38(1):162. doi:10.1186/s13046-019-1153-8
32. Miyai Y, Esaki N, Takahashi M, Enomoto A Cancer‐associated fibroblasts that restrain cancer progression: hypotheses and perspectives. Cancer Science. 2020;111(4):1047–1057. doi:10.1111/cas.14346
33. Djurec M, Graña O, Lee A, et al. Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors. Proceed Nat Acad Sci. 2018;115(6):E1147–E1156. doi:10.1073/pnas.1717802115
34. Bhowmick NA, Chytil A, Plieth D, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303(5659):848–51. doi:10.1126/science.1090922
35. Shimura T, Sasatani M, Kawai H, et al. Radiation-induced myofibroblasts promote tumor growth via mitochondrial ROS-Activated TGFβ Signaling. Molec Can Res. 2018;16(11):1676–1686. doi:10.1158/1541-7786.Mcr-18-0321
36. Baulida J Epithelial-to-mesenchymal transition transcription factors in cancer-associated fibroblasts. Molec Oncol. 2017;11(7):847–859. doi:10.1002/1878-0261.12080
37. Eck SM, Côté AL, Winkelman WD, Brinckerhoff CE CXCR4 and matrix metalloproteinase-1 are elevated in breast carcinoma-associated fibroblasts and in normal mammary fibroblasts exposed to factors secreted by breast cancer cells. Molec Can Res. 2009;7(7):1033–1044. doi:10.1158/1541-7786.Mcr-09-0015
38. Zhu X, Wang K, Zhang K, et al. Galectin-1 knockdown in carcinoma-associated fibroblasts inhibits migration and invasion of human MDA-MB-231 breast cancer cells by modulating MMP-9 expression. Acta Biochim Et Biophy Sin. 2016;48(5):462–467. doi:10.1093/abbs/gmw019
39. Molina-Infante J, Romano M, Fernandez-Bermejo M, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 2013;145(1):121–128.e1. doi:10.1053/j.gastro.2013.03.050
40. Lee HO, Mullins SR, Franco-Barraza J, Valianou M, Cukierman E, Cheng JD FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer. 2011;11:245. doi:10.1186/1471-2407-11-245
41. Kawase T, Yasui Y, Nishina S, et al. Fibroblast activation protein-α-expressing fibroblasts promote the progression of pancreatic ductal adenocarcinoma. BMC Gastroenterol. 2015;15:109. doi:10.1186/s12876-015-0340-0
42. McAndrews KM, Chen Y, Darpolor JK, et al. Identification of functional heterogeneity of carcinoma-associated fibroblasts with distinct IL6-mediated therapy resistance in pancreatic cancer. Cancer Discovery. 2022;12(6):1580–1597. doi:10.1158/2159-8290.Cd-20-1484
43. Knudsen ES, Balaji U, Freinkman E, McCue P, Witkiewicz AK Unique metabolic features of pancreatic cancer stroma: relevance to the tumor compartment, prognosis, and invasive potential. Oncotarget. 2016;7(48):78396–78411. doi:10.18632/oncotarget.11893
44. Zhao H, Yang L, Baddour J, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife. 2016;5:e10250. doi:10.7554/eLife.10250
45. Auciello FR, Bulusu V, Oon C, et al. A stromal lysolipid-autotaxin signaling axis promotes pancreatic tumor progression. Cancer Discovery. 2019;9(5):617–627. doi:10.1158/2159-8290.Cd-18-1212
46. Morrison AH, Byrne KT, Vonderheide RH Immunotherapy and prevention of pancreatic cancer. Trends in Cancer. 2018;4(6):418–428. doi:10.1016/j.trecan.2018.04.001
47. Gupta VK, Sharma NS, Durden B, et al. Hypoxia-driven oncometabolite L-2HG maintains stemness-differentiation balance and facilitates immune evasion in pancreatic cancer. Cancer Research. 2021;81(15):4001–4013. doi:10.1158/0008-5472.Can-20-2562
48. Kim EJ, Sahai V, Abel EV, et al. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Can Res. 2014;20(23):5937–5945. doi:10.1158/1078-0432.Ccr-14-1269
49. Lee JJ, Perera RM, Wang H, et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proceed Nat Acad Sci United States Am. 2014;111(30):E3091–100. doi:10.1073/pnas.1411679111
50. Fitzgerald AA, Weiner LM The role of fibroblast activation protein in health and malignancy. Can Metast Rev. 2020;39(3):783–803. doi:10.1007/s10555-020-09909-3
51. Mizutani Y, Kobayashi H, Iida T, et al. Meflin-positive cancer-associated fibroblasts inhibit pancreatic carcinogenesis. Cancer Res. 2019;79(20):5367–5381. doi:10.1158/0008-5472.Can-19-0454
52. Hu G, Wang S, Xu F, et al. Tumor-infiltrating podoplanin+ fibroblasts predict worse outcome in solid tumors. Cellular Physiol Biochem. 2018;51(3):1041–1050. doi:10.1159/000495484
53. DuFort CC, DelGiorno KE, Carlson MA, et al. Interstitial pressure in pancreatic ductal adenocarcinoma is dominated by a gel-fluid phase. Bioph J. 2016;110(9):2106–2119. doi:10.1016/j.bpj.2016.03.040
54. Cheng XB, Sato N, Kohi S, Yamaguchi K Prognostic impact of hyaluronan and its regulators in pancreatic ductal adenocarcinoma. PLoS One. 2013;8(11):e80765. doi:10.1371/journal.pone.0080765
55. Amrutkar M, Aasrum M, Verbeke CS, Gladhaug IP Secretion of fibronectin by human pancreatic stellate cells promotes chemoresistance to gemcitabine in pancreatic cancer cells. BMC Can. 2019;19(1):596. doi:10.1186/s12885-019-5803-1
56. Ireland L, Santos A, Ahmed MS, et al. Chemoresistance in pancreatic cancer is driven by stroma-derived insulin-like growth factors. Can Res. 2016;76(23):6851–6863. doi:10.1158/0008-5472.Can-16-1201
57. Zhang H, Wu H, Guan J, et al. Paracrine SDF-1α signaling mediates the effects of PSCs on GEM chemoresistance through an IL-6 autocrine loop in pancreatic cancer cells. Oncotarget. 2015;6(5):3085–3097. doi:10.18632/oncotarget.3099
58. Singh S, Srivastava SK, Bhardwaj A, Owen LB, Singh AP CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br J Can. 2010;103(11):1671–1679. doi:10.1038/sj.bjc.6605968
59. Vennin C, Mélénec P, Rouet R, et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat Communic. 2019;10(1):3637. doi:10.1038/s41467-019-10968-6
60. Huanwen W, Zhiyong L, Xiaohua S, Xinyu R, Kai W, Tonghua L Intrinsic chemoresistance to gemcitabine is associated with constitutive and laminin-induced phosphorylation of FAK in pancreatic cancer cell lines. Molec Can. 2009;8:125. doi:10.1186/1476-4598-8-125
61. Richards KE, Zeleniak AE, Fishel ML, Wu J, Littlepage LE, Hill R Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36(13):1770–1778. doi:10.1038/onc.2016.353
62. Toste PA, Nguyen AH, Kadera BE, et al. Chemotherapy-induced inflammatory gene signature and protumorigenic phenotype in pancreatic CAFs via stress-associated MAPK. Molec Can Res. 2016;14(5):437–447. doi:10.1158/1541-7786.Mcr-15-0348
63. Fang Y, Zhou W, Rong Y, et al. Exosomal miRNA-106b from cancer-associated fibroblast promotes gemcitabine resistance in pancreatic cancer. Experim Cell Res. 2019;383(1):111543. doi:10.1016/j.yexcr.2019.111543
64. Takikawa T, Masamune A, Yoshida N, Hamada S, Kogure T, Shimosegawa T Exosomes derived from pancreatic stellate cells: microRNA signature and effects on pancreatic cancer cells. Pancreas. 2017;46(1):19–27. doi:10.1097/mpa.0000000000000722
65. van Jaarsveld MT, Helleman J, Berns EM, Wiemer EA MicroRNAs in ovarian cancer biology and therapy resistance. Internat J Biochem Cell Biol. 2010;42(8):1282–1290. doi:10.1016/j.biocel.2010.01.014
66. Ohuchida K, Mizumoto K, Murakami M, et al. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Can Res. 2004;64(9):3215–3222. doi:10.1158/0008-5472.can-03-2464
67. Li D, Qu C, Ning Z, et al. Radiation promotes epithelial-to-mesenchymal transition and invasion of pancreatic cancer cell by activating carcinoma-associated fibroblasts. Am J Can Res. 2016;6(10):2192–2206.
68. Zhang H, Xie C, Yue J, et al. Cancer-associated fibroblasts mediated chemoresistance by a FOXO1/TGFβ1 signaling loop in esophageal squamous cell carcinoma. Molec Carcinog 2017;56(3):1150–1163. doi:10.1002/mc.22581
69. Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Can Res. 2011;71(10):3453–3458. doi:10.1158/0008-5472.Can-10-1633
70. Horsman MR, Overgaard J The impact of hypoxia and its modification of the outcome of radiotherapy. J Rad Res. 2016;57(Suppl 1):i90–i98. doi:10.1093/jrr/rrw007
71. Wang Y, Gan G, Wang B, et al. Cancer-associated fibroblasts promote irradiated cancer cell recovery through autophagy. EBioMed. 2017;17:45–56. doi:10.1016/j.ebiom.2017.02.019
72. Harper J, Sainson RC Regulation of the anti-tumour immune response by cancer-associated fibroblasts. Sem Can Biol 2014;25:69–77. doi:10.1016/j.semcancer.2013.12.005
73. Liu T, Han C, Wang S, et al. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019;12(1):86. doi:10.1186/s13045-019-0770-1
74. Kim R, Emi M, Tanabe K Cancer immunosuppression and autoimmune disease: beyond immunosuppressive networks for tumour immunity. Immunology. 2006;119(2):254–264. doi:10.1111/j.1365-2567.2006.02430.x
75. Ziani L, Chouaib S, Thiery J Alteration of the antitumor immune response by cancer-associated fibroblasts. Frontiers in immunology. 2018;9:414. doi:10.3389/fimmu.2018.00414
76. Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers. 2015;7(4):2443–2458. doi:10.3390/cancers7040902
77. Sun Q, Zhang B, Hu Q, et al. The impact of cancer-associated fibroblasts on major hallmarks of pancreatic cancer. Theranostics. 2018;8(18):5072–5087. doi:10.7150/thno.26546
78. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M The chemokine system in diverse forms of macrophage activation and polarization. Tren Immunol. 2004;25(12):677–686. doi:10.1016/j.it.2004.09.015
79. Yugawa K, Itoh S, Yoshizumi T, et al. CMTM6 Stabilizes PD-L1 expression and is a new prognostic impact factor in hepatocellular carcinoma. Hepatol Communic. 2021;5(2):334–348. doi:10.1002/hep4.1643
80. Hu B, Wang Z, Zeng H, et al. Blockade of DC-SIGN(+) tumor-associated macrophages reactivates antitumor immunity and improves immunotherapy in muscle-invasive bladder cancer. Can Res. 2020;80(8):1707–1719. doi:10.1158/0008-5472.Can-19-2254
81. Herrera M, Herrera A, Domínguez G, et al. Cancer-associated fibroblast and M2 macrophage markers together predict outcome in colorectal cancer patients. Can Sci 2013;104(4):437–444. doi:10.1111/cas.12096
82. Coffelt SB, Wellenstein MD, de Visser KE Neutrophils in cancer: neutral no more. Nat Rev Can. 2016;16(7):431–446. doi:10.1038/nrc.2016.52
83. Wu L, Saxena S, Awaji M, Singh RK Tumor-associated neutrophils in cancer: going pro. Cancers. 2019;11:4. doi:10.3390/cancers11040564
84. Song M, He J, Pan QZ, et al. Cancer-associated fibroblast-mediated cellular crosstalk supports hepatocellular carcinoma progression. Hepatology. 2021;73(5):1717–1735. doi:10.1002/hep.31792
85. Cheng Y, Li H, Deng Y, et al. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Dea Dis. 2018;9(4):422. doi:10.1038/s41419-018-0458-4
86. Dalton DK, Noelle RJ The roles of mast cells in anticancer immunity. Can Immunol. 2012;61(9):1511–1520. doi:10.1007/s00262-012-1246-0
87. Liu J, Zhang Y, Zhao J, et al. Mast cell: insight into remodeling a tumor microenvironment. Can Metast Rev. 2011;30(2):177–184. doi:10.1007/s10555-011-9276-1
88. Yang FC, Chen S, Clegg T, et al. Nf1± mast cells induce neurofibroma like phenotypes through secreted TGF-beta signaling. Human Molec Genet. 2006;15(16):2421–2437. doi:10.1093/hmg/ddl165
89. Chiossone L, Dumas PY, Vienne M, Vivier E Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18(11):671–688. doi:10.1038/s41577-018-0061-z
90. Turley SJ, Cremasco V, Astarita JL Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15(11):669–682. doi:10.1038/nri3902
91. Chen X, Song E Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18(2):99–115. doi:10.1038/s41573-018-0004-1
92. Rahma OE, Hodi FS The intersection between tumor angiogenesis and immune suppression. Clin Can Res. 2019;25(18):5449–5457. doi:10.1158/1078-0432.Ccr-18-1543
93. Kumar BV, Connors TJ, Farber DL Human T cell development, localization, and function throughout life. Immunity. 2018;48(2):202–213. doi:10.1016/j.immuni.2018.01.007
94. Tanaka A, Sakaguchi S Regulatory T cells in cancer immunotherapy. Cell Research. 2017;27(1):109–118. doi:10.1038/cr.2016.151
95. Kinoshita T, Ishii G, Hiraoka N, et al. Forkhead box P3 regulatory T cells coexisting with cancer associated fibroblasts are correlated with a poor outcome in lung adenocarcinoma. Can Sci 2013;104(4):409–415. doi:10.1111/cas.12099
96. Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4(11):e7965. doi:10.1371/journal.pone.0007965
97. Freeman P, Mielgo A Cancer-associated fibroblast mediated inhibition of CD8+ Cytotoxic T cell accumulation in tumours: mechanisms and therapeutic opportunities. Cancers. 2020;12(9)2687 doi:10.3390/cancers12092687
98. Thommen DS, Schumacher TN T cell dysfunction in cancer. Cancer Cell. 2018;33(4):547–562. doi:10.1016/j.ccell.2018.03.012
99. Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD Cancer-associated fibroblasts induce antigen-specific deletion of CD8 (+) T Cells to protect tumour cells. Nat Communic 2018;9(1):948. doi:10.1038/s41467-018-03347-0
100. Gorchs L, Fernández Moro C, Bankhead P, et al. Human pancreatic carcinoma-associated fibroblasts promote expression of co-inhibitory markers on CD4(+) and CD8(+) T-Cells. Front Immunol. 2019;10:847. doi:10.3389/fimmu.2019.00847
101. Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi:10.1016/j.cell.2009.10.027
102. Liu T, Zhou L, Li D, Andl T, Zhang Y Cancer-associated fibroblasts build and secure the tumor microenvironment. Front Cell Devel Biol. 2019;7:60. doi:10.3389/fcell.2019.00060
103. Murphy G, Nagase H Progress in matrix metalloproteinase research. Molec Asp Med 2008;29(5):290–308. doi:10.1016/j.mam.2008.05.002
104. Truffi M, Sorrentino L, Corsi F Fibroblasts in the tumor microenvironment. Adv Experim MedBiol. 2020;1234:15–29. doi:10.1007/978-3-030-37184-5_2
105. Sorokin L The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10(10):712–723. doi:10.1038/nri2852
106. Varol C Tumorigenic interplay between macrophages and collagenous matrix in the tumor microenvironment. Meth Molec Biol. 2019;1944:203–220. doi:10.1007/978-1-4939-9095-5_15
107. Tran E, Chinnasamy D, Yu Z, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med. 2013;210(6):1125–1135. doi:10.1084/jem.20130110
108. Truffi M, Mazzucchelli S, Bonizzi A, et al. Nano-strategies to target breast cancer-associated fibroblasts: rearranging the tumor microenvironment to achieve antitumor efficacy. Int J Mol Sci. 2019;20(6)doi:10.3390/ijms20061263 1263
109. Yang Z, Zhang L, Zhu H, et al. Nanoparticle formulation of mycophenolate mofetil achieves enhanced efficacy against hepatocellular carcinoma by targeting tumour-associated fibroblast. J Cell Mol Med 2021;25(7):3511–3523. doi:10.1111/jcmm.16434
110. Long KB, Tooker G, Tooker E, et al. IL6 receptor blockade enhances chemotherapy efficacy in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2017;16(9):1898–1908. doi:10.1158/1535-7163.MCT-16-0899
111. Steele NG, Biffi G, Kemp SB, et al. Inhibition of hedgehog signaling alters fibroblast composition in pancreatic cancer. Clin Cancer Res. 2021;27(7):2023–2037. doi:10.1158/1078-0432.CCR-20-3715
112. Brunetto E, De Monte L, Balzano G, et al. The IL-1/IL-1 receptor axis and tumor cell released inflammasome adaptor ASC are key regulators of TSLP secretion by cancer associated fibroblasts in pancreatic cancer. J Immun Can. 2019;7(1):45. doi:10.1186/s40425-019-0521-4
113. Kojima Y, Acar A, Eaton EN, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107(46):20009–20014. doi:10.1073/pnas.1013805107
114. Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110(50):20212–20217. doi:10.1073/pnas.1320318110
115. Hingorani SR, Zheng L, Bullock AJ, et al. HALO 202: randomized Phase II Study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2018;36(4):359–366. doi:10.1200/JCO.2017.74.9564
116. Mucciolo G, Araos Henriquez J, Jihad M, et al. EGFR-activated myofibroblasts promote metastasis of pancreatic cancer. Cancer Cell. 2024;42(1):101–118 e11. doi:10.1016/j.ccell.2023.12.002
117. Aggarwal S, Brennen WN, Kole TP, et al. Fibroblast activation protein peptide substrates identified from human collagen I derived gelatin cleavage sites. Biochemistry. 2008;47(3):1076–1086. doi:10.1021/bi701921b
118. Yu Q, Qiu Y, Li J, et al. Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy. J Cont Rel. 2020;321:564–575. doi:10.1016/j.jconrel.2020.02.040
119. Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi:10.1126/science.1171362
120. Wei L, Ye H, Li G, et al. Correction: cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer. Cell Death Dis. 2021;12(3):232. doi:10.1038/s41419-021-03420-5
121. Chen W, Ayala-Orozco C, Biswal NC, et al. Targeting pancreatic cancer with magneto-fluorescent theranostic gold nanoshells. Nanomedicine. 2014;9(8):1209–1222. doi:10.2217/nnm.13.84
122. David KI, Ravikumar TS, Sethuraman S, Krishnan UM Development and evaluation of a multi-functional organic-inorganic nanotheranostic hybrid for pancreatic cancer therapy. Biom Mat. 2021;16(5)055016 doi:10.1088/1748-605X/ac177c
123. Carofiglio M, Conte M, Racca L, Cauda V Synergistic phenomena between iron-doped ZnO nanoparticles and shock waves exploited against pancreatic cancer cells. ACS Appl Nano Mater. 2022;5(11):17212–17225. doi:10.1021/acsanm.2c04211
124. Khalifehzadeh R, Arami H Biodegradable calcium phosphate nanoparticles for cancer therapy. Adv Colloid Interface Sci. 2020;279:102157. doi:10.1016/j.cis.2020.102157
125. Gao F, Wu J, Niu S, et al. Biodegradable, pH-sensitive hollow mesoporous organosilica nanoparticle (HMON) with controlled release of pirfenidone and ultrasound-target-microbubble-destruction (UTMD) for pancreatic cancer treatment. Theranostics. 2019;9(20):6002–6018. doi:10.7150/thno.36135
126. Vallet-Regi M, Colilla M, Izquierdo-Barba I, Manzano M Mesoporous silica nanoparticles for drug delivery: current insights. Molecules. 2017;23(1)47 doi:10.3390/molecules23010047
127. Alsaiari SK, Qutub SS, Sun S, et al. Sustained and targeted delivery of checkpoint inhibitors by metal-organic frameworks for cancer immunotherapy. Sci Adv. 2021;7(4)doi:10.1126/sciadv.abe7174
128. Xu Y, Hu B, Xu J, Wu J, Ye B Preparation of biodegradable polymeric nanocapsules for treatment of malignant tumor using coaxial capillary microfluidic device. Can Bio Radioph. 2020;35(8):570–580. doi:10.1089/cbr.2019.3412
129. Zhao W, Yang S, Li C, et al. Amphiphilic dendritic nanomicelle-mediated delivery of gemcitabine for enhancing the specificity and effectiveness. Int J Nanomedicine. 2022;17:3239–3249. doi:10.2147/IJN.S371775
130. Kim CE, Lim SK, Kim JS In vivo antitumor effect of cromolyn in PEGylated liposomes for pancreatic cancer. J Control Release. 2012;157(2):190–195. doi:10.1016/j.jconrel.2011.09.066
131. Tang Z, Niu Y, Xu Z, et al. Anti-tumor and anti-metastasis effects of berbamine-loaded lipid nanoparticles on pancreatic cancer. Anticancer Agents Med Chem. 2022;22(18):3097–3106. doi:10.2174/1871520622666220501161636
132. Moloney C, Roy chaudhuri T, Spernyak JA, Straubinger RM, Brougham DF Long-circulating magnetoliposomes as surrogates for assessing pancreatic tumour permeability and nanoparticle deposition. Acta Biomater. 2023;158:611–624. doi:10.1016/j.actbio.2022.12.057
133. Lu J, Liu X, Liao YP, et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat Commun. 2017;8(1):1811. doi:10.1038/s41467-017-01651-9
134. Lu L, Jie L, Zhou Y, et al. Preparation and characterization of PLGA-based magnetic polymer nanoparticles for targeting pancreatic adenocarcinoma. Curr Pharm Des. 2023;29(9):686–696. doi:10.2174/1381612829666230324091555
135. Tomitaka A, Takemura Y, Huang Z, Roy U, Nair M Magnetoliposomes in controlled-release drug delivery systems. Crit Rev Biomed Engin. 2019;47(6):495–505. doi:10.1615/CritRevBiomedEng.2020033002
136. Rommasi F, Esfandiari N. Liposomal nanomedicine: applications for drug delivery in cancer therapy. Nanos Res Lett. 2021;16(1):95. doi:10.1186/s11671-021-03553-8
137. Beltrán-Gracia E, López-Camacho A, Higuera-Ciapara I, Velázquez-Fernández JB, Vallejo-Cardona AA Nanomedicine review: clinical developments in liposomal applications. Can Nanotech. 2019;10(1) doi:10.1186/s12645-019-0055-y
138. Suk JS, Xu Q, Kim N, Hanes J, Ensign LM PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Advan Drug Deliv Rev. 2016;99(Pt A):28–51. doi:10.1016/j.addr.2015.09.012
139. Ndemazie NB, Bulusu R, Zhu XY, et al. Evaluation of anticancer activity of Zhubech, a New 5-FU analog liposomal formulation, against pancreatic cancer. Internat J Molec Sci. 2023;24(5)4288 doi:10.3390/ijms24054288
140. Shang L, Xie Q, Yang C, Kong L, Zhang Z Extracellular vesicles facilitate the transportation of nanoparticles within and between cells for enhanced tumor therapy. ACS Appl Mater Interf. 2023;15(36):42378–42394. doi:10.1021/acsami.3c10237
141. Shimada S, Yamaguchi K, Takahashi M, Ogawa M Pancreatic elastase IIIA and its variants are expressed in pancreatic carcinoma cells. Internat J Molec Med. 2002;10(5):599–603.
142. Wang XQ, Zhang Q pH-sensitive polymeric nanoparticles to improve oral bioavailability of peptide/protein drugs and poorly water-soluble drugs. Europ J Pharma Biopharm. 2012;82(2):219–229. doi:10.1016/j.ejpb.2012.07.014
143. Du J, Lane LA, Nie S Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J Cont Rel. 2015;219:205–214. doi:10.1016/j.jconrel.2015.08.050
144. Tagami T, Foltz WD, Ernsting MJ, et al. MRI monitoring of intratumoral drug delivery and prediction of the therapeutic effect with a multifunctional thermosensitive liposome. Biomaterials. 2011;32(27):6570–6578. doi:10.1016/j.biomaterials.2011.05.029
145. Pereira BA, Vennin C, Papanicolaou M, et al. CAF subpopulations: a new reservoir of stromal targets in pancreatic cancer. Trend Can. 2019;5(11):724–741. doi:10.1016/j.trecan.2019.09.010
146. Linares J, Marín-Jiménez JA, Badia-Ramentol J, Calon A Determinants and functions of cafs secretome during cancer progression and therapy. Front Cell Develop Biol. 2020;8:621070. doi:10.3389/fcell.2020.621070
147. Wang M, Li Y, Wang M, et al. Synergistic interventional photothermal therapy and immunotherapy using an iron oxide nanoplatform for the treatment of pancreatic cancer. Acta Biomater. 2022;138:453–462. doi:10.1016/j.actbio.2021.10.048
148. Lakiotaki E, Sakellariou S, Evangelou K, Liapis G, Patsouris E, Delladetsima I Vascular and ductal elastotic changes in pancreatic cancer. APMIS. 2016;124(3):181–187. doi:10.1111/apm.12482
149. Chen Q, Liu G, Liu S, et al. Remodeling the tumor microenvironment with emerging nanotherapeutics. Trend Pharmacol Sci. 2018;39(1):59–74. doi:10.1016/j.tips.2017.10.009
150. Feng J, Xu M, Wang J, et al. Sequential delivery of nanoformulated α-mangostin and triptolide overcomes permeation obstacles and improves therapeutic effects in pancreatic cancer. Biomaterials. 2020;241:119907. doi:10.1016/j.biomaterials.2020.119907
151. Kunz-Schughart LA, Knuechel R Tumor-associated fibroblasts (part II): functional impact on tumor tissue. Histol Histopathol 2002;17(2):623–637. doi:10.14670/hh-17.623
152. Alili L, Sack M, Karakoti AS, et al. Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor-stroma interactions. Biomaterials. 2011;32(11):2918–2929. doi:10.1016/j.biomaterials.2010.12.056
153. Mardhian DF, Storm G, Bansal R, Prakash J Nano-targeted relaxin impairs fibrosis and tumor growth in pancreatic cancer and improves the efficacy of gemcitabine in vivo. Journal of Controlled Release. 2018;290:1–10. doi:10.1016/j.jconrel.2018.09.031
154. Hu C, Liu X, Ran W, et al. Regulating cancer associated fibroblasts with losartan-loaded injectable peptide hydrogel to potentiate chemotherapy in inhibiting growth and lung metastasis of triple negative breast cancer. Biomaterials. 2017;144:60–72. doi:10.1016/j.biomaterials.2017.08.009
155. Chen X, Zhou W, Liang C, et al. Codelivery nanosystem targeting the deep microenvironment of pancreatic cancer. Nano Letters. 2019;19(6):3527–3534. doi:10.1021/acs.nanolett.9b00374
156. Kim DJ, Dunleavey JM, Xiao L, et al. Suppression of TGFβ-mediated conversion of endothelial cells and fibroblasts into cancer associated (myo)fibroblasts via HDAC inhibition. British Journal of Cancer. 2018;118(10):1359–1368. doi:10.1038/s41416-018-0072-3
157. Albrengues J, Bertero T, Grasset E, et al. Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nature Communications. 2015;6:10204. doi:10.1038/ncomms10204
158. Eckert MA, Coscia F, Chryplewicz A, et al. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature. 2019;569(7758):723–728. doi:10.1038/s41586-019-1173-8
159. Gabasa M, Ikemori R, Hilberg F, Reguart N, Alcaraz J Nintedanib selectively inhibits the activation and tumour-promoting effects of fibroblasts from lung adenocarcinoma patients. Br J Can. 2017;117(8):1128–1138. doi:10.1038/bjc.2017.270
160. Borriello L, Nakata R, Sheard MA, et al. Cancer-associated fibroblasts share characteristics and protumorigenic activity with mesenchymal stromal cells. Can Res. 2017;77(18):5142–5157. doi:10.1158/0008-5472.Can-16-2586
161. Ford K, Hanley CJ, Mellone M, et al. NOX4 inhibition potentiates immunotherapy by overcoming cancer-associated fibroblast-mediated CD8 T-cell exclusion from tumors. Can Res. 2020;80(9):1846–1860. doi:10.1158/0008-5472.Can-19-3158
162. Miao L, Liu Q, Lin CM, et al. Targeting tumor-associated fibroblasts for therapeutic delivery in desmoplastic tumors. Can Res. 2017;77(3):719–731. doi:10.1158/0008-5472.Can-16-0866
163. Han X, Li Y, Xu Y, et al. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat Communic. 2018;9(1):3390. doi:10.1038/s41467-018-05906-x
164. Duan H, Li L, He S Advances and Prospects in the Treatment of Pancreatic Cancer. Internat J Nanomed. 2023;18:3973–3988. doi:10.2147/ijn.S413496
165. Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15(6):333–348. doi:10.1038/s41575-018-0005-x
166. Muller M, Haghnejad V, Schaefer M, et al. The immune landscape of human pancreatic ductal carcinoma: key players, clinical implications, and challenges. Cancers. 2022;14:4.
167. Siegel RL, Miller KD, Fuchs HE, Jemal A Cancer statistics, 2022. CA. 2022;72(1):7–33. doi:10.3322/caac.21708
168. Raj S, Khurana S, Choudhari R, et al. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. Semin Can Biol. 2021;69:166–177. doi:10.1016/j.semcancer.2019.11.002
169. Ernsting MJ, Hoang B, Lohse I, et al. Targeting of metastasis-promoting tumor-associated fibroblasts and modulation of pancreatic tumor-associated stroma with a carboxymethylcellulose-docetaxel nanoparticle. J Control Release. 2015;206:122–130. doi:10.1016/j.jconrel.2015.03.023
170. Wang L, Liu X, Zhou Q, et al. Terminating the criminal collaboration in pancreatic cancer: nanoparticle-based synergistic therapy for overcoming fibroblast-induced drug resistance. Biomaterials. 2017;144:105–118. doi:10.1016/j.biomaterials.2017.08.002
171. Zhao J, Wang H, Hsiao CH, et al. Simultaneous inhibition of hedgehog signaling and tumor proliferation remodels stroma and enhances pancreatic cancer therapy. Biomaterials. 2018;159:215–228. doi:10.1016/j.biomaterials.2018.01.014
172. Cun X, Chen J, Li M, et al. Tumor-associated fibroblast-targeted regulation and deep tumor delivery of chemotherapeutic drugs with a multifunctional size-switchable nanoparticle. ACS Appl Mater Interf. 2019;11(43):39545–39559. doi:10.1021/acsami.9b13957
173. Schnittert J, Kuninty PR, Bystry TF, Brock R, Storm G, Prakash J Anti-microRNA targeting using peptide-based nanocomplexes to inhibit differentiation of human pancreatic stellate cells. Nanomedicine. 2017;12(12):1369–1384. doi:10.2217/nnm-2017-0054
174. Shi Y, Luo Z, You J Subcellular delivery of lipid nanoparticles to endoplasmic reticulum and mitochondria. Wiley Interdiscip Rev Nanom Nanob. 2022;14(5):e1803. doi:10.1002/wnan.1803
175. Qiao L, Yan S, Dou X, et al. Biogenic selenium nanoparticles alleviate intestinal epithelial barrier damage through regulating endoplasmic reticulum stress-mediated mitophagy. Oxid Med Cell Longev. 2022;2022:3982613. doi:10.1155/2022/3982613
176. Wang H, Mu X, He H, Zhang XD. Cancer Radiosensitizers. Trends Pharmacol Sci. 2018;39(1):24–48. doi:10.1016/j.tips.2017.11.003
177. Chi A, Chen H, Wen S, Yan H, Liao Z Comparison of particle beam therapy and stereotactic body radiotherapy for early stage non-small cell lung cancer: a systematic review and hypothesis-generating meta-analysis. Radiother Oncol. 2017;123(3):346–354. doi:10.1016/j.radonc.2017.05.007
178. Chen Q, Chen J, Yang Z, et al. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy. Adv Mater. 2019;31(10):e1802228. doi:10.1002/adma.201802228
179. Jin J, Zhao Q Engineering nanoparticles to reprogram radiotherapy and immunotherapy: recent advances and future challenges. J Nanobiotechnology. 2020;18(1):75. doi:10.1186/s12951-020-00629-y
180. Gao J, Wang Z, Guo Q, et al. Mitochondrion-targeted supramolecular ”nano-boat” simultaneously inhibiting dual energy metabolism for tumor selective and synergistic chemo-radiotherapy. Theranostics. 2022;12(3):1286–1302. doi:10.7150/thno.67543
181. Inamdar S, Pushpavanam K, Lentz JM, Bues M, Anand A, Rege K Hydrogel nanosensors for colorimetric detection and dosimetry in proton beam radiotherapy. ACS Appl Mater Interfaces. 2018;10(4):3274–3281. doi:10.1021/acsami.7b15127
182. Zhao Z, Zheng L, Chen W, Weng W, Song J, Ji J Delivery strategies of cancer immunotherapy: recent advances and future perspectives. J Hematol Oncol. 2019;12(1):126. doi:10.1186/s13045-019-0817-3
183. van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJM, Lammers T Smart cancer nanomedicine. Nat Nanotechnol. 2019;14(11):1007–1017. doi:10.1038/s41565-019-0567-y
184. Kitamura T, Qian BZ, Pollard JW Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15(2):73–86. doi:10.1038/nri3789
185. Raman D, Baugher PJ, Thu YM, Richmond A Role of chemokines in tumor growth. Cancer Lett. 2007;256(2):137–165. doi:10.1016/j.canlet.2007.05.013
186. Cheng K, Ding Y, Zhao Y, et al. Sequentially responsive therapeutic peptide assembling nanoparticles for dual-targeted cancer immunotherapy. Nano Lett. 2018;18(5):3250–3258. doi:10.1021/acs.nanolett.8b01071
187. Suto A, Kudo D, Yoshida E, et al. Increase of tumor infiltrating gammadelta T-cells in pancreatic ductal adenocarcinoma through remodeling of the extracellular matrix by a hyaluronan synthesis suppressor, 4-methylumbelliferone. Pancreas. 2019;48(2):292–298. doi:10.1097/MPA.0000000000001211
188. Zhang Y, Han X, Wang K, et al. Co-delivery nanomicelles for potentiating TNBC immunotherapy by synergetically reshaping CAFs-mediated tumor stroma and reprogramming immunosuppressive microenvironment. Int J Nanomedicine. 2023;18:4329–4346. doi:10.2147/IJN.S418100
189. Zhang H, Chen L, Zhao Y, et al. Relaxin-encapsulated polymeric metformin nanoparticles remodel tumor immune microenvironment by reducing CAFs for efficient triple-negative breast cancer immunotherapy. Asian J Pharm Sci. 2023;18(2):100796. doi:10.1016/j.ajps.2023.100796
190. Hwang WL, Pike LRG, Royce TJ, Mahal BA, Loeffler JS Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol. 2018;15(8):477–494. doi:10.1038/s41571-018-0046-7
191. Guan X, Sun L, Shen Y, et al. Nanoparticle-enhanced radiotherapy synergizes with PD-L1 blockade to limit post-surgical cancer recurrence and metastasis. Nat Commun. 2022;13(1):2834. doi:10.1038/s41467-022-30543-w
192. Li S, Kang Y, Zeng Y Targeting tumor and bone microenvironment: novel therapeutic opportunities for castration-resistant prostate cancer patients with bone metastasis. Biochim Biophys Acta Rev Cancer. 2024;1879(1):189033. doi:10.1016/j.bbcan.2023.189033
193. Au M, Emeto TI, Power J, Vangaveti VN, Lai HC Emerging therapeutic potential of nanoparticles in pancreatic cancer: a systematic review of clinical trials. Biomedicines. 2016;4(3)20 doi:10.3390/biomedicines4030020
194. Xu M, Li S Nano-drug delivery system targeting tumor microenvironment: a prospective strategy for melanoma treatment. Cancer Lett. 2023;574:216397. doi:10.1016/j.canlet.2023.216397
195. Galanis E, Carlson SK, Foster NR, et al. Phase I trial of a pathotropic retroviral vector expressing a cytocidal cyclin G1 construct (Rexin-G) in patients with advanced pancreatic cancer. Mol Ther. 2008;16(5):979–984. doi:10.1038/mt.2008.29
196. Guo X, Gao C, Yang DH, Li S Exosomal circular RNAs: a chief culprit in cancer chemotherapy resistance. Drug Resist Updat. 2023;67:100937. doi:10.1016/j.drup.2023.100937
197. Baigude H, Rana TM Delivery of therapeutic RNAi by nanovehicles. Chembiochem. 2009;10(15):2449–2454. doi:10.1002/cbic.200900252
198. Eriksson M, Taskinen M, Leppa S Mitogen activated protein kinase-dependent activation of c-Jun and c-Fos is required for neuronal differentiation but not for growth and stress response in PC12 cells. J Cell Physiol. 2007;210(2):538–548. doi:10.1002/jcp.20907
199. Rana TM Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol. 2007;8(1):23–36. doi:10.1038/nrm2085
200. Davis ME, Zuckerman JE, Choi CH, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi:10.1038/nature08956
201. Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG Lipid-based nanotherapeutics for siRNA delivery. J Intern Med. 2010;267(1):9–21. doi:10.1111/j.1365-2796.2009.02189.x
202. Zimmermann TS, Lee AC, Akinc A, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–4. doi:10.1038/nature04688
203. Jindal A, Sarkar S, Alam A Nanomaterials-mediated immunomodulation for cancer therapeutics. Front Chem. 2021;9:629635. doi:10.3389/fchem.2021.629635
204. Liu L, Kshirsagar PG, Gautam SK, et al. Nanocarriers for pancreatic cancer imaging, treatments, and immunotherapies. Theranostics. 2022;12(3):1030–1060. doi:10.7150/thno.64805
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.