Back to Journals » International Journal of Nanomedicine » Volume 19
Nano-Drug Delivery Systems Based on Natural Products
Authors Lv Y , Li W, Liao W, Jiang H, Liu Y, Cao J, Lu W, Feng Y
Received 8 October 2023
Accepted for publication 9 January 2024
Published 18 January 2024 Volume 2024:19 Pages 541—569
DOI https://doi.org/10.2147/IJN.S443692
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Ying Lv, Wenqing Li, Wei Liao, Haibo Jiang, Yuwei Liu, Jiansheng Cao, Wenfei Lu, Yufei Feng
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040, People’s Republic of China
Correspondence: Yufei Feng, 150040, Tel +86-18503653988, Email [email protected]
Abstract: Natural products have proven to have significant curative effects and are increasingly considered as potential candidates for clinical prevention, diagnosis, and treatment. Compared with synthetic drugs, natural products not only have diverse structures but also exhibit a range of biological activities against different disease states and molecular targets, making them attractive for development in the field of medicine. Despite advancements in the use of natural products for clinical purposes, there remain obstacles that hinder their full potential. These challenges include issues such as limited solubility and stability when administered orally, as well as short durations of effectiveness. To address these concerns, nano-drug delivery systems have emerged as a promising solution to overcome the barriers faced in the clinical application of natural products. These systems offer notable advantages, such as a large specific surface area, enhanced targeting capabilities, and the ability to achieve sustained and controlled release. Extensive in vitro and in vivo studies have provided further evidence supporting the efficacy and safety of nanoparticle-based systems in delivering natural products in preclinical disease models. This review describes the limitations of natural product applications and the current status of natural products combined with nanotechnology. The latest advances in nano-drug delivery systems for delivery of natural products are considered from three aspects: connecting targeting warheads, self-assembly, and co-delivery. Finally, the challenges faced in the clinical translation of nano-drugs are discussed.
Keywords: natural products, nanotechnology, drug delivery, targeting
Graphical Abstract:
Introduction
Over the course of human evolution, natural products have been a source of necessities for human life. They have been used in numerous fields, ranging from ancient applications in paper-making to modern use in the production of perfumes and spices, and recently for the precaution and treatment of diverse diseases.1,2 With the continued advances in separation techniques and methods for evaluating pharmacological effects, an increasing number of active components have been identified in natural products. Due to the structural diversity of natural products and their biological activities on multiple tissues and molecular targets, modulating various signaling or functional pathways, they have significant clinical applications, especially in the treatment of complex diseases such as cancer and cardiovascular disorders.3,4
The effectiveness of natural products in clinical trials, however, has not been particularly impressive, and most pharmaceutical companies are currently reluctant to pursue their development.5–7 This is mainly due to concerns regarding their poor solubility, limited biological distribution, or rapid metabolic clearance. These factors may result in drug plasma concentrations falling below therapeutic levels. Additionally, permeability and absorption across biological membranes and barriers can be challenging for compounds with relatively high molecular weight and low lipophilicity, resulting in poor drug transport efficiency and short half-lives. Therefore, finding new drug delivery methods may provide solutions to address these key issues.8,9 Currently, identifying the most effective means to maximize the efficacy of natural products is an important focus of research in the scientific community. These studies will lead to the emergence of more innovative drugs with fewer side effects.10
Nano-Carriers
Nanotechnology has proven to be instrumental in connecting the realms of biological and physical sciences. Specifically, nano-drug delivery systems have garnered considerable interest within the pharmaceutical industry.7,11,12 Carriers based on nanostructures can facilitate drug delivery by encapsulating or attaching therapeutic agents and precisely targeting tissues or organs through controlled release.9,13 Compared to larger materials, nano-drugs designed at the atomic or molecular level have the ability to move more unrestrictedly within the human body, thus enabling them to attain therapeutic effects.14–19 However, the scope of application of these methods has huge limitations compared to the delivery of drugs through nanostructures. Drug delivery systems based on nanoparticles (NPs) not only improve the mean residence times of drugs in the body, but also have unique advantages, such as rapid extravasation and reduced likelihood of macrophage phagocytosis. Nanostructures can also be utilized successfully to co-load hydrophilic or hydrophobic substances. It has been reported that first-generation drugs based on nano-drug delivery systems have been approved for use by relevant authorities.20 Liposomes and micelles containing inorganic NPs (such as gold NPs) have been developed, focusing on drug delivery, imaging, and therapeutic functions.21 Nano-formulations can incorporate natural products into nano-carriers using various methods, such as thin film hydration, high-pressure homogenization, nanoprecipitation, and self-assembly.22 These approaches represent significant advances in improving treatment outcomes by controlling drug release, enhancing drug solubility and permeability, improving drug targeting, reducing adverse reactions, and increasing physical and chemical stability. In summary, the application of nanotechnology offers substantial backing for the advancement of natural products and serves as a technological foundation for modernization (Figure 1).
 |
Figure 1 The combination of natural products and nanotechnology promotes the development of the pharmaceutical field. |
Nano-Drug Delivery Systems and Natural Products
The application of nanotechnology in natural products is a rapidly developing field. The use of nanocarriers to successfully deliver drugs is increasingly considered a potential way to safely transport therapeutic agents to targeted areas within organs, specific tissues, or cells.6 The combination of natural products and nanotechnology holds promising prospects, as nanotechnology provides the possibility to load one or multiple natural products. The presence of nano-drug delivery systems can enhance the bioavailability, targeting, and controlled release properties of natural products. This greatly improves the shortcomings in the clinical application of natural products and amplifies their pharmacological advantages, making significant contributions to clinical practice.
Design of the Review
This review covers strategies for developing natural product-based nano-drug delivery systems, which fall into the following three categories: connecting targeting warheads, self-assembly, and co-delivery. It specifically describes the characteristics of nano-systems applied to one or more natural product components, as well as their routes of administration. We summarize the in vitro and in vivo studies of natural product nano-drug delivery systems and highlight their potential advantages for improving biodistribution and efficacy and reducing adverse reactions and toxicity. Finally, the challenges faced in the clinical translation of nano-drugs are discussed. Table 1 provides a comprehensive summary of the natural products and their nano-drug delivery systems mentioned throughout the entire text, including the mechanisms of drug delivery or structural composition, treatment of diseases, and application dosages.
 |
Table 1 Natural Product Nano-Drug Delivery System Drug Delivery/Structure Composition Mechanism, Dosage |
Study Design
In January 2023, we performed a literature search in PubMed for natural products, research on nanomedicine delivery systems, and systematic reviews. The search terms we used were “natural products” and “nano-carriers”, “natural products” and “targeting payload” and “targeting”, “natural products” and “self-assembly” or “carrier-free”, “natural products” and “co-delivery” and “nano” (since 2018.01–2022.12). Due to the classic and typical nature of the experimental content, three original research articles have been specially cited (2012, 2015, 2016).
Criteria for inclusion: Original articles related to the subject matter, scientific research in relevant fields, and key reference literature.
Criteria for exclusion: Irrelevant literature, duplicate publications, conference proceedings, lectures, letters to the editor, non-English publications.
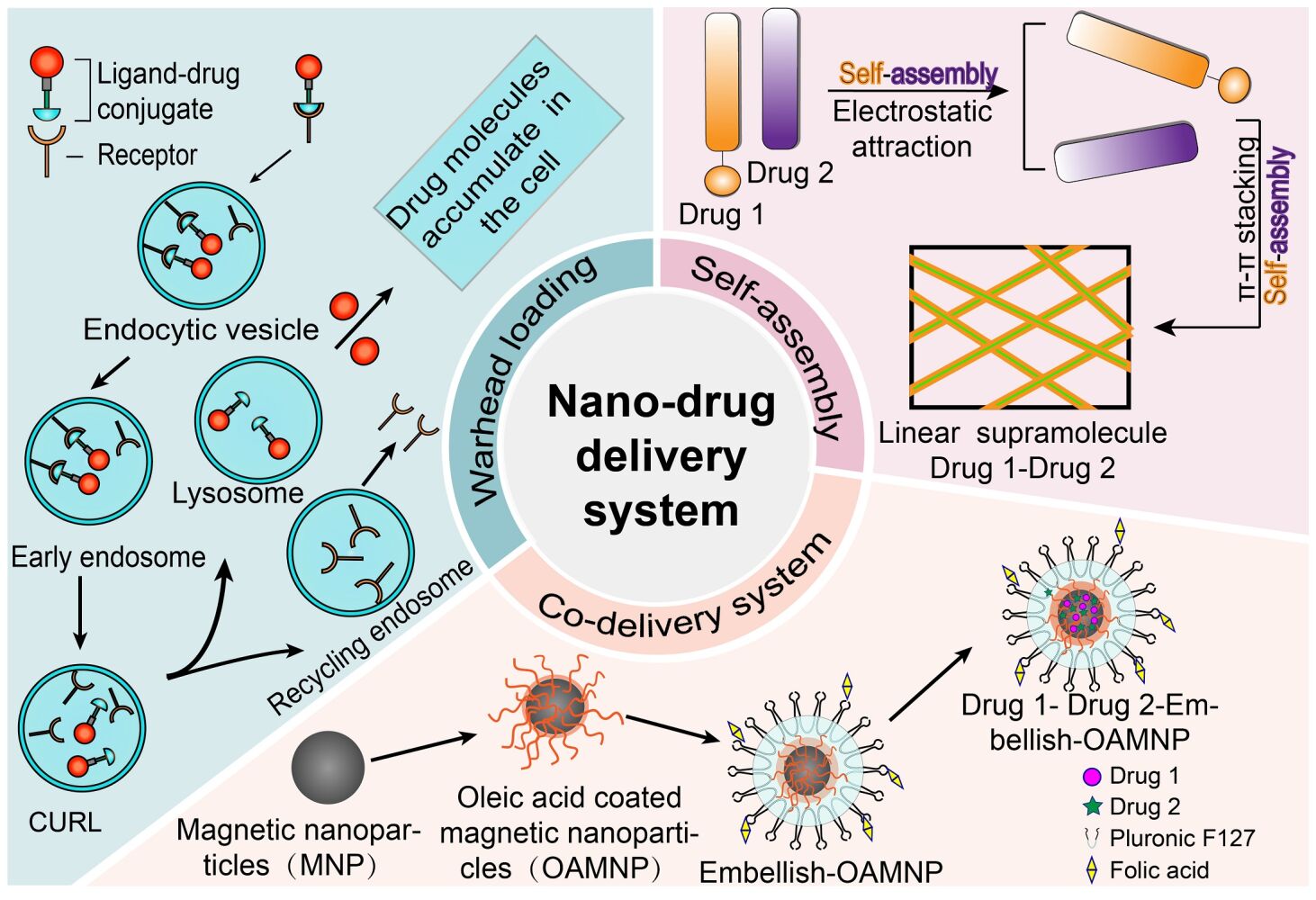
Natural Product-Based Nano-Drug Delivery Systems with Targeting Warheads
Mechanisms and Advantages
The world is entering a new era of personalized healthcare, where drugs are prepared based on patient needs and then targeted and delivered to non-healthy cells. This is currently a research hotspot.23 Nano-drug delivery systems, prepared through innovative nanotechnology, have received widespread attention for their capacity to enhance the bioavailability of drugs that are insoluble. To further optimize the nano-drug delivery system, researchers often attach targeting warheads to the nano-carriers through different strategies. These warheads can selectively deliver the drug to pathological cells through receptor-mediated endocytosis.24,25 The attachment of targeting warheads can be achieved using various reaction mechanisms, including nucleophilic addition, nucleophilic substitution, and addition elimination.26 Among them, nucleophilic addition and addition-elimination are the two most widely used mechanisms. These mechanisms make it possible for drugs to be selectively delivered; for example, aromatic electrophilic substitution occurs only with tyrosine and tryptophan.26
Drug delivery systems loaded with targeting warheads have many advantages over their non-targeted counterparts. Firstly, targeted systems can selectively deliver effective therapeutic drugs to particular organs or tissues, thereby avoiding non-specific uptake and toxicity in healthy cells.27 Secondly, when drugs administered in the non-targeted form at the MTD show no significant efficacy, efficacy may be improved by connecting them to highly effective therapeutic warheads.27 For example, nano-conjugates of polyethylene glycol or methoxy polyethylene glycol-modified with the targeting peptide T7 and covalently linked to podophyllotoxin (PPT) (Pep-SS-NPs) have been developed to target the transferrin receptor, resulting in a significant increase (5.3 times) in the MTD compared with free PPT.28 Last advantage is that the targeting warheads can be connected to diagnostic agents, such as imaging agents, which can be used to identify overexpression of target receptors in pathological cells.29 In the context of drug delivery using drug carriers containing warheads that specifically target folate receptors, successful development has taken place in the creation of folate-linked 99mTc and 68Ga radiopharmaceutical imaging agents. These agents serve the purpose of identifying whether tumor cells in patients exhibit an ample expression of folate receptors, which is necessary for the absorption of therapeutic drug doses.30–33
Currently, warheads that specifically bind to receptors are being synthesized and studied in both in vitro and in vivo experiments. Integrins (α2β3, ανβ3, and α5β1) and aminopeptidase N (CD13) have been identified as the most common targets in tumor neovascular systems. They can be recognized by derivatives of cyclic RGD peptide and linear NGR peptide, respectively.34 Another example that is commonly referred to is the specific targeting of the folate receptor, which can be found on the outer membrane of different types of breast cancer cells.35 In this case, the folate receptor is targeted by synthesizing FA-drug conjugates and grafting FA onto nano-carriers to promote internalization by cancer cells. Focusing on natural products, this section presents an extensive overview of advancements in the investigation of nano-drug delivery systems containing targeting warheads. Table 1 presents the clinical applications of natural products and the advantages of nano-drug delivery systems loaded with targeting warheads.
Natural Products Delivered by Nano-Drug Delivery Systems with Targeting Warheads
Terpenoids
Terpenoids are widely used in diversified industries, such as medicine, and food. They are consisted of several C5H8 units.36 In the late 1990s, research on the separation and purification of active ingredients, component analysis, and structural research of terpenoids received widespread attention. Research has highlighted their prominent role in medicine, with terpenoids exhibiting multiple pharmacological effects, including anti-tumor, anti-inflammatory, antimicrobial, antiviral properties, and utility for treatment of cardiovascular diseases.37–41 This section discusses research on nano-drug delivery systems for typical natural terpenoid products, triptolide and ginsenoside, aiming to provide a theoretical basis for subsequent studies.
Triptolide (TP), derived mainly from Tripterygium wilfordii Hook.f., is considered to be a potential candidate for the next “blockbuster” anticancer drug. It is a highly oxidized diterpenoid trioxide that has analgesic, anti-inflammatory, and immunomodulatory activities. Several triptolide derivatives have entered clinical research.22,42,43 With the advancement of research, a variety of drug delivery systems have been developed to optimize the clinical adverse factors of TP (Figure 2). One study44 focused on the design of galactosylated chitosan-triptolide NPs with a high capacity for loading the drug, aiming for targeted delivery to hepatocellular carcinoma. As anticipated, the GC-TP-NPs demonstrated sustained release properties. In laboratory experiments, the NPs were internalized by cells through the asialoglycoprotein receptor (ASGPR), and in vivo they accumulated significantly in liver tumors. Furthermore, compared with free drug administration, nanoparticle-based delivery with targeting warheads resulted in less systemic toxicity and reduced male reproductive toxicity. The galactose moiety of the NPs attached specifically to receptors on liver cancer cells, enabling significant uptake of TP through ligand-receptor interactions. This dramatically demonstrates the superiority of nano-drug delivery systems with targeting warheads. Hydrophobic toxic drugs can be conjugated with specific targeting warheads, which enhance the drug concentration inside cells by receptor-mediated endocytosis on the membrane of liver cancer cells, reducing nonspecific distribution and significantly improving drug safety. Huang et al45 also conducted research on delivery of TP and developed Pluronic F127/P123 NPs (TP-FPNPs) with folate as a targeting moiety, modified with the toxic drug TP. The drug-loaded NPs exhibited high kidney targeting, prolonged in vivo residence, and improved cellular uptake. TP-FPNPs gave significantly better therapeutic effects on renal ischemia/reperfusion injury (IRI) than TP alone. The NPs entered cells by binding to folate receptors on the renal tubular cell membrane via the folate moiety. TP was slowly released from TP-FPNPs by a controlled diffusion process, followed by degradation of the NPs, thereby affecting treatment of renal IRI. This indicates that folate-functionalized drug-loaded NPs have potential as delivery platforms for hydrophobic drugs in the treatment of renal diseases.
 |
Figure 2 Nano-formulated triptolide for hepatocellular through EPR effect and active targeting. |
Ginsenosides is also known as triterpenoid saponin, which mainly is found in the dried root of ginseng. Due to their chemical structure and biological effects similar to glucocorticoids, they have huge potential for application. However, their unsatisfactory solubility and bioavailability greatly impede their clinical application.46,47 Many nano-drug delivery systems have been utilized to maximize the pharmacological effects of these compounds.48 Zhang et al49 employed A54 peptide to construct micelles loaded with ginsenoside compound K (CK) for liver targeting. The vehicle used for this purpose was deoxycholic acid-O-carboxymethyl chitosan. Compared with the use of CK alone, polymer micelles significantly enhanced their anti-proliferative effect on two types of liver cancer cell lines, exhibiting better anti-cancer effects. This is because A54 peptide is a liver cancer-specific binding peptide that enables modified drug nano-systems to recognize specific cell surface receptors, targeting liver cancer cells, and being rapidly absorbed. As CK is gradually released from the micellar carrier into solution, its residence time in the bloodstream is increased, which improves bioavailability (Figure 3).
 |
Figure 3 Schematic diagram of the preparation of polymer micelles, cellular uptake of CK, and the anticancer mechanism. Notes: Reprinted from Carbohydrate Polymers, Volume 230, Zhang J, Jiang Y, Li Y, et al. Micelles modified with a chitosan-derived homing peptide for targeted intracellular delivery of ginsenoside compound K to liver cancer cells, Pages 115576, Copyright 2020, with permission from Elsevier.49 |
Flavonoids
Flavonoids have a C15 backbone and contain two phenyl rings (C6-C3-C6). Flavonoids have a wide range of therapeutic applications and can help delay the aging of various life systems and organs to varying degrees. They also help prevent cardiovascular diseases, Alzheimer’s disease and breast cancer.50–52 For these reasons, many methods have been taken to increase their solubility, improve gastrointestinal absorption, and reduce metabolic clearance. In addition, when consumed with other food components, flavonoids can undergo enhanced complexation or precipitation, ultimately leading to degradation by microbial flora and reducing their stability and bioavailability. To overcome these limitations, researchers have focused on developing nano-carrier systems to deliver flavonoid compounds to specific target sites. This approach has significantly improved the pharmacokinetics and pharmacodynamics of the drugs by altering their free form.53,54 Haroon Khan et al55 have extensively discussed the improved bioavailability of flavonoid nano-drugs and their safety implications. Paola Aiello and his team56 evaluated in vivo studies of dietary flavonoid NPs with anticancer activity. This section focuses on the recent progress made in nano-drug delivery systems for flavonoid compounds, with the specific mention of two flavonoids, gambogic acid, and baicalin.
Gambogic acid (GA) is a novel flavonoid-based anticancer drug that has been found to exhibit strong tumor activity against colon, pancreatic, and breast cancers.57–60 Unfortunately, its clinical use is hindered by inadequate solubility, brief duration of action within the body, toxicity that is dependent on the dosage, and discomfort experienced at the site of injection.61,62 Therefore, there have been many attempts to construct new GA nano-formulations to improve bioavailability and clinical efficacy. In a study,63 hyaluronic acid (HA) was used to modify redox-sensitive chitosan NPs. By controlling a well-established synthesis procedure, HA-coated redox-sensitive chitosan NPs (HA(HECS-ss-OA)/GA) were prepared for tumor-specific intracellular delivery of GA (Figure 4). The system exhibited excellent loading of GA, and in vivo and in vitro experiments demonstrated that compared with the non-sensitive control and HA-uncoated counterparts, HA(HECS-ss-OA)/GA NPs exhibited the strongest anti-tumor efficacy. The low-solubility and toxic drug GA can be efficiently delivered into cells by binding of the surface-modified hyaluronic acid to the CD44 receptor on cancer cells. This approach not only greatly enhances drug safety but also underscores the need to develop nano-drug delivery systems that utilize natural products as targeting agents. It provides a promising new avenue for effective tumor treatment.
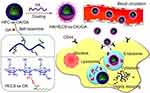 |
Figure 4 The design and targeted delivery of HA(HECs-ss-OA)/GA nanoparticles for tumor treatment. Notes: Copyright ©2013. Dove Medical Press. Reproduced from Xu W, Wang H, Dong L, et al. Hyaluronic acid-decorated redox-sensitive chitosan micelles for tumor-specific intracellular delivery of gambogic acid. Int J Nanomed. 2019;14:4649–4666.63 |
Baicalin (BAI) is derived from the roots and stems of Scutellaria baicalensis. Extensive research has demonstrated its remarkable ability to inhibit the growth and proliferation of different types of cancer cells, including those found in breast and ovarian cancers.64–66 Researchers67 have developed folate-conjugated bovine serum albumin NPs (FA-BSANPs/BAI) with BAI as a model drug, using the desolvation-crosslinking method. The folate-modified albumin delivery system prolongs the in vivo residence of BAI and enhances its tumor-targeting ability. By making use of the elevated levels of folate receptors present on the superficial breast cancer cells, targeted recognition and folate receptor-mediated endocytosis substantially enhance the concentration of hydrophobic medications within tumor cells. As a result, this approach significantly improves the efficacy of breast cancer treatment. Research team68 optimized a nano-carrier for neuroprotective agents by obtaining RVG29 peptide-modified polyethylene glycol-polylactic acid-co-glycolic acid nanoparticles with favorable physicochemical properties. The carrier provided drug stability and continuous release, thus enhancing the effectiveness of neuroprotective agents for delivering medication from the nose to the brain. It was discovered that administering BAI-PEG-PLGA RNPs through the nasal route resulted in significant improvement in neurological function in rats with ischemic brain injury. The RVG29 peptide, derived from the rabies virus glycoprotein with a sequence of 29 amino acids, serves as a brain-targeting molecule and is incorporated into the drug delivery system. It exploits receptor-mediated endocytosis through binding of the RVG29 peptide to receptors on the BBB to transport the hydrophobic baicalin from the bloodstream to the brain, achieving brain-targeted drug delivery. BAI-PEG-PLGA RNPs effectively delivered BAI in rats, and the safety of the delivery system was improved.
Polyphenols
Polyphenols are widely recognized as highly effective natural substances for the clinical use of chronic diseases due to their exceptional antioxidant and anti-inflammatory characteristics.69 They are substances found in various vascular plants that consist of numerous hydroxyl groups on the aromatic ring.70 For the past few years, clinical research on the pharmacological effects of polyphenols has gradually expanded, and they have been found to have antioxidant, anti-inflammatory, anti-cancer, hepatoprotective, and anti-obesity properties.71–75 Despite their significant potential, polyphenols have a tendency to become structurally unstable when exposed to alkaline, light, and thermal conditions. This instability is attributed to the presence of multiple hydroxyl groups. Moreover, the majority of polyphenols have low solubility, leading to limited gastrointestinal absorption and bioavailability. Consequently, these limitations restrict their usage as pharmaceuticals and functional foods.76 To address these difficulties, nano-drug delivery systems have been created to counteract the occurrence of precipitation, quick degradation, and elimination of natural polyphenolic compounds. A substantial body of studies has demonstrated the successful development of nano-carrier systems for polyphenolic compounds, thereby improving their stability and bioavailability.77,78 This section provides an overview of recent advances in nano-drug delivery systems, focusing on two polyphenolic natural compounds, resveratrol and curcumin. These findings lay a theoretical foundation for future research in this field.
Resveratrol (RES) is a polyphenol antioxidant of natural origin, characterized by its trans-3,4’,5-trihydroxystilbene structure. It is present in different plants, including grapes, mulberries, peanuts, and rhubarb roots. RES plays a crucial role in safeguarding blood vessels and DNA against oxidative harm, making it an important element in the precaution and treatment of chronic diseases associated with inflammation. Nevertheless, the limited physicochemical properties of RES pose significant constraints on its application as a standalone medication.79 Encapsulation and controlled release of RES using different carriers have emerged as promising strategies to overcome the limitation of low bioavailability.80 Jhaveri et al81 first used transferrin-modified polyethylene glycol liposomes (Tf-RES-L) as a delivery system for RES. Compared with free drugs, functionalized drug-loaded liposomes significantly increased apoptosis of glioblastoma multiforme cells (GBMs) by activating the caspase 3/7 pathway. Since transferrin receptors (TfRs) are upregulated in GBMs, the surface modification of liposomes with transferrin confers cancer cell specificity. In the presence of excess free transferrin, competitive inhibition confirmed that Tf-RES-L exploits the receptor-mediated endocytosis pathway mediated by TfRs for cell internalization. As expected, Tf-RES-L exhibited uniform size distribution and zeta potential, and its stability increased. The effectiveness of Tf-RES-L is largely attributed to the surface modification by transferrin. This also demonstrates that the increased bioavailability and targeting of natural product accumulation are due to the cumulative effects of the nano-drug delivery system targeting warheads.
Curcumin (Cur) is a polyphenol isolated from the rhizome of Curcuma longa. It demonstrates remarkable antioxidant, wound healing, and anticancer properties.82,83 However, the use of it is restricted due to low bioavailability, high hydrophobicity, and vulnerability to degradation when exposed to light. One approach to address these issues is to enclose natural plant metabolites within biodegradable and biocompatible NPs. Feng and colleagues84 developed a CD44-targeted drug delivery system by covalently linking hyaluronic acid to propylene glycol-based ethosomes (HA-ES). The HA-ES surface was coated with a HA gel network, resulting in decreased leakage and release of curcumin. This improved the targeted delivery of Cur to inflamed psoriatic skin that overexpressed CD44 protein, thereby enhancing the therapeutic effect on the affected area. By encapsulating lipophilic natural products within the gel structure, the stability of the drug is ensured, as well as its safety in non-target tissues. This promising local drug delivery system, which releases the drug in areas of inflamed skin that highly express CD44 protein, can also be used to enhance the accumulation of other drugs.
Alkaloids
Alkaloids are generally small nitrogenous organic compounds, derived from amino acids, that are isolated from plants. They can be categorized into 80 groups according to their chemical core structures, such as isoquinoline alkaloids, quinoline alkaloids, indole alkaloids, and pyridine alkaloids.85 A significant number of studies conducted in vivo and in clinical settings have shown that alkaloids possess diverse pharmacological properties, including anti-cancer, antiviral, anti-inflammatory.86–88 Many alkaloid drugs have been developed and are widely used in clinical practice, such as camptothecin and paclitaxel for anticancer therapy, and berberine hydrochloride for antibacterial therapy. However, research has shown that despite their significant pharmacological activities, alkaloids often exhibit poor pharmacokinetic characteristics, incompatibility in biological media, and the formation of aggregates that can hinder cellular uptake, thus limiting their clinical applications.89,90 A prominent focus of contemporary scientific investigation lies in the advancement of nano-drug delivery systems. These systems possess distinctive physical and chemical attributes that safeguard encapsulated drugs from enzymatic and mechanical deterioration, heighten permeability, and exhibit retention effects. Such properties offer an array of advantages in drug delivery.91,92 Consequently, designing novel nano-drug delivery systems to improve the therapeutic effects of alkaloids in diseases such as cancer holds great potential. In this section, we have chosen the natural product paclitaxel as a representative drug and discuss recent research on relevant delivery systems.
Paclitaxel (PTX), a natural compound derived from the bark of Taxus brevifolia, is known for its remarkable anti-tumor properties, especially in the treatment of ovarian and uterine cancers.93,94 However, because of its limited ability to dissolve in water, tendency to become unstable, propensity to precipitate, and toxic nature, its application in clinical settings has proven to be quite difficult.95,96 To address these drawbacks, researchers97 have successfully devised and produced an innovative Tn-Lipo-PTX, a PEGylated paclitaxel nano-liposome with ASGPR targeting capabilities, specifically tailored for the treatment of hepatocellular carcinoma. A configurationally defined N-acetylgalactosamine was synthesized and used as a targeting warhead, significantly enhancing the cytotoxicity of paclitaxel towards liver cancer cells. This study highlights the superiority of nano-carriers with warhead-modifications for delivery of lipophilic drugs. Human H-chain ferritin (HFtn), with its excellent solubility in water, biodegradability, multifunctionality, and uniform size distribution, has gained increasing attention in drug delivery applications.98–101 Ma et al102 confirmed that the N-terminus of human HFtn can be functionalized with the C-terminal cyclic peptide tLyP-1 as the targeting moiety for PTX delivery. By adjusting the pH, PTX was encapsulated into HFtn NPs through disassembly/reassembly, forming a dual-targeting NP delivery system. This method greatly improved the ability of PTX to inhibit cancer cells and demonstrated the promise of utilizing protein-functionalized targeting peptides as vehicles for delivering hydrophobic drugs to tumor tissues. The hydrophobic drugs are encapsulated within functionalized proteins, maintaining relative stability under normal physiological conditions, and then released from the nanostructures (Figure 5).
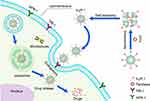 |
Figure 5 Method for preparing PTX nanoparticles; schematic illustration of the tLyP-1 peptide functionalized HFtn for targeted delivery. |
Natural Product-Based Self-Assembled Nano-Drug Delivery Systems
Self-assembly refers to the process in which fundamental units, such as molecules and nano-structured materials, spontaneously come together to form a stable and closely-knit structure. This process is primarily propelled by feeble non-covalent interactions, encompassing hydrogen bonding, van der Waals forces, π–π stacking, and electrostatic interactions, with the intention of generating durable self-assembled systems featuring distinct structures. The resulting products can take forms such as micelles, liposomes, or helical belts.103,104 In 1993, Ghadiri and others105 first used self-assembly techniques to synthesize peptide nanotubes from a novel cyclic peptide. Subsequently, Zhang and others106 discovered ionizable short peptides that could undergo self-assembly, and the field of peptide self-assembly gradually developed. Natural product self-assembly has shown apparent advantages in terms of low cost, low energy consumption, and low pollution in the field of nanomaterials, and can also improve drug solubility, targeting, and other properties.
In recent years, supramolecular self-assembly technology has attracted wide attention both domestically and internationally because of its ability to prepare nanomaterials with unique physical and chemical properties. Research has found that nano-drug delivery systems may suffer from problems such as non-ideal biocompatibility and biodegradability in clinical applications. Therefore, researchers are dedicated to developing biologically safe drug carrier materials and formulating drugs with minimal side effects. The construction of nanostructures mainly abides by two strategies: top-down and bottom-up approaches (Figure 6). Top-down strategies involve creating nanostructures of specific shapes and sizes from larger structures. On the other hand, bottom-up methods produce the desired nanostructures by using basic components and utilizing molecular recognition and self-assembly processes. The latter approach heavily relies on the interactions between these basic units to form highly organized structures. Consequently, by effectively controlling the structure of self-assembled molecular units, it becomes possible to manipulate the formation of nanostructures at the atomic or molecular level.103 This section provides an overview of the study and practical uses of various types of self-assembled nano-carriers.
 |
Figure 6 Two strategies for self-assembly: top-down and bottom-up. |
Drug Self-Delivery Systems Based on Natural Small-Molecule Drugs
Drug Self-Delivery Systems (DSDSs) are novel ways to deliver drugs that do not require additional nano-catalysts to achieve drug delivery.107 The self-assembled drugs serve as both carriers and medications, resulting in an increased rate of drug encapsulation and drug loading capacity, while also minimizing drug wastage. This technology eliminates the necessity for additional nano-catalysts, thus preventing drug degradation, metabolism, and excretion, while also avoiding any potential toxicity or immunogenicity caused by the carriers. As a result, the drug remains protected from damage, and the system demonstrates excellent biocompatibility and is relatively safe.108,109 Optimization of key characteristics such as size, surface properties, and circulatory stability in vivo of the nanomaterials allows effective improvement of their clinical safety, enabling timely application in clinical practice.110
Ursolic acid (UA) is a pentacyclic triterpenoid compound commonly found in plants and food. It has promising bio-nanotechnology applications and pharmacological activity. UA exhibits potent anticancer effects by inhibiting tumor cell differentiation, angiogenesis, invasion, and metastasis through various pathways with low toxicity to normal cells.111–114 In the realm of drug delivery, self-assembled peptide-based nano-drug delivery The progress and utilization of this compound encounter obstacles such as inadequate drug levels within the body and unsatisfactory distribution throughout the body. Nano-drug delivery systems hold promise in augmenting the pharmacokinetics, bioavailability, and therapeutic efficacy of targeted medications. Based on the robust hydrophobic interactions and hydrogen bonding interactions among UA molecules, certain scholars have employed self-assembly technology to devise a carrier-free and highly loaded nano-drug system.115 UA allows for self-assembly at a small size and negative charge without any additional assistance, which helps improve its solubility and concentration-dependent inhibition of HeLa and A549 cell growth. Additionally, it significantly increases the deposition of UA in tumor tissues. This suggests that the novel carrier-free nano-drug delivery system holds potential as a strategy to improve the efficacy of unsatisfactory solubility of anticancer drugs. Hydrophobic drugs have the ability to spontaneously form Drug Self-Assembled Delivery Systems (DSDSs) by means of intermolecular interactions. These drugs can then gather at the specific site of the disease, influenced by the permeability of blood vessels and the EPR effect that tumors exhibit. The systems could release load in response to the acidic tumor microenvironment. Several reports have described the successful development of supramolecular hydrogels containing drug complexes in the laboratory, which have been used for alleviating inflammation, wound healing, and other applications.116–119 Rhein (Rhe) is an anthraquinone compound extracted from the dried roots and rhizomes of Rheum palmatum L. or Rheum tanguticum Maxim, which has been provided evidence of its remarkable potential in the field of medical imaging, as well as in the treatment of neurodegenerative diseases.120–123 However, the difficulties faced in clinical development of Rhe arise from its limited solubility in water and low bioavailability. To settle these problems, nano-drug delivery systems have been employed to improve the pharmacological effectiveness of Rhe. Zheng and his group116 have successfully fabricated a controlled-release hydrogel by means of the directed self-assembly of Rhe molecules, which is facilitated through π–π stacking and hydrogen bond interactions. The hydrogel has good biological stability and exhibits sustained release and stimulus responsiveness, which is attributed to its stability and fiber brittleness. The self-assembly of Rhe monomers, devoid of any structural modification or delivery carriers, provides significant insights into the self-assembly mechanisms of other small molecules. Ke and others124 found that licorice protein could self-assemble into NPs and load 28.2% aconitine at pH 5 and 20–25 °C. The preparation of high-bioavailability protein NPs expands the application of protein components and provides a basis for preparation and targeted action of self-assembled NPs of active ingredients from natural products.
In one study,125 the concept of a “self-contained bioactive nano-carrier” system has been proposed, revealing that van der Waals forces and electrostatic interactions are important driving forces in the assembly of UA and PTX, and that the drugs are almost fully loaded. The experiments have indicated a time-dependent effect, resulting in a tumor inhibition rate of 90.2%. Furthermore, the experimental data provide convincing evidence for the biocompatibility of UA. UA initially forms a double-layered vesicle structure, providing hydrophobic positions for the self-assembly of UA and PTX. The self-assembly of the two drugs compensates for the lack of bioactivity of the nano-carrier, endowing it with excellent therapeutic activity, and achieving synergy of the carrier and drug treatment. Tian et al126 constructed a nano-drug delivery system based on self-assembly of the natural products Rhe and Berberine (Ber) to enhance antibacterial activity against Staphylococcus aureus. The experimental results showed that at the lowest bactericidal concentration of 0.1 µmol/mL, Ber-Rhe NPs gave significantly higher inhibition of S. aureus compared with the same concentration of free drugs. That is, after Ber and Rhe were self-assembled into NPs, the synergistic antibacterial effect was significantly enhanced, and good biocompatibility and safety were demonstrated. These molar complex NPs relying on ectopic stacking and electrostatic interactions could form stable layered stacking three-dimensional configurations in an aqueous solution (Figure 7). The weak bond-driven effective structural self-assembly offers several advantages over nano-carriers. These advantages include enhanced drug delivery efficiency and the ability to evade the potential metabolism and toxicity issues relevant to nano-carriers. Chen and others127 investigated the thermodynamic mechanism of the interaction between the active components of rhubarb and Coptis chinensis, tannic acid and berberine, and found that their combination was a spontaneous process. They constructed a supramolecular self-assembly system of tannic acid-berberine host molecules and drug conjugate HA for active targeted delivery to the colon. The constructed nano-drug delivery system exhibited a powerful synergy of the two drugs, enhanced uptake and accumulation of drugs in lesions, suppressed levels of pro-inflammatory cytokines, and showed substantial anti-inflammatory effects against acute colitis induced by dextran sodium sulfate. In comparison to previously reported drug carriers, these possess distinct benefits in preventive healthcare, highlighting the promising growth potential of self-assembly in utilizing natural products.
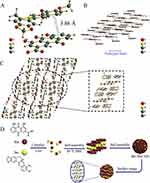 |
Figure 7 (A) The basic unit of the crystal formed by the π-π bond of Ber-Rhe nanoparticles; (B) the layered skeletal structure formed under the hydrogen bonding by Rhe; (C) the accumulation structure of Ber-Rhe NPs crystals; (D) schematic diagram of self-assembly of Ber and Rhe. Notes: Reproduced wthn permission from Tian X, Wang P, Li T, et al. Self-assembled natural phytochemicals for synergistically antibacterial application from the enlightenment of traditional Chinese medicine combination. Acta Pharm Sin B. 2020;10(9):1784–1795.126 © 2020 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V. |
Self-Assembled Nano-Drug Delivery Systems Based on Natural Products and Peptides
Peptides are bioactive compounds situated between proteins and amino acids, which are generated by linking numerous amino acids in a specific sequence through amide bonds. Although peptides have simple structures and are small, they often have important biological functions. Compared to traditional biomedical materials, peptides possess excellent biocompatibility and chemical versatility. They are also biodegradable and exhibit remarkable self-assembly characteristics.128,129 By modifying the pH and ion strength, and incorporating enzymes or light, it becomes feasible to initiate self-assembly and create a variety of distinct nanostructures. These nanostructures have been found utility in the realm of controlled drug delivery.130 Peptide-based NPs have unique advantages, such as customizable molecular design, effective functional regulation, and peptide-specific biomolecular recognition capabilities, making them stand out among many nano drugs.131,132 There have been numerous studies on the design, synthesis, and application of peptide-based nano-drugs. For example, in their comprehensive review, Wang et al133 concisely outline the design methodologies employed in the development of self-assembled peptide nano-drug delivery systems. The authors place particular emphasis on elucidating the various strategies utilized for drug delivery, all of which are based on the self-assembly properties of peptides. In the realm of drug delivery, self-assembled peptide-based nano-drug delivery systems have gained significant traction. This can be attributed to their remarkable attributes, such as their ability to carry a large quantity of drugs, heightened bioavailability, effective targeting capabilities, and capacity to permeate cellular structures.
The diverse chemical properties of non-covalent interactions have promoted rapid development of self-assembly for drug delivery. Sis and Webber134 provide a deep-going overview of the basic design of peptide self-assembled systems and methods. Amphiphilic peptides have extended the application of self-assembling peptides in drug delivery. Hsieh and Liaw135 proposed safety measures to ensure the precise size and length of nanotubes for effective delivery of active drug ingredients utilizing multifunctional cyclic peptides. In a separate study, Ji Hwan Park et al136 formed amphiphilic peptides R7L10 by self-assembly of additional arginine and leucine residues at the amino and carboxyl ends of peptide R3L6, which stably self-assembles with plasmid DNA to form complexes. The model drug curcumin, a lipophilic anti-inflammatory drug, was loaded into the hydrophobic core of the complex formed by the amphiphilic peptide R7L10 and plasmid DNA, significantly improving the solubility. Additionally, the system enhanced cellular uptake in a model of acute lung injury without inducing hepatotoxicity, demonstrating its potential.
Peptide-based hydrogels are a class of drug delivery carriers that involve encapsulation of drugs and covalent linkage of therapeutic agents. Typically, small peptide molecules generally form specific secondary structures in solution and have the ability to spontaneously form fibrous networks in response to different physical factors.137–139 Hydrogels are hydrophilic networks with strong water-retaining capacity that resemble biological tissues.140 Based on supramolecular chemistry, Faisal Raza and his colleagues141 designed a pH-responsive octapeptide, FER-8, for targeted delivery of the anti-tumor drug PTX. As a consequence of the compatibility of the peptide self-assembly and cross-linking with physiological conditions, FER-8 hydrogel exhibited exceptional efficacy as a drug carrier. It effectively increased accumulation of PTX in tumor cells, prolonged residence time, and reduced systemic side effects through intratumoral injection. This suggests the superiority of peptide self-assembling hydrogels as carriers for insoluble natural products. Drugs loaded in hydrogels exhibit biphasic release behavior and diffuse from the surface pores of the hydrogel. Wei et al142 confirmed that the self-assembling peptides RADA16-I and RVDV16-I interacted with emodin. Both of these peptides that assemble themselves formed hydrogels in aqueous solution with emodin while being influenced by magnetic stirring. The hydrogels significantly increased the effectiveness of inhibiting tumor cells in vitro compared to emodin on its own. This also fully showcases the capability of self-assembling peptides as carriers for hydrophobic drugs. Hydrophobic drugs are enclosed within the self-assembled peptides, ensuring their stability in the circulatory system, and then released from the hydrogel.
Self-Assembly Based on Metal-Coordinated Polyphenols
Recently, researchers have discovered that natural polyphenols can coordinate with metals to self-assemble into metal-polyphenol nanonetworks (MPNs) with a thickness of two nanometers. Like most self-assembled systems, MPNs can not only exist as thin films but also take on various other forms as a consequence of their flexible structures.143 Polyphenols exhibit a range of powerful physical, chemical, and biological properties, such as antioxidant activity, high adhesion, and the ability to absorb ultraviolet radiation.144,145 Adriamycin, peroxidases, and some natural products can be stably embedded in the MPN framework. Many natural polyphenols, such as catechin, shikonin, curcumin, epigallocatechin, and tannic acid, have been utilized for constructing MPNs in cancer treatment.146 Feng et al147 constructed a Fe (III)-shikonin metal-polyphenol-coordinated supramolecular nanomedicine (FSSN) for comprehensive tumor therapy involving iron-dependent apoptosis and necroptosis (Figure 8). The preparation of FSSN not only improved the solubility and loading of shikonin but also reduced toxicity to normal cells. When the FSSN is engulfed by tumor cells, Fe3+ is reduced by glutathione, causing decomposition of the FSSN and release of the drug, triggering the effective biological activity of tumor cell deferrization. This demonstrates the promising potential of nano-drug delivery systems derived from metal-polyphenol coordination interactions. Wen et al148 reported a degradable carrier-free curcumin-based MPN theranostic agent (ICG @Cur-Gd NPs) combining indocyanine green (ICG), Cur, and Gd3+. In the tumor microenvironment, ICG @Cur-Gd NPs were degraded, resulting in the release of Cur, ICG, and Gd3+ to enhance therapeutic efficacy and reduce biotoxicity. In vivo experiments confirmed that laser treatment in combination with ICG @Cur-Gd NPs significantly inhibited tumor growth without apparent cytotoxicity, indicating great potential for clinical applications. This investigation presents an innovative and efficient approach for the development of tumor imaging-guided therapeutic drugs, without the need for carrier molecules.
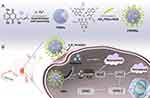 |
Figure 8 (A) Schematic diagram illustrating the supramolecular nano-drug FSSNs formed by Shikonin and Fe3+, as well as further modifications; (B) FSRSN specifically binds to the βvβ3 receptor, significantly enhancing its accumulation in tumor cells. Notes: Reproduced with permission from Feng W, Shi W, Liu S, et al. Fe(III)-Shikonin Supramolecular Nanomedicine for Combined Therapy of Tumor via Ferroptosis and Necroptosis. Adv Healthc Mater. 2022;11(2):90.147 © 2021 Wiley-VCH GmbH. |
Dietary flavonoids have a wide range of biological functions, including acting as antioxidants, contributing to pigmentation, and providing defense against radiation damage. Flavonoids have garnered considerable interest due to their notable advantages for human health, with applications in areas such as cancer treatment, prevention of cardiovascular diseases, and mitigation of inflammation. Nadja Bertleff-Zieschang et al149 assembled four flavonoids containing a common ortho-dihydroxybenzene structure, namely myricetin (Myr), quercetin (Que), fisetin (Fis), and luteolin (Lut), into solid surface coatings by coordinating with Fe (III). The mechanism is based on pH-dependent rapid coordination assembly between the flavonoid ligands and Fe (III). The results indicated that the films formed by Que, Myr, Lut, and Fis had free radical scavenging activity (Figure 9), and could be reused in multiple cycles.
 |
Figure 9 (A) Molecular structure of flavonoids and their main sources; (B) schematic diagram of the self-assembly of flavonoids and Fe3+ on the template surface. Notes: Reproduced from Bertleff-Zieschang N, Arifur Rahim M, Ju Y, et al. Biofunctional metal–phenolic films from dietary flavonoids. Chem Commun. 2017;53(6):1068–1071.149 |
Co-Delivery Nano-Drug Delivery Systems Based on Natural Products
Natural products exhibit therapeutic effects in clinical applications through the comprehensive actions of multiple components. The fundamental aspect of this is the role played by the pharmacologically bioactive constituents. The key to preventing and treating diseases lies in effective delivery of multiple natural small-molecule components. Consequently, taking advantage of modern nanotechnology to achieve effective delivery and distribution of multiple components, leveraging synergistic effects, reversing multidrug resistance, and reducing toxicity is a scientifically rational choice. Modern research has found that co-delivery systems based on nano-carriers clearly demonstrate potential to overcome problems associated with the use of single drugs or traditional treatment methods. Nano-drug co-delivery systems are specifically developed for clinical purposes, wherein a minimum of two drugs possessing distinct physicochemical and pharmacological characteristics are incorporated into a system.150 According to reports, co-delivery technologies have numerous advantages, such as administration of different natural products in specific ratios and doses, controlled release, and potential synergistic effects.151,152 The combination of nanotechnology and co-delivery strategies has not only become an ideal drug delivery approach but also represents one of the cutting-edge research areas in the quest for effective drug delivery systems.153–155 The segment furnishes a thorough review and analysis of the present state of research and utilization of nano delivery carriers for drug co-delivery. Its objective is to establish a theoretical foundation for nano systems that can effectively transport multiple natural active ingredients concurrently.
Solid Lipid Nano-Carriers
The application of lipid NPs is an emerging field at the forefront of rapidly developing nanotechnology.156,157 In the early days, solid lipid nanoparticles (SLNs) were formulated as colloidal vehicles for drug delivery, exhibiting a particle size distribution spanning from 50 to 1000 nanometers.158 They offer numerous advantages, such as reducing adverse reactions, permitting encapsulation and entrapment of various molecules, accelerating targeted delivery of therapeutic drugs to specific tissues and cells, and feasibility of large-scale production.159,160 SLNs could be composed of a mixture of solid lipids or lipids and surfactants. In addition, aqueous phases, co-surfactants, and cryoprotectants may also be present.161–163 Hydrophobic drug combinations are encapsulated in a solid lipid matrix to protect the drugs from chemical degradation, thereby increasing their stability.
In a scholarly investigation,164 the objective was to elevate the therapeutic effectiveness of tanshinone (TAN) and achieve a synergistic effect with puerarin (PUE). This was accomplished by co-encapsulating PUE-prodrug and TAN in solid lipid nanoparticles (SLNs) to treat myocardial infarction. Compared with administration of free TAN and PUE, SLNs loaded with both drugs were significantly more efficacious in the treatment of infarction. Single emulsification and solvent evaporation methods were employed for the formulation of co-loaded solid lipid nanoparticles (SLNs), resulting in reduced cytotoxicity and extended-release characteristics attributed to the incorporation of PEG. This significantly improved drug safety, demonstrating significant advantages of co-delivery nano-drug delivery systems with synergistic effects. The drugs, present independently on the surface layer of the particles, were released by rapid dissolution from the surface of the SLN. In another study, Wang et al165 formulated solid lipid nanoparticles (SLNs) loaded with paclitaxel and naringenin in order to address glioblastoma multiforme. To enhance the targeting efficiency towards cancerous sites, the SLNs were modified with cyclic RGD peptide sequences. The research demonstrated that the pharmacokinetic properties of the peptide-functionalized SLNs, including maximum plasma concentration (Cmax), time to Cmax, and relative bioavailability, outperformed those of regular SLNs and drug suspensions. Furthermore, the functionalized SLNs exhibited greater cytotoxicity against U87MG glioma cells in comparison to the free drug suspension. This study highlights the immense benefits of co-delivery nano-drug delivery systems in the context of cancer treatment.
Liposomes
Some of the most important nanotechnology carrier systems are lipid-based,166 including widely used liposomes, and closely related lipid nanodiscs. Lipid-based vesicles such as liposomes are highly attractive for drug delivery because of their high drug-loading capacity, ability to deliver both hydrophilic and hydrophobic drugs, good biodegradability and biocompatibility, prolonged residence in the body, and ease of controlling drug delivery.167 Liposomes are primarily composed of phospholipids that allow self-assembly of non-ionic surfactants and other amphiphilic chemicals, or are composed of amphiphilic lipids, which form spherical vesicles. This structure creates two microenvironments, facilitating co-delivery of drugs.168,169 The drug encapsulation efficiency of liposomes is contingent upon factors such as the size and quantity of bilayers, as well as the vesicle size. Modifying the structure of liposomes can be an effective method to attain the desired therapeutic outcome.170,171
According to recent research,172 it has been discovered that the combination of paclitaxel and tanshinone IIA (TanIIA) has a cooperative effect on the induction of apoptosis in human acute promyelocytic leukemia NB4 cells. For the sake of enhancing the effectiveness of this combination and minimizing any adverse effects, the authors of the study developed a drug delivery system using mesoporous silica nanoparticles that were coated with FA-modified PEGylated lipid-bilayer membranes. It was found that the NPs exhibited sustained release characteristics, with both paclitaxel and TanIIA being released simultaneously from the carrier. This finding highlights the potential of nano-carrier systems to accommodate multiple hydrophilic and hydrophobic drugs while maintaining a high drug-loading capacity. Through specific binding between ligands and receptors, multiple drugs enter cells through endocytosis to exert synergistic anticancer effects. Wang et al173 used ginsenoside Rg3 as a membrane material to prepare unique Rg3-based liposomes loaded with PTX and evaluated their stability, anticancer activity, and mechanisms. The study showed that Rg3-PTX-LPs were specifically distributed to human breast cancer cells and the tumor microenvironment synchronously. Compared with conventional liposomes, Rg3-PTX-LPs exhibited significantly improved reversal of drug resistance and in vivo antitumor efficacy.
Polymeric Nanoparticles
Polymer nanoparticles (PNPs) offer several advantages as drug carriers in terms of controlled release, protection of drugs and bioactive molecules from environmental factors, and enhancement of bioavailability and therapeutic index.167,174 Various methods can be employed to produce PNPs for drug delivery, depending on the specific drug and its administration requirements. These methods can be broadly categorized into two main strategies that are commonly utilized in the field. The first strategy involves the dispersion of preformed polymers, while the second strategy involves the polymerization of monomers. The selection of the appropriate method depends on the type of drug to be loaded into the PNPs and the desired pathway of administration.175,176 To extend the duration of drug circulation in the body and minimize their interaction with blood proteins, PEG can be employed to modify the drugs or carriers. PEGylation of PNPs considerably prolongs drug circulation in the body and enhances their stability.177
Leila Khoshravan Azar et al178 co-encapsulated two natural products, artemisinin (Art) and chrysin (Chr), in PEG-modified poly (lactic-co-glycolic acid) NPs to investigate their combined effects against T47D breast cancer cells. The administration of the Art/Chr-PLGA/PEG NPs demonstrated superior synergistic effects in inhibiting cell proliferation when compared to the individual drugs administered separately. The modification reduced the size of the co-delivery system, which significantly improved drug solubility and showed good stability. Co-encapsulation of the two natural products in PEG-PLGA biodegradable NPs significantly amplified the synergistic anti-tumor effects of artemisinin and chrysin while ensuring drug safety.
Polymeric Micelles
Polymer-based delivery systems present themselves as stable and dependable substitutes for carriers based on lipids.179,180 Polymer micelles (PMs) are biodegradable, flexible, and spherical nanoparticles formed by combining different amphiphilic diblock or triblock copolymers. The hydrophobic interior of PMs provides sufficient space for water-insoluble drugs, while the exterior shell effectively safeguards the drugs from the external environment, thus impeding rapid degradation.181 Research has shown that PMs are versatile drug carriers due to increased drug solubility, excellent biocompatibility, enhanced permeability, and reduced toxicity.182 Commonly used hydrophilic segments in PMs include PEG and poly-N-isopropyl acrylamide, while hydrophobic segments include polypropylene glycol and polycaprolactone.183
Zhou et al184 successfully constructed glutathione (GSH)/reactive oxygen species (ROS) dual-responsive hybrid PMs composed of PTX and honokiol (HK) prodrug that self-assembled. The resulting micelles inhibited the growth of laryngeal cancer. Following optimization and characterization, PTX and HK were precisely loaded in the PMs in a defined mass ratio, enabling proportional drug loading and synchronous drug release in response to the high ROS and GSH levels in tumor cells. The PMs exhibited prolonged circulation and significantly increased accumulation in tumors (Figure 10). Through the covalent linkage of different drugs with biocompatible polymers that possess diselenium bonds, the result was the formation of amphiphilic polymers. These polymers, in turn, exhibited self-assembly properties, leading to the creation of core-shell micelles when placed in an aqueous solution. This significantly increased the drug loading capacity of the nano-drug delivery system while maintaining excellent biomimetic properties and demonstrating the advantages of multi-drug co-delivery and synergistic effects.
 |
Figure 10 The preparation of PHPPM and the schematic illustration of the intracellular glutathione/reactive oxygen species (ROS) dual-responsive drug delivery system. |
Metal Nanoparticles
Many studies have reported the potential of metal nanoparticles (MNPs) with different compositions, such as platinum, gold, silver, iron, selenium, and their oxides, for disease treatment through various mechanisms, including controlled drug release and localized hyperthermia.185,186 These NPs possess remarkable attributes such as surface plasmon resonance properties, structural variability, minimal toxicity, and remarkable compatibility with biological systems.158 These characteristics render them well-suited for drug encapsulation through non-covalent attractive interactions or covalent bonding. Moreover, scientific investigations have demonstrated that MNPs exhibit exceptional capabilities in targeting specific areas, gene silencing, and facilitating drug transport.167,187 Various MNPs that have been used for disease treatment include gold nanoparticles (Au NPs), silver nanoparticles (Ag NPs), and iron oxide nanoparticles (IO NPs).188
Iron oxide nanoparticles (IO NPs) have garnered considerable attention in the emerging field of magnetic nanoparticle technologies due to their remarkable ability to selectively target specific areas under the influence of external magnetic fields.189 In a study by Hiremath C. et al190 folate-modified Pluronic F127-coated oleic acid-stabilized IO NPs were developed as a means of concurrently delivering PTX and Cur to combat breast cancer. The modification of the NPs resulted in enhanced toxicity towards cancer cells, which was further augmented when an external magnetic field was applied, indicating an increased uptake of the NPs by the cancer cells. The transportation of medications by IO NPs is facilitated by the interaction between the hydrophobic cavities of the NPs and hydrophobic drugs. Through the specific binding of folate to folate receptors on the surface of breast cancer cells, a substantial amount of medication enters the cells, resulting in synergistic effects that induce apoptosis in the cancer cells. This underscores the significance of developing co-delivery systems.
Conclusions
It has been discovered that natural products have significant pharmacological effects against most diseases. Despite their great therapeutic potential, the less-than-ideal physicochemical and pharmacokinetic properties of these natural product compounds have limited clinical application. Nanotechnology-based delivery systems have successfully resolved concerns related to natural products through the enhancement of tissue targeting, reduction of off-target side effects, and improvement of local bioavailability. It is anticipated that nano-drugs will emerge as a viable method for diagnosis, imaging, as well as the treatment and control of diverse diseases, representing a significant advancement in the modernization of natural product research. The utilization of nano-drug delivery systems alters the particle size of natural products, elevates drug solubility, and enhances cellular or tissue absorption. Combining natural products with different nano-carriers can solve most of the challenges they face. Nano-carriers not only reduce the hydrophobicity of natural products but also enhance component stability and bioavailability.
Nevertheless, research on natural product-based nano-drug delivery systems is still in its early stages, and foundational research is currently weak. The pharmacological mechanisms of natural products in the treatment of certain diseases are not yet clear, making their clinical application relatively complex. The loading or modifying of natural products with nanomaterials may enhance their effectiveness, but it may also lead to new adverse reactions. Nanotechnology has fundamentally improved the physical or chemical properties of some natural products, resulting in significant uncertainty regarding their composition. It is crucial to better understand the metabolic pathways and choose appropriate and safe nano-carriers. The widespread application of nanotechnology provides new opportunities for the modernization of natural products. However, foundational and exploratory research still needs to be strengthened. Immaturity in modern separation techniques for natural products, lagging nano-drug preparation techniques, and imperfect methods for evaluating pharmacological efficacy are key issues that need to be addressed. Currently, there are numerous controversies surrounding the effectiveness and safety of nano-carriers. Some nano-drugs lack specific targeting in disease treatment, and the EPR effect fails to achieve drug accumulation at the site of the lesion. Nano delivery rates need to be improved, but current research is mainly focused on assembly of the carriers, with insufficient in-depth research on the interaction of drugs involved in the assembly process. Although binding to specific receptors may somewhat enhance targeting, off-target effects and other issues still exist. Henceforth, it is imperative to give precedence to the advancement of carrier materials that possess heightened tissue targeting specificity, superior biocompatibility, and augmented stability. Insufficient understanding of nanotoxicity is an important issue that requires further research to improve efficacy and safety, enabling safer application of these drugs. Currently, the basic knowledge of nano delivery systems and the evaluation of their effectiveness and safety are continuously expanding. Therefore, cautious design of these nano-systems may help address issues associated with their use. Figure 11 is employed to demonstrate the constraints observed in current clinical utilization of natural products, the benefits offered by nanotechnology, the clinical implementation of nano-drug delivery systems derived from natural products, and the pressing concerns that require immediate attention.
Advances in technology will undoubtedly become an important driving force for the modernization of natural products. In the field of natural product research, once the key issues of nanotechnology are overcome, tremendous social and economic benefits can be achieved through clinical application of natural products, promoting their continuous development.
Acknowledgments
The authors acknowledge funding by Heilongjiang Touyan Innovation Team Program, the National Natural Science Foundation of China (Nos. 81703944 and 82174232), the Heilongjiang Natural Science Foundation Project (YQ2019H031), the Postdoctoral Researchers Settled in Heilongjiang Scientific Research Startup Fund (2020), Excellent Scholar of the Qihuang Project of Heilongjiang University of Chinese medicine (2023) and Heilongjiang Province Youth Qihuang Scholar Training Project (2023).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Khan IA, Smillie T. Implementing a “Quality by Design” Approach to Assure the Safety and Integrity of Botanical Dietary Supplements. J Nat Prod. 2012;75(9):1665–1673. doi:10.1021/np300434j
2. Mehta P, Shah R, Lohidasan S, Mahadik KR. Pharmacokinetic profile of phytoconstituent(s) isolated from medicinal plants-A comprehensive review. J Tradit Complement Med. 2015;5(4):207–227. doi:10.1016/j.jtcme.2014.11.041
3. Rodrigues T, Reker D, Schneider P, Schneider G. Counting on natural products for drug design. Nat Chem. 2016;8(6):531–541. doi:10.1038/nchem.2479
4. Vanti G. Recent strategies in nanodelivery systems for natural products: a review. Environ Chem Lett. 2021;19(6):4311–4326. doi:10.1007/s10311-021-01276-x
5. Beutler JA. Natural Products as a Foundation for Drug Discovery. Curr Protoc Pharmacol. 2009;46:9.11.1–9.11.21. doi:10.1002/0471141755.ph0911s46
6. Watkins R, Wu L, Zhang C, Davis RM, Xu B. Natural product-based nanomedicine: recent advances and issues. Int J Nanomed. 2015;10:6055–6074. doi:10.2147/IJN.S92162
7. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16(1):71. doi:10.1186/s12951-018-0392-8
8. Martinho N, Damgé C, Reis CP. Recent Advances in Drug Delivery Systems. J Biomater Nanobiotechnol. 2011;02(05):510–526. doi:10.4236/jbnb.2011.225062
9. Jahangirian H, Lemraski EG, Webster TJ, Rafiee-Moghaddam R, Abdollahi Y. A review of drug delivery systems based on nanotechnology and green chemistry: green nanomedicine. Int J Nanomed. 2017;12:2957–2978. doi:10.2147/IJN.S127683
10. Bonifácio BV, Silva PB, Ramos MADS, Negri KMS, Bauab TM, Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomed. 2014;9:1–15. doi:10.2147/IJN.S52634
11. Liu Z, Tabakman S, Welsher K, Dai H. Carbon Nanotubes in Biology and Medicine: in vitro and in vivo Detection, Imaging and Drug Delivery. Nano Res. 2009;2(2):85–120. doi:10.1007/s12274-009-9009-8
12. Razzacki SZ, Thwar PK, Yang M, Ugaz VM, Burns MA. Integrated microsystems for controlled drug delivery. Adv Drug Deliv Rev. 2004;56(2):185–198. doi:10.1016/j.addr.2003.08.012
13. Lam PL, Wong WY, Bian Z, Chui CH, Gambari R. Recent advances in green nanoparticulate systems for drug delivery: efficient delivery and safety concern. Nanomed. 2017;12(4):357–385. doi:10.2217/nnm-2016-0305
14. Rudramurthy GR, Swamy MK, Sinniah UR, Ghasemzadeh A. Nanoparticles: alternatives Against Drug-Resistant Pathogenic Microbes. Mol. 2016;21(7):836.
15. Saka R, Chella N. Nanotechnology for delivery of natural therapeutic substances: a review. Environ Chem Lett. 2021;19(2):1097–1106. doi:10.1007/s10311-020-01103-9
16. Jain H, Chella N. Methods to improve the solubility of therapeutical natural products: a review. Environ Chem Lett. 2021;19(1):111–121. doi:10.1007/s10311-020-01082-x
17. Paroha S, Dewangan RP, Dubey RD, Sahoo PK. Conventional and nanomaterial-based techniques to increase the bioavailability of therapeutic natural products: a review. Environ Chem Lett. 2020;18(6):1767–1778. doi:10.1007/s10311-020-01038-1
18. Ita KB. Prodrugs for transdermal drug delivery – trends and challenges. J Drug Target. 2016;24(8):671–678. doi:10.3109/1061186X.2016.1154562
19. Fang JY, Leu YL. Prodrug strategy for enhancing drug delivery via skin. Curr Drug Discov Technol. 2006;3(3):211–224. doi:10.2174/157016306780136772
20. Shi X, Sun K, Baker JR. Spontaneous Formation of Functionalized Dendrimer-Stabilized Gold Nanoparticles. J Phys Chem C Nanomater Interfaces. 2009;112(22):8251–8258. doi:10.1021/jp801293a
21. Park SH, Oh SG, Mun JY, Han SS. Loading of gold nanoparticles inside the DPPC bilayers of liposome and their effects on membrane fluidities. Colloids Surf B Biointerfaces. 2006;48(2):112–118. doi:10.1016/j.colsurfb.2006.01.006
22. Qiao L, Han M, Gao S, et al. Research progress on nanotechnology for delivery of active ingredients from traditional Chinese medicines. J Mater Chem B. 2020;8(30):6333–6351. doi:10.1039/D0TB01260B
23. Ashley EA. Towards precision medicine. Nat Rev Genet. 2016;17(9):507–522. doi:10.1038/nrg.2016.86
24. Muro S. Challenges in design and characterization of ligand-targeted drug delivery systems. J Control Release off J Control Release Soc. 2012;164(2):125–137. doi:10.1016/j.jconrel.2012.05.052
25. Srinivasarao M, Galliford CV, Low PS. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat Rev Drug Discov. 2015;14(3):203–219. doi:10.1038/nrd4519
26. Péczka N, Orgován Z, Ábrányi-Balogh P, Keserű GM. Electrophilic warheads in covalent drug discovery: an overview. Expert Opin Drug Discov. 2022;17(4):413–422. doi:10.1080/17460441.2022.2034783
27. Srinivasarao M, Low PS. Ligand-Targeted Drug Delivery. Chem Rev. 2017;117(19):12133–12164. doi:10.1021/acs.chemrev.7b00013
28. Li Y, Chen M, Yao B, et al. Transferrin receptor-targeted redox/pH-sensitive podophyllotoxin prodrug micelles for multidrug-resistant breast cancer therapy. J Mater Chem B. 2019;7(38):5814–5824. doi:10.1039/C9TB00651F
29. Van Heertum RL, Scarimbolo R, Ford R, Berdougo E, O’Neal M. Companion diagnostics and molecular imaging-enhanced approaches for oncology clinical trials. Drug Des Devel Ther. 2015;9:5215–5223. doi:10.2147/DDDT.S87561
30. Maurer AH, Elsinga P, Fanti S, Nguyen B, Oyen WJG, Weber WA. Imaging the folate receptor on cancer cells with 99mTc-etarfolatide: properties, clinical use, and future potential of folate receptor imaging. J Nucl Med off Publ Soc Nucl Med. 2014;55(5):701–704.
31. Farkas R, Siwowska K, Ametamey SM, Schibli R, van der Meulen NP, Müller C. 64Cu- and 68Ga-Based PET Imaging of Folate Receptor-Positive Tumors: development and Evaluation of an Albumin-Binding NODAGA−Folate. Mol Pharm. 2016;13(6):1979–1987. doi:10.1021/acs.molpharmaceut.6b00143
32. Fani M, Tamma ML, Nicolas GP, et al. In Vivo Imaging of Folate Receptor Positive Tumor Xenografts Using Novel 68Ga-NODAGA-Folate Conjugates. Mol Pharm. 2012;9(5):1136–1145. doi:10.1021/mp200418f
33. Müller C, Schibli R. Folic Acid Conjugates for Nuclear Imaging of Folate Receptor–Positive Cancer. J Nucl Med off Publ Soc Nucl Med. 2011;52(1):1–4.
34. Jin SE, Jin HE, Hong SS. Targeted delivery system of nanobiomaterials in anticancer therapy: from cells to clinics. BioMed Res Int. 2014;2014:814208. doi:10.1155/2014/814208
35. Scaranti M, Cojocaru E, Banerjee S, Banerji U. Exploiting the folate receptor α in oncology. Nat Rev Clin Oncol. 2020;17(6):349–359. doi:10.1038/s41571-020-0339-5
36. Li-chao SUN, Shu-ying LI, Feng-zhong W, Feng-jiao XIN. Research Progresses in the Synthetic Biology of Terpenoids. Biotechnol Bull. 2017;33(1):64.
37. Lage H, Duarte N, Coburger C, Hilgeroth A, Ferreira MJU. Antitumor activity of terpenoids against classical and atypical multidrug resistant cancer cells. Phytomedicine. 2010;17(6):441–448. doi:10.1016/j.phymed.2009.07.009
38. Ge J, Liu Z, Zhong Z, et al. Natural terpenoids with anti-inflammatory activities: potential leads for anti-inflammatory drug discovery. Bioorg Chem. 2022;124:105817. doi:10.1016/j.bioorg.2022.105817
39. Yamaguchi T. Antibacterial effect of the combination of terpenoids. Arch Microbiol. 2022;204(8):520. doi:10.1007/s00203-022-03142-y
40. Lin LT, Chung CY, Hsu WC, et al. Saikosaponin b2 is a Naturally Occurring Terpenoid That Efficiently Inhibits Hepatitis C Virus Entry. J Hepatol. 2015;62(3):541–548. doi:10.1016/j.jhep.2014.10.040
41. Abdul Ghani MA, Ugusman A, Latip J, Zainalabidin S. Role of Terpenophenolics in Modulating Inflammation and Apoptosis in Cardiovascular Diseases: a Review. Int J Mol Sci. 2023;24(6):5339. doi:10.3390/ijms24065339
42. Gao J, Zhang Y, Liu X, Wu X, Huang L, Gao W. Triptolide: pharmacological spectrum, biosynthesis, chemical synthesis and derivatives. Theranostics. 2021;11(15):7199–7221. doi:10.7150/thno.57745
43. Xu H, Liu B. Triptolide-targeted delivery methods. Eur J Med Chem. 2019;164:342–351. doi:10.1016/j.ejmech.2018.12.058
44. Zhang YQ, Shen Y, Liao MM, et al. Galactosylated chitosan triptolide nanoparticles for overcoming hepatocellular carcinoma: enhanced therapeutic efficacy, low toxicity, and validated network regulatory mechanisms. Nanomedicine. 2019;15(1):86–97. doi:10.1016/j.nano.2018.09.002
45. Huang C, Zeng T, Li J, et al. Folate Receptor-Mediated Renal-Targeting Nanoplatform for the Specific Delivery of Triptolide to Treat Renal Ischemia/Reperfusion Injury. ACS Biomater Sci Eng. 2019;5(6):2877–2886. doi:10.1021/acsbiomaterials.9b00119
46. Qian T, Cai Z, Wong RNS, Mak NK, Jiang ZH. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg3. J Chromatogr B. 2005;816(1):223–232. doi:10.1016/j.jchromb.2004.11.036
47. Xu QF, Fang XL, Chen DF. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol. 2003;84(2):187–192. doi:10.1016/S0378-8741(02)00317-3
48. Kim H, Lee JH, Kim JE, et al. Micro-/nano-sized delivery systems of ginsenosides for improved systemic bioavailability. J Ginseng Res. 2018;42(3):361–369. doi:10.1016/j.jgr.2017.12.003
49. Zhang J, Jiang Y, Li Y, et al. Micelles modified with a chitosan-derived homing peptide for targeted intracellular delivery of ginsenoside compound K to liver cancer cells. Carbohydr Polym. 2020;230:115576. doi:10.1016/j.carbpol.2019.115576
50. Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126(2):485–493. doi:10.1104/pp.126.2.485
51. Selvakumar P, Badgeley A, Murphy P, et al. Flavonoids and Other Polyphenols Act as Epigenetic Modifiers in Breast Cancer. Nutrients. 2020;12(3):761. doi:10.3390/nu12030761
52. Fernandes I, Pérez-Gregorio R, Soares S, Mateus N, De Freitas V. Wine Flavonoids in Health and Disease Prevention. Mol. 2017;22(2):292.
53. Amawi H, Ashby CR, Tiwari AK. Cancer chemoprevention through dietary flavonoids: what’s limiting? Chin J Cancer. 2017;36(1):50. doi:10.1186/s40880-017-0217-4
54. Gao S, Hu M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini Rev Med Chem. 2010;10(6):550–567. doi:10.2174/138955710791384081
55. Khan H, Ullah H, Martorell M, et al. Flavonoids nanoparticles in cancer: treatment, prevention and clinical prospects. Semin Cancer Biol. 2021;69:200–211. doi:10.1016/j.semcancer.2019.07.023
56. Aiello P, Consalvi S, Poce G, et al. Dietary flavonoids: nano delivery and nanoparticles for cancer therapy. Semin Cancer Biol. 2021;69:150–165. doi:10.1016/j.semcancer.2019.08.029
57. Zhou Z, Ma J. Gambogic acid suppresses colon cancer cell activity in vitro. Exp Ther Med. 2019;18(4):2917–2923. doi:10.3892/etm.2019.7912
58. Lin D, Lin X, He T, Xie G. Gambogic Acid Inhibits the Progression of Gastric Cancer via circRNA_ASAP2/miR-33a-5p/CDK7 Axis. Cancer Manag Res. 2020;12:9221–9233. doi:10.2147/CMAR.S269768
59. Wang H, Zhao Z, Lei S, et al. Gambogic acid induces autophagy and combines synergistically with chloroquine to suppress pancreatic cancer by increasing the accumulation of reactive oxygen species. Cancer Cell Int. 2019;19:7. doi:10.1186/s12935-018-0705-x
60. Wang Y, Liang X, Tong R, et al. Gambogic Acid-Loaded Polymeric Micelles for Improved Therapeutic Effect in Breast Cancer. J Biomed Nanotechnol. 2018;14(10):1695–1704. doi:10.1166/jbn.2018.2626
61. Li M, Su F, Zhu M, et al. Research Progress in the Field of Gambogic Acid and Its Derivatives as Antineoplastic Drugs. Mol. 2022;27(9):2937.
62. Wang X, Chen W. Gambogic acid is a novel anti-cancer agent that inhibits cell proliferation, angiogenesis and metastasis. Anticancer Agents Med Chem. 2012;12(8):994–1000. doi:10.2174/187152012802650066
63. Xu W, Wang H, Dong L, et al. Hyaluronic acid-decorated redox-sensitive chitosan micelles for tumor-specific intracellular delivery of gambogic acid. Int J Nanomed. 2019;14:4649–4666. doi:10.2147/IJN.S201110
64. Peng Y, Zhao FZ, Guo CS, et al. Effects and Mechanism of Baicalin on Apoptosis of Cervical Cancer HeLa Cells In-vitro. Iran J Pharm Res IJPR. 2015;14(1):251–261.
65. Mei ZQ, Wang S, Zhang H, et al. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol Sin. 2009;30(12):1648–1658. doi:10.1038/aps.2009.166
66. Gao C, Zhou Y, Li H, et al. Antitumor effects of baicalin on ovarian cancer cells through induction of cell apoptosis and inhibition of cell migration in vitro. Mol Med Rep. 2017;16(6):8729–8734. doi:10.3892/mmr.2017.7757
67. Meng F, Liu F, Lan M, et al. Preparation and evaluation of folate-modified albumin baicalin-loaded nanoparticles for the targeted treatment of breast cancer. J Drug Deliv Sci Technol. 2021;65:102603. doi:10.1016/j.jddst.2021.102603
68. Li X, Li S, Ma C, Li T, Yang L. Preparation of baicalin-loaded ligand-modified nanoparticles for nose-to-brain delivery for neuroprotection in cerebral ischemia. Drug Deliv. 2022;29(1):1282–1298. doi:10.1080/10717544.2022.2064564
69. Zhang Z, Qiu C, Li X, et al. Advances in research on interactions between polyphenols and biology-based nano-delivery systems and their applications in improving the bioavailability of polyphenols. Trends Food Sci Technol. 2021;116:492–500. doi:10.1016/j.tifs.2021.08.009
70. Kostić AŽ, Milinčić DD, Gašić UM, et al. Polyphenolic profile and antioxidant properties of bee-collected pollen from sunflower (Helianthus annuus L.) plant. LWT. 2019;112:108244. doi:10.1016/j.lwt.2019.06.011
71. Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: antioxidants and beyond. Am J Clin Nutr. 2005;81(1):215S–217S. doi:10.1093/ajcn/81.1.215S
72. Davatgaran-Taghipour Y, Masoomzadeh S, Farzaei MH, et al. Polyphenol nanoformulations for cancer therapy: experimental evidence and clinical perspective. Int J Nanomed. 2017;12:2689–2702. doi:10.2147/IJN.S131973
73. Ignat I, Radu DG, Volf I, Pag AI, Popa VI. Antioxidant and antibacterial activities of some natural polyphenol. Cellulose Chem Technol. 2013;47(5–6):387–399.
74. Yahfoufi N, Alsadi N, Jambi M, Matar C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients. 2018;10(11):1618. doi:10.3390/nu10111618
75. Zhao C, Wan X, Zhou S, Cao H. Natural Polyphenols: a Potential Therapeutic Approach to Hypoglycemia. eFood. 2020;1(2):107–118. doi:10.2991/efood.k.200302.001
76. Kumar Vivekanandhan D, Ranjan Prasad Verma P, Kumar Singh S. Emerging Technologies for Improving Bioavailability of Polyphenols. Curr Nutr Food Sci. 2016;12(1):12–22. doi:10.2174/1573401311666151015213704
77. Han Y, Zhou J, Hu Y, et al. Polyphenol-Based Nanoparticles for Intracellular Protein Delivery via Competing Supramolecular Interactions. ACS Nano. 2020;14(10):12972–12981. doi:10.1021/acsnano.0c04197
78. Neethirajan S, Jayas DS. Nanotechnology for the Food and Bioprocessing Industries. Food Bioprocess Technol. 2011;4(1):39–47. doi:10.1007/s11947-010-0328-2
79. Gowd V, Kanika JC, et al. Resveratrol and resveratrol nano-delivery systems in the treatment of inflammatory bowel disease. J Nutr Biochem. 2022;109:109101. doi:10.1016/j.jnutbio.2022.109101
80. Liang M, Guo M, Saw PE, Yao Y. Fully Natural Lecithin Encapsulated Nano-Resveratrol for Anti-Cancer Therapy. Int J Nanomed. 2022;17:2069–2078. doi:10.2147/IJN.S362418
81. Jhaveri A, Deshpande P, Pattni B, Torchilin V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J Control Release. 2018;277:89–101. doi:10.1016/j.jconrel.2018.03.006
82. Naksuriya O, Okonogi S. Comparison and combination effects on antioxidant power of curcumin with gallic acid, ascorbic acid, and xanthone. Drug Discov Ther. 2015;9(2):136–141. doi:10.5582/ddt.2015.01013
83. Vallianou NG, Evangelopoulos A, Schizas N, Kazazis C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 2015;35(2):645–651.
84. Zhang Y, Xia Q, Li Y, et al. CD44 Assists the Topical Anti-Psoriatic Efficacy of Curcumin-Loaded Hyaluronan-Modified Ethosomes: a New Strategy for Clustering Drug in Inflammatory Skin. Theranostics. 2019;9(1):48. doi:10.7150/thno.29715
85. Yan Y, Li X, Zhang C, Lv L, Gao B, Li M. Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: a Review. Antibiot Basel Switz. 2021;10(3):318.
86. Qing ZX, Huang JL, Yang XY, et al. Anticancer and Reversing Multidrug Resistance Activities of Natural Isoquinoline Alkaloids and their Structure-activity Relationship. Curr Med Chem. 2018;25(38):5088–5114. doi:10.2174/0929867324666170920125135
87. Gorpenchenko TY, Grigorchuk VP, Bulgakov DV, Tchernoded GK, Bulgakov VP. Tempo-Spatial Pattern of Stepharine Accumulation in Stephania Glabra Morphogenic Tissues. Int J Mol Sci. 2019;20(4):808. doi:10.3390/ijms20040808
88. Souza CRM, Bezerra WP, Souto JT. Marine Alkaloids with Anti-Inflammatory Activity: current Knowledge and Future Perspectives. Mar Drugs. 2020;18(3):147. doi:10.3390/md18030147
89. Ma X, Zhou J, Zhang CX, et al. Modulation of drug-resistant membrane and apoptosis proteins of breast cancer stem cells by targeting berberine liposomes. Biomaterials. 2013;34(18):4452–4465. doi:10.1016/j.biomaterials.2013.02.066
90. Majidzadeh H, Araj-Khodaei M, Ghaffari M, Torbati M, Ezzati Nazhad Dolatabadi J, Hamblin MR. Nano-based delivery systems for berberine: a modern anti-cancer herbal medicine. Colloids Surf B Biointerfaces. 2020;194:111188. doi:10.1016/j.colsurfb.2020.111188
91. Bregoli L, Movia D, Gavigan-Imedio JD, Lysaght J, Reynolds J, Prina-Mello A. Nanomedicine applied to translational oncology: a future perspective on cancer treatment. Nanomedicine. 2016;12(1):81–103. doi:10.1016/j.nano.2015.08.006
92. Jacob J, Haponiuk JT, Thomas S, Gopi S. Biopolymer based nanomaterials in drug delivery systems: a review. Mater Today Chem. 2018;9:43–55. doi:10.1016/j.mtchem.2018.05.002
93. Ingle SG, Pai RV, Monpara JD, Vavia PR. Liposils: an effective strategy for stabilizing Paclitaxel loaded liposomes by surface coating with silica. Eur J Pharm Sci off J Eur Fed Pharm Sci. 2018;122:51–63.
94. Wang F, Porter M, Konstantopoulos A, Zhang P, Cui H. Preclinical development of drug delivery systems for paclitaxel-based cancer chemotherapy. J Control Release off J Control Release Soc. 2017;267:100–118. doi:10.1016/j.jconrel.2017.09.026
95. Geisler JP, Linnemeier GC, Thomas AJ, Manahan KJ. Extreme drug resistance is common after prior exposure to paclitaxel. Gynecol Oncol. 2007;106(3):538–540. doi:10.1016/j.ygyno.2007.05.002
96. Ganta S, Amiji M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 2009;6(3):928–939. doi:10.1021/mp800240j
97. Li T, Yu P, Chen Y, et al. N-acetylgalactosamine-decorated nanoliposomes for targeted delivery of paclitaxel to hepatocellular carcinoma. Eur J Med Chem. 2021;222:113605. doi:10.1016/j.ejmech.2021.113605
98. Khoshnejad M, Parhiz H, Shuvaev VV, Dmochowski IJ, Muzykantov VR. Ferritin-based drug delivery systems: hybrid nanocarriers for vascular immunotargeting. J Control Release off J Control Release Soc. 2018;282:13–24. doi:10.1016/j.jconrel.2018.02.042
99. Liang M, Fan K, Zhou M, et al. H-ferritin-nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc Natl Acad Sci U S A. 2014;111(41):14900–14905. doi:10.1073/pnas.1407808111
100. Fan K, Jia X, Zhou M, et al. Ferritin Nanocarrier Traverses the Blood Brain Barrier and Kills Glioma. ACS Nano. 2018;12(5):4105–4115. doi:10.1021/acsnano.7b06969
101. Pandolfi L, Bellini M, Vanna R, et al. H-Ferritin Enriches the Curcumin Uptake and Improves the Therapeutic Efficacy in Triple Negative Breast Cancer Cells. Biomacromolecules. 2017;18(10):3318–3330. doi:10.1021/acs.biomac.7b00974
102. Ma Y, Li R, Dong Y, et al. tLyP-1 Peptide Functionalized Human H Chain Ferritin for Targeted Delivery of Paclitaxel. Int J Nanomed. 2021;16:789. doi:10.2147/IJN.S289005
103. Yadav S, Sharma AK, Kumar P. Nanoscale Self-Assembly for Therapeutic Delivery. Front Bioeng Biotechnol. 2020;8:127. doi:10.3389/fbioe.2020.00127
104. Mendes AC, Baran ET, Reis RL, Azevedo HS. Self-assembly in nature: using the principles of nature to create complex nanobiomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(6):582–612. doi:10.1002/wnan.1238
105. Ghadiri MR, Granja JR, Milligan RA, McRee DE, Khazanovich N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature. 1993;366(6453):324–327. doi:10.1038/366324a0
106. Zhang S, Holmes T, Lockshin C, Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc Natl Acad Sci U S A. 1993;90(8):3334–3338. doi:10.1073/pnas.90.8.3334
107. Das RP, Singh BG, Kunwar A. Preparation of a size selective nanocomposite through temperature assisted co-assembly of gelatin and pluronic F127 for passive targeting of doxorubicin. Biomater Sci. 2020;8(15):4251–4265. doi:10.1039/D0BM00725K
108. Qiao L, Yang H, Gao S, Li L, Fu X, Wei Q. Research progress on self-assembled nanodrug delivery systems. J Mater Chem B. 2022;10(12):1908–1922. doi:10.1039/D1TB02470A
109. Jiang Y, Wang X, Liu X, et al. Enhanced Antiglioma Efficacy of Ultrahigh Loading Capacity Paclitaxel Prodrug Conjugate Self-Assembled Targeted Nanoparticles. ACS Appl Mater Interfaces. 2017;9(1):211–217. doi:10.1021/acsami.6b13805
110. Karaosmanoglu S, Zhou M, Shi B, Zhang X, Williams GR, Chen X. Carrier-free nanodrugs for safe and effective cancer treatment. J Control Release off J Control Release Soc. 2021;329:805–832. doi:10.1016/j.jconrel.2020.10.014
111. Nguyen HN, Ullevig SL, Short JD, Wang L, Ahn YJ, Asmis R. Ursolic Acid and Related Analogues: triterpenoids with Broad Health Benefits. Antioxid Basel Switz. 2021;10(8):1161. doi:10.3390/antiox10081161
112. Khwaza V, Oyedeji OO, Aderibigbe BA. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: an Update. Int J Mol Sci. 2020;21(16):5920. doi:10.3390/ijms21165920
113. Chen X, Chen J, Li B, et al. PLGA-PEG-PLGA triblock copolymeric micelles as oral drug delivery system: in vitro drug release and in vivo pharmacokinetics assessment. J Colloid Interface Sci. 2017;490:542–552. doi:10.1016/j.jcis.2016.11.089
114. Prasad S, Yadav VR, Sung B, et al. Ursolic acid inhibits growth and metastasis of human colorectal cancer in an orthotopic nude mouse model by targeting multiple cell signaling pathways: chemosensitization with capecitabine. Clin Cancer Res off J Am Assoc Cancer Res. 2012;18(18):4942–4953. doi:10.1158/1078-0432.CCR-11-2805
115. Fan L, Zhang B, Xu A, et al. Carrier-Free, Pure Nanodrug Formed by the Self-Assembly of an Anticancer Drug for Cancer Immune Therapy. Mol Pharm. 2018;15(6):2466–2478. doi:10.1021/acs.molpharmaceut.8b00444
116. Zheng J, Fan R, Wu H, et al. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nat Commun. 2020;11(1):3815. doi:10.1038/s41467-020-17712-5
117. Li J, Kuang Y, Gao Y, Du X, Shi J, Xu B. D-amino acids boost the selectivity and confer supramolecular hydrogels of a nonsteroidal anti-inflammatory drug (NSAID). J Am Chem Soc. 2013;135(2):542–545. doi:10.1021/ja310019x
118. Pappas CG, Shafi R, Sasselli IR, et al. Dynamic peptide libraries for the discovery of supramolecular nanomaterials. Nat Nanotechnol. 2016;11(11):960–967. doi:10.1038/nnano.2016.169
119. Brown TE, Anseth KS. Spatiotemporal hydrogel biomaterials for regenerative medicine. Chem Soc Rev. 2017;46(21):6532–6552. doi:10.1039/C7CS00445A
120. Luo Q, Jin Q, Su C, et al. Radiolabeled Rhein as Small-Molecule Necrosis Avid Agents for Imaging of Necrotic Myocardium. Anal Chem. 2017;89(2):1260–1266. doi:10.1021/acs.analchem.6b03959
121. Bian L, Gao M, Zhang D, et al. Synthesis and Biological Evaluation of Rhein-Based MRI Contrast Agents for in Vivo Visualization of Necrosis. Anal Chem. 2018;90(22):13249–13256. doi:10.1021/acs.analchem.8b01868
122. Liu J, Hu G, Xu R, et al. Rhein lysinate decreases the generation of β-amyloid in the brain tissues of Alzheimer’s disease model mice by inhibiting inflammatory response and oxidative stress. J Asian Nat Prod Res. 2013;15(7):756–763. doi:10.1080/10286020.2013.800972
123. Wang A, Jiang H, Liu Y, et al. Rhein induces liver cancer cells apoptosis via activating ROS-dependent JNK/Jun/caspase-3 signaling pathway. J Cancer. 2020;11(2):500–507. doi:10.7150/jca.30381
124. Jing KL, Zhen GG, Shen Y, Wu ZJ, fan RP. Encapsulation of Aconitine in Self-Assembled Licorice Protein Nanoparticles Reduces the Toxicity In Vivo. Nanoscale Res Lett. 2015;10(1):1–8. doi:10.1186/1556-276X-10-1
125. Wang J, Zhao H, Zhi K, Yang X. Exploration of the Natural Active Small-Molecule Drug-Loading Process and Highly Efficient Synergistic Antitumor Efficacy. ACS Appl Mater Interfaces. 2020;12(6):6827–6839. doi:10.1021/acsami.9b18443
126. Tian X, Wang P, Li T, et al. Self-assembled natural phytochemicals for synergistically antibacterial application from the enlightenment of traditional Chinese medicine combination. Acta Pharm Sin B. 2020;10(9):1784–1795. doi:10.1016/j.apsb.2019.12.014
127. Chen S, Chen Z, Wang Y, et al. Targeted delivery of Chinese herb pair-based berberine/tannin acid self-assemblies for the treatment of ulcerative colitis. J Adv Res. 2022;40:263–276. doi:10.1016/j.jare.2021.11.017
128. Singh N, Kumar M, Miravet JF, Ulijn RV, Escuder B. Peptide-Based Molecular Hydrogels as Supramolecular Protein Mimics. Chem Weinh Bergstr Ger. 2017;23(5):981–993.
129. Habibi N, Kamaly N, Memic A, Shafiee H. Self-assembled peptide-based nanostructures: smart nanomaterials toward targeted drug delivery. Nano Today. 2016;11(1):41–60. doi:10.1016/j.nantod.2016.02.004
130. Dehsorkhi A, Castelletto V, Hamley IW. Self-assembling amphiphilic peptides. J Pept Sci off Publ Eur Pept Soc. 2014;20(7):453–467.
131. Zhang L, Huang Y, Lindstrom AR, Lin TY, Lam KS, Li Y. Peptide-based materials for cancer immunotherapy. Theranostics. 2019;9(25):7807–7825. doi:10.7150/thno.37194
132. Eskandari S, Guerin T, Toth I, Stephenson RJ. Recent advances in self-assembled peptides: implications for targeted drug delivery and vaccine engineering. Adv Drug Deliv Rev. 2017;110-111:169–187. doi:10.1016/j.addr.2016.06.013
133. Wang Q, Jiang N, Fu B, Huang F, Liu J. Self-assembling peptide-based nanodrug delivery systems. Biomater Sci. 2019;7(12):4888–4911. doi:10.1039/C9BM01212E
134. Sis MJ, Webber MJ. Drug Delivery with Designed Peptide Assemblies. Trends Pharmacol Sci. 2019;40(10):747–762. doi:10.1016/j.tips.2019.08.003
135. Hsieh WH, Liaw J. Applications of cyclic peptide nanotubes (cPNTs). J Food Drug Anal. 2019;27(1):32–47. doi:10.1016/j.jfda.2018.09.004
136. Park JH, Kim HA, Park JH, Lee M. Amphiphilic peptide carrier for the combined delivery of curcumin and plasmid DNA into the lungs. Biomaterials. 2012;33(27):6542–6550. doi:10.1016/j.biomaterials.2012.05.046
137. Fu Y, Li B, Huang Z, Li Y, Yang Y. Terminal is important for the helicity of the self-assemblies of dipeptides derived from alanine. Langmuir ACS J Surf Colloids. 2013;29(20):6013–6017. doi:10.1021/la400910g
138. Tomasini C, Castellucci N. Peptides and peptidomimetics that behave as low molecular weight gelators. Chem Soc Rev. 2013;42(1):156–172. doi:10.1039/C2CS35284B
139. Zhang L, Wang X, Wang T, Liu M. Tuning soft nanostructures in self-assembled supramolecular gels: from morphology control to morphology-dependent functions. Small Weinh Bergstr Ger. 2015;11(9–10):64.
140. Rivas M, Del Valle LJ, Alemán C, Puiggalí J. Peptide Self-Assembly into Hydrogels for Biomedical Applications Related to Hydroxyapatite. Gels. 2019;5(1):14. doi:10.3390/gels5010014
141. Raza F, Zhu Y, Chen L, et al. Paclitaxel-loaded pH responsive hydrogel based on self-assembled peptides for tumor targeting. Biomater Sci. 2019;7(5):2023–2036. doi:10.1039/C9BM00139E
142. Wei W, Meng C, Wang Y, et al. The interaction between self - assembling peptides and emodin and the controlled release of emodin from in-situ hydrogel. Cells Nanomed Biotechnol. 2019;47(1):3961–3975. doi:10.1080/21691401.2019.1673768
143. Zhang X, Parekh G, Guo B, et al. Polyphenol and self-assembly: metal polyphenol nanonetwork for drug delivery and pharmaceutical applications. Future Drug Discov. 2019;1(1):FDD7. doi:10.4155/fdd-2019-0001
144. Zhang X, Li Z, Yang P, et al. Polyphenol scaffolds in tissue engineering. Mater Horiz. 2021;8(1):145–167. doi:10.1039/D0MH01317J
145. Z J, L Z, J Y, Ma R, Jj R, C F. Polyphenol-Mediated Assembly for Particle Engineering. Acc Chem Res. 2020;53(7):45.
146. Li Q, Dong Z, Chen M, Feng L. Phenolic molecules constructed nanomedicine for innovative cancer treatment. Coord Chem Rev. 2021;439:213912. doi:10.1016/j.ccr.2021.213912
147. Feng W, Shi W, Liu S, et al. Fe(III)-Shikonin Supramolecular Nanomedicine for Combined Therapy of Tumor via Ferroptosis and Necroptosis. Adv Healthc Mater. 2022;11(2):90.
148. Wen Y, Hu J, Liu J, Li M. Degradable Carrier-Free Metal–Phenolic Network Theranostic Agent with Targeted Mitochondrial Damage for Efficient Cancer Theranostics. Chem Mater. 2021;33(17):7089–7099. doi:10.1021/acs.chemmater.1c02267
149. Bertleff-Zieschang N, Arifur Rahim M, Ju Y, et al. Biofunctional metal–phenolic films from dietary flavonoids. Chem Commun. 2017;53(6):1068–1071. doi:10.1039/C6CC08607A
150. Li B, Shao H, Gao L, Li H, Sheng H, Zhu L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: a review. Drug Deliv. 2022;29(1):2130–2161. doi:10.1080/10717544.2022.2094498
151. Wang M, Fu Y, Chen G, et al. Fabrication and characterization of carboxymethyl chitosan and tea polyphenols coating on zein nanoparticles to encapsulate β-carotene by anti-solvent precipitation method. Food Hydrocoll. 2018;77:577–587. doi:10.1016/j.foodhyd.2017.10.036
152. Shi F, Zhao JH, Liu Y, Wang Z, Zhang YT, Feng NP. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int J Nanomed. 2012;7:2033–2043. doi:10.2147/IJN.S30085
153. Wu Y, Lv S, Li Y, et al. Co-delivery of dual chemo-drugs with precisely controlled, high drug loading polymeric micelles for synergistic anti-cancer therapy. Biomater Sci. 2020;8(3):949–959. doi:10.1039/C9BM01662G
154. Fumoto S, Nishida K. Co-delivery Systems of Multiple Drugs Using Nanotechnology for Future Cancer Therapy. Chem Pharm Bull (Tokyo). 2020;68(7):603–612. doi:10.1248/cpb.c20-00008
155. Al Bostami RD, Abuwatfa WH, Husseini GA. Recent Advances in Nanoparticle-Based Co-Delivery Systems for Cancer Therapy. Nanomater Basel Switz. 2022;12(15):2672. doi:10.3390/nano12152672
156. Tagde P, Najda A, Nagpal K, et al. Nanomedicine-Based Delivery Strategies for Breast Cancer Treatment and Management. Int J Mol Sci. 2022;23(5):2856. doi:10.3390/ijms23052856
157. Scioli Montoto S, Muraca G, Ruiz ME. Solid Lipid Nanoparticles for Drug Delivery: pharmacological and Biopharmaceutical Aspects. Front Mol Biosci. 2020;7:587997. doi:10.3389/fmolb.2020.587997
158. Rahman HS, Othman HH, Hammadi NI, et al. Novel Drug Delivery Systems for Loading of Natural Plant Extracts and Their Biomedical Applications. Int J Nanomed. 2020;15:2439–2483. doi:10.2147/IJN.S227805
159. Luo CF, Yuan M, Chen MS, et al. Pharmacokinetics, tissue distribution and relative bioavailability of puerarin solid lipid nanoparticles following oral administration. Int J Pharm. 2011;410(1–2):138–144. doi:10.1016/j.ijpharm.2011.02.064
160. Rostami E, Kashanian S, Azandaryani AH, Faramarzi H, Dolatabadi JEN, Omidfar K. Drug targeting using solid lipid nanoparticles. Chem Phys Lipids. 2014;181:56–61. doi:10.1016/j.chemphyslip.2014.03.006
161. Bayón-Cordero L, Alkorta I, Arana L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomater Basel Switz. 2019;9(3):474. doi:10.3390/nano9030474
162. Fathy Abd-Ellatef GE, Gazzano E, Chirio D, et al. Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells. Pharmaceutics. 2020;12(2):96. doi:10.3390/pharmaceutics12020096
163. Harish V, Tewari D, Mohd S, et al. Quality by Design Based Formulation of Xanthohumol Loaded Solid Lipid Nanoparticles with Improved Bioavailability and Anticancer Effect against PC-3 Cells. Pharmaceutics. 2022;14(11):2403. doi:10.3390/pharmaceutics14112403
164. Guo J, Xing X, Lv N, et al. Therapy for myocardial infarction: in vitro and in vivo evaluation of puerarin-prodrug and tanshinone co-loaded lipid nanoparticulate system. Biomed Pharmacother. 2019;120:109480. doi:10.1016/j.biopha.2019.109480
165. Wang L, Wang X, Shen L, et al. Paclitaxel and naringenin-loaded solid lipid nanoparticles surface modified with cyclic peptides with improved tumor targeting ability in glioblastoma multiforme. Biomed Pharmacother Biomedecine Pharmacother. 2021;138:111461. doi:10.1016/j.biopha.2021.111461
166. Stiepel RT, Duggan E, Batty CJ, Ainslie KM. Micro and nanotechnologies: the little formulations that could. Bioeng Transl Med. 2023;8(2):e10421. doi:10.1002/btm2.10421
167. Kumar G, Virmani T, Sharma A, Pathak K. Codelivery of Phytochemicals with Conventional Anticancer Drugs in Form of Nanocarriers. Pharmaceutics. 2023;15(3):889. doi:10.3390/pharmaceutics15030889
168. Taléns-Visconti R, Díez-Sales O, De julián-ortiz JV, Nácher A. Nanoliposomes in Cancer Therapy: marketed Products and Current Clinical Trials. Int J Mol Sci. 2022;23(8):4249. doi:10.3390/ijms23084249
169. Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci off J Eur Fed Pharm Sci. 2013;48(3):416–427.
170. Kumar P, Huo P, Liu B. Formulation Strategies for Folate-Targeted Liposomes and Their Biomedical Applications. Pharmaceutics. 2019;11(8):381. doi:10.3390/pharmaceutics11080381
171. Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022;8(5):e09394. doi:10.1016/j.heliyon.2022.e09394
172. Li Z, Zhang Y, Zhu C, et al. Folic acid modified lipid-bilayer coated mesoporous silica nanoparticles co-loading paclitaxel and tanshinone IIA for the treatment of acute promyelocytic leukemia. Int J Pharm. 2020;586:119576. doi:10.1016/j.ijpharm.2020.119576
173. Zhu Y, Wang A, Zhang S, et al. Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells. J Adv Res. 2023;49:159–173. doi:10.1016/j.jare.2022.09.007
174. Perumal S. Polymer Nanoparticles: synthesis and Applications. Polymers. 2022;14(24):5449. doi:10.3390/polym14245449
175. Amgoth C, Phan C, Banavoth M, et al. Polymer Properties: functionalization and Surface Modified Nanoparticles. Role Novel Drug Delivery Vehicles Nanobiomed IntechOpen. 2019.
176. Zielińska A, Carreiró F, Oliveira AM, et al. Polymeric Nanoparticles: production, Characterization. Toxicol Ecotoxicol Mol Basel Switz. 2020;25(16):3731.
177. Truong NP, Whittaker MR, Mak CW, Davis TP. The importance of nanoparticle shape in cancer drug delivery. Expert Opin Drug Deliv. 2015;12(1):129–142. doi:10.1517/17425247.2014.950564
178. Khoshravan Azar L, Dadashpour M, Hashemi M, Zarghami N. Design and Development of Nanostructured Co Delivery of Artemisinin and Chrysin for Targeting hTERT Gene Expression in Breast Cancer Cell Line: possible Clinical Application in Cancer Treatment. Asian Pac J Cancer Prev APJCP. 2022;23(3):919–927. doi:10.31557/APJCP.2022.23.3.919
179. Afsharzadeh M, Hashemi M, Mokhtarzadeh A, Abnous K, Ramezani M. Recent advances in co-delivery systems based on polymeric nanoparticle for cancer treatment. Cells Nanomed Biotechnol. 2018;46(6):1095–1110. doi:10.1080/21691401.2017.1376675
180. Alven S, Aderibigbe BA. Efficacy of Polymer-Based Nanocarriers for Co-Delivery of Curcumin and Selected Anticancer Drugs. Nanomater Basel Switz. 2020;10(8):1556. doi:10.3390/nano10081556
181. Yu G, Ning Q, Mo Z, Tang S. Intelligent polymeric micelles for multidrug co-delivery and cancer therapy. Cells Nanomed Biotechnol. 2019;47(1):1476–1487. doi:10.1080/21691401.2019.1601104
182. Kar A, Rout SR, Singh V, et al. Chapter 22 - Triblock polymeric micelles as an emerging nanocarrier for drug delivery. In: Kesharwani P, Greish K, editors. Polymeric Micelles for Drug Delivery. Woodhead Publishing Series in Biomaterials. Woodhead Publishing; 2022. 561–590.
183. Perumal S, Atchudan R, Lee W. A Review of Polymeric Micelles and Their Applications. Polymers. 2022;14(12):2510. doi:10.3390/polym14122510
184. Zhou L, Wu J, Sun Z, Wang W. Oxidation and Reduction Dual-Responsive Polymeric Prodrug Micelles Co-delivery Precisely Prescribed Paclitaxel and Honokiol for Laryngeal Carcinoma Combination Therapy. Front Pharmacol. 2022;13:934632. doi:10.3389/fphar.2022.934632
185. Maier-Hauff K, Ulrich F, Nestler D, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103(2):317–324. doi:10.1007/s11060-010-0389-0
186. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi:10.1038/s41573-020-0090-8
187. Sharma A, Goyal AK, Rath G. Recent advances in metal nanoparticles in cancer therapy. J Drug Target. 2018;26(8):617–632. doi:10.1080/1061186X.2017.1400553
188. Xu JJ, Zhang WC, Guo YW, Chen XY, Zhang YN. Metal nanoparticles as a promising technology in targeted cancer treatment. Drug Deliv. 2022;29(1):664–678. doi:10.1080/10717544.2022.2039804
189. Canese R, Vurro F, Marzola P. Iron Oxide Nanoparticles as Theranostic Agents in Cancer Immunotherapy. Nanomater Basel Switz. 2021;11(8):1950. doi:10.3390/nano11081950
190. Hiremath CG, Heggnnavar GB, Kariduraganavar MY, Hiremath MB. Co-delivery of paclitaxel and curcumin to foliate positive cancer cells using Pluronic-coated iron oxide nanoparticles. Prog Biomater. 2019;8(3):155–168. doi:10.1007/s40204-019-0118-5
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

