Back to Journals » Clinical Interventions in Aging » Volume 9
N-palmitoylethanolamine and N-acetylethanolamine are effective in asteatotic eczema: results of a randomized, double-blind, controlled study in 60 patients
Authors Yuan C, Wang X, Guichard A, Tan Y, Qian C, Yang L, Humbert P
Received 3 April 2014
Accepted for publication 17 June 2014
Published 17 July 2014 Volume 2014:9 Pages 1163—1169
DOI https://doi.org/10.2147/CIA.S65448
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Chao Yuan,1,* Xue-Min Wang,1,* Alexandre Guichard,2 Yi-Mei Tan,1 Chun-Yan Qian,1 Li-Jie Yang,1 Philippe Humbert2
1Department of Skin and Cosmetic Research, Shanghai Skin Disease Hospital, Shanghai, People’s Republic of China; 2Research and Studies Center on the Integument, Department of Dermatology, Besançon University Hospital, University of Franche-Comté, Franche-Comté, France
*These authors contributed equally to this work
Background: Asteatotic eczema (AE) is characterized by itchy, dry, rough, and scaling skin. The treatments for AE are mainly emollients, usually containing urea, lactic acid, or a lactate salt. N-palmitoylethanolamine (PEA) and N-acetylethanolamine (AEA) are both endogenous lipids used as novel therapeutic tools in the treatment of many skin diseases. The purpose of this study was to compare a PEA/AEA emollient with a traditional emollient in the treatment of AE.
Methods: A monocentric, randomized, double-blind, comparative trial was conducted in 60 AE patients to evaluate and compare the efficacy of the two emollients. The level of skin dryness among the subjects ranged from mild to moderate. The subjects’ skin barrier function and the current perception threshold were tested for 28 days by clinical scoring and bioengineering technology.
Results: The results showed that, although some aspects were improved in both groups, the group using the emollient containing PEA/AEA presented a better skin surface change in capacitance. However, the most impressive finding was the ability of the PEA/AEA emollient to increase the 5 Hz current perception threshold to a normal level after 7 days, with a significant difference between values at baseline and after 14 days. A current perception threshold of 5 Hz was positively and significantly correlated with skin surface hydration and negatively correlated with transepidermal water loss in the PEA/AEA emollient group.
Conclusion: Compared with traditional emollients, regular application of a topical PEA/AEA emollient could improve both passive and active skin functions simultaneously.
Keywords: N-palmitoylethanolamine, N-acetylethanolamine, current perception threshold, skin barrier, pruritus, asteatotic eczema
Introduction
Asteatotic eczema (AE) is characterized by itchy, dry, rough, and scaling skin, and is often aggravated during the dry winter season as a result of the interaction between environmental agents such as soap and other detergents,1 especially for the elderly. Some researchers suggest that the itchy sensation can be persistent in its effects.2 The itchy sensation is produced via a complex process that involves stimulation of free nerve endings very close to the surface of the skin. The sensation is transmitted through the C-fibers in the skin to the dorsal horn of the spinal cord, and finally to the cerebral cortex for processing via the spinothalamic tract.3 An increasing number of studies have shown that daily use of emollients is a vital part of the management of patients with dry skin conditions.4,5
Endogenous phospholipids are universal among mammalian organisms. Among them, phospholipids like N-palmitoylethanolamine (PEA) and N-acetylethanolamine (AEA), which both belong to the functional endocannabinoid system, are present in high amounts in the stratum granulosum of the skin.6 A number of researchers have shown in recent years that the endocannabinoid system could play a role in providing substantial relief for objective and subjective symptoms of some skin diseases, including atopic eczema,7,8 facial postherpetic neuralgia,9 and contact allergic dermatitis.10 It has also been documented that the endocannabinoid system has the ability to prevent skin damage as a result of exposure to ultraviolet rays.11 Its main physiological function is to constitutively control and balance the proliferation, differentiation, and survival of skin cells.12
Considering that the endocannabinoid system could be used as a novel therapeutic tool to remedy dry skin by enhancing lipid production in the stratum granulosum, the scope of this clinical trial was to evaluate the advantages of using an emollient containing PEA/AEA over a traditional emollient. The neuroselective transcutaneous electrical stimulator, Neurometer® CPT (Neurotron Inc., Baltimore, MD, USA), is a useful tool that was recently created to assess the threshold for itch sensation noninvasively.13 To investigate the differences between the PEA/AEA emollient and a traditional emollient, we measured and compared the difference in skin barrier function and current perception threshold (CPT) in AE patients.
Materials and methods
Participants
This monocentric, prospective, double-blind, randomized study enrolled 66 participants with mild to moderate AE on the lower leg, which is the predilection site for AE. Additionally, AE was determined for each subject by clinical scoring, with scores ranging from mild to moderate erythema, scaling, or dryness. Individuals with active psoriasis or a history of psoriasis, active allergic skin responses, or severe eczema were excluded. Subjects treated for any type of cancer within the last 6 months and those who had used anti-inflammatory, immunosuppressive, or antihistamine medications were also excluded. In total, six subjects withdrew or dropped out of the study. The 60 subjects who completed the trial were evaluated for efficacy parameters. They were all female, with a mean age of 51.17±10.28 years. Each subject signed their informed consent before entering the study, which was conducted strictly in accordance with the instructions laid down by the ethics committee of the Shanghai Skin Disease Hospital (No. 2012-011) and carried out between October 23 and November 20, 2012.
Test materials
For the test, we used an emollient PEA/AEA cream, hereafter referred to as product P, manufactured by Stiefel Laboratories (Ireland) Ltd (Sligo, Ireland), containing purified water, Olea europaea, glycerol, pentylene glycol, palm glycerides, olus, hydrogenated lecithin, squalane, 0.3% PEA, 0.21% AEA, acetamide, monoethanolamine, betaine, sarcosine, hydroxyethylcellulose, sodium carbomer, carbomer, and xanthan gum. The control product used was an emollient devoid of PEA/AEA (thereafter referred to as product A, manufactured by Laboratoires Pierre Fabre, Aignan, France) containing purified water, liquid paraffin, cyclomethicone, butylene glycol, glycerin, glyceryl stearate, squalane, carbomer, triethanolamine, phenoxyethanol, tetrasodium ethylenediaminetetraacetic acid, and benzoic acid. For each subject, according to randomization, only one type of product was used to treat the AE lesion on both legs at a dose of 2 mg/cm2. Each subject was exposed to the product twice a day for 28 days. During the study, we asked the subjects to visit the laboratory where the product would then be applied for the first time in the morning. For the second application in the evening, they were instructed to self-apply the emollient at home.
Clinical scoring
This comparative study was controlled, randomized, and double-blind. Clinical assessments were performed using the Eczema Area and Severity Index,8 which is widely used to assess atopic dermatitis. Two dermatologists assessed the lesion parameters, ie, erythema, skin integrity, scaling, dryness, and itching scores, at the same time. Dermatological assessments were performed on day 1 (before using the product), and on days 3, 7, 14, and 28 thereafter. For this study, we chose patients with mild to moderate erythematous, scaling, or dry skin, with a total score from 0 to 30. The grading details are shown in Table 1.
Stratum corneum function and electrical CPT
The barrier function of the stratum corneum was assessed by measuring both skin surface hydration and transepidermal water loss (TEWL) on each subject’s lower front leg on days 1, 3, 7, 14, and 28. Skin surface hydration was evaluated by capacitance using the Corneometer CM820® (Courage and Khazaka Electronic GmbH, Köln, Germany). TEWL was measured by detecting the evaporated water with a TM210® device (Courage and Khazaka Electronic GmbH). We measured surface hydration and TEWL on the same skin area at each visit.
A 5 Hz CPT has been reported to be able to quantify the sensory threshold of C-fibers, believed to transmit sensory information from the skin, which is then perceived as itch.14,15 The CPT of the sensory nerves was tested using the Neurometer. CPT was performed in a sitting position on the first toe of the foot. The electrical current was delivered to the skin using a pair of 1 cm diameter gold surface electrodes covered by a thin layer of electroconductive gel. The neurometer delivered a 5 Hz alternating sinusoid waveform current, and its intensity was increased until the participant perceived any sensation such as pain, itch, or painful itch. A transcutaneous electric current stimulus at 5 Hz can stimulate C-fibers.16 CPT of the first toe is a reliable site to assess pruritus of the lower leg because it contains the peroneal nerve.
Statistical analysis
Statistical Package for the Social Sciences version 13.0 software (SPSS Inc., Chicago, IL, USA) was used for calculation of statistical data, which were expressed as the mean ± standard deviation and assessed for statistical significance. We used the Student’s t-test to compare skin surface hydration, TEWL, and CPT. We used Pearson’s correlation coefficient and conducted a linear regression analysis to determine correlations between skin surface hydration and CPT. For most of the tests, a value of P<0.05 was considered to be statistically significant (a value of P<0.10 was considered to be statistically significant for CPT).
Results
Clinical evaluation
The lower leg was evaluated for each parameter prior to testing (on day 1), and on days 3, 7, 14, and 28 thereafter. The details for clinical assessment of erythema, skin integrity, scaling, dryness, and itching are shown in Table 2. When the results are compared with baseline, they show that both products were highly capable of decreasing skin erythema, scaling, dryness, and itching, while also improving skin integrity. However, the results for day 28 show that product P was better at improving skin scaling and dryness, and also better at reducing itching (P<0.05). The recovery rate (RR) was calculated by the formula: RRx = (Dx − D0)/D0*100% (D, day). Table 3 shows the clinical evaluation per parameter and the mean RR for each group at each visit. No subject had any adverse event or severe adverse event during 28 days of use of either product.
Skin barrier function
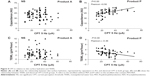
After application of the emollients, the skin surface hydration was increased in both groups. There were significant differences on day 1 and days 3, 7, 14, and 28 in both groups (P<0.05). Comparing the change in capacitance of the skin surface (minus the baseline) between the two groups, product P was better than product A in treating AE (Figure 1).
The TEWL values were decreased in both groups after application of emollient. There was a significant difference between baseline values for the two groups and the values on days 3, 7, 14, and 28 (P<0.05). However, we found no statistically significant difference between the two products (P>0.05) with regard to change in TEWL (Figure 2).
Current perception threshold
There was no significant difference in CPT between the two groups on days 1, 3, 7, and 28. However, on day 14, a higher CPT was recorded for product P (P<0.10). In a pilot study carried out on the skin of the toes of healthy patients, the mean 5 Hz CPT was 33.82±16.57 μA (72 female and five male subjects, mean age 51.3±10.0 years). Figure 3 shows the mean CPT in each group of the clinical trial at each visit. On baseline and day 3, the mean 5 Hz CPT was less than 33.82 μA in each group. However, on days 7 and 14, the mean 5 Hz CPT in the product P group was considerably increased and higher than 33.82 μA, and was also higher than 33.82 μA on day 28. In contrast, the mean 5 Hz CPT in the product A group was no higher than 33.82 μA, ie, below the normal level, until day 28.
The results of the analysis of CPT in relation to skin surface hydration for the product P group indicate that there was a high degree of correlation between CPT and skin surface hydration (Pearson ratio 0.5, P<0.01). This suggests that early treatment has a moisturizing effect on the stratum corneum layer that helps to alleviate skin itching. While these characteristics were present with application of product P, the same results were not found with product A (Pearson ratio −0.05, P>0.10). When CPT was analyzed in relation to TEWL, it was observed that TEWL for the product P group was inversely correlated with CPT (Pearson ratio −0.36, P<0.01). This result suggests that in healthy skin the skin barrier contributes to its resistance to external stimuli and this resistance is expressed as itching and pain. Figure 4 shows these relationships.
Discussion
In recent years, more evidence has emerged suggesting that the skin and its appendages are active neuroimmunoendocrine organs.17 Skin has both a “passive” function (as a physicochemical barrier) and an “active” function (as a neuroimmunoendocrine barrier). The use of traditional emollients over a long time would help to restore the lipid lamellae, improve skin hydration and elasticity, and support epidermal differentiation.18 These elements are all skin physicochemical barrier functions.
The endocannabinoid system is believed to be actively involved in endogenous protective mechanisms in skin barrier function; application of PEA/AEA could augment local production of lipids and inhibit their degradation (via fatty acid amide hydrolase) in the stratum granulosum, and could therefore serve as another therapeutic method for combating dry skin and reducing itching. PEA could markedly augment the effect of AEA at cannabinoid receptor type 1 and/or 2, as well as directly activate peroxisome proliferator-activated receptor alpha constitutively, and simultaneously control both “passive” and “active” skin functions, including regeneration of skin and restoration of lipid lamellae, skin sensation, and immune competence.19–21 Activation of cannabinoid receptor type 2 in the sebaceous glands by AEA could markedly enhance lipid synthesis. The literature indicates that the endocannabinoid system is a good treatment for uremic pruritus. It was not only the result of dry skin improvement but also addition of endocannabinoids that may have played a role in affecting the sensation nerves.22 In a model of passive immunoglobulin E-induced cutaneous anaphylaxis, researchers found that PEA and AEA are both bioactive signaling lipids capable of downregulating inflammation in the skin.23 In the immune system, PEA could downmodulate activation of skin mast cells and inhibit release of histamine, prostaglandin D2, and tumor necrosis factor alpha.24
Compared with the traditional emollient, the main physiological function of the cutaneous endocannabinoid system is to constitutively control and balance the proliferation, differentiation, and survival of immune cells and tolerance of skin cells (Figure 5).
AE is characterized by itchy, dry, rough, and scaling skin. With aging, the activity of the sebaceous and sweat glands decreases, and the elderly are at increased risk of AE.25 There is evidence that AE is associated with a defect in skin barrier function, and this dysfunction results in increased permeability, ultimately causing inflammation and pruritus.26 The rational treatment of AE is the use of a complete emollient therapy. The aim of this study was to determine whether an emollient cream containing PEA/AEA is more efficient than a traditional emollient in treating AE. The results show that both emollient creams could restore the skin barrier function, improve skin surface hydration, and decrease TEWL values, but the emollient containing PEA/AEA performed better than its traditional counterpart in terms of change in capacitance at the skin surface.
However, the most impressive finding was that application of PEA/AEA emollient could increase 5 Hz CPT to normal levels after 7 days, with a significant difference between baseline values and those after 14 days. This is the remarkable “active” function of PEA/AEA in rebuilding the skin neuroimmunoendocrine barrier. The commercially available neuroselective stimulator can provoke itch in healthy individuals and there are body area-specific differences in the itch perception induced by this device. C-fibers are the sensory nerves that transmit the sensation of pruritus. CPT decreases when hydration levels in the stratum corneum are low, suggesting that sensitivity to itching is amplified when the skin is dryer. When the emollient containing PEA/AEA was applied to the skin, there was an inverse relationship between CPT and TEWL values. In contrast, these relationships were absent in the group using the emollient without PEA/AEA, suggesting that even though the traditional emollient may be an acceptable substitute for maintaining healthy skin, it cannot be relied on to increase 5 Hz CPT values or alleviate itching in the short term.
Conclusion
This study shows that, compared with a traditional emollient, regular application of a topical PEA/AEA emollient in individuals with mild or moderate AE can significantly improve skin barrier function, increase 5 Hz CPT values, and reduce itching. Topical application of an emollient containing PEA/AEA is an effective tool that could guide prophylactic and therapeutic measures for AE, along with dry and itchy skin, in the elderly, in the future. However, this study was carried out only in female Shanghainese patients, and without checking their immunoglobulin E levels.
Acknowledgments
The authors thank all the subjects who participated in this study. We appreciate the concern and support provided by the staff of our department. Our deepest gratitude goes to Ms PL Wu and Ms J Li.
Disclosure
This research was supported by GlaxoSmithKline (China) Investment Co Ltd. The authors report no other conflicts of interest in this work.
References
Cork MJ, Danby S. Skin barrier breakdown: a renaissance in emollient therapy. Br J Nurs. 2009;18(14):872-874, 876-877. | ||
Kini SP, DeLong LK, Veledar E, McKenzie-Brown AM, Schaufele M, Chen SC. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147(10):1153-1156. | ||
Ikoma A, Cevikbas F, Kempkes C, Steinhoff M. Anatomy and neurophysiology of pruritus. Semin Cutan Med Surg. 2011;30(2):64-70. | ||
Lodén M. Effect of moisturizers on epidermal barrier function. Clin Dermatol. 2012;30(3):286-296. | ||
Chandar P, Nole G, Johnson AW. Understanding natural moisturizing mechanisms: implications for moisturizer technology. Cutis. 2009;84(1 Suppl):2-15. | ||
Lo Verme J, Fu J, Astarita G, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67(1):15-19. | ||
Kircik L. A nonsteroidal lamellar matrix cream containing palmitoylethanolamide for the treatment of atopic dermatitis. J Drugs Dermatol. 2010;9(4):334-338. | ||
Eberlein B, Eicke C, Reinhardt HW, Ring J. Adjuvant treatment of atopic eczema: assessment of an emollient containing N-palmitoylethanolamine (ATOPA study). J Eur Acad Dermatol Venereol. 2008;22(1):73-82. | ||
Phan NQ, Siepmann D, Gralow I, Ständer S. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8(2):88-91. | ||
Petrosino S, Cristino L, Karsak M, et al. Protective role of palmitoylethanolamide in contact allergic dermatitis. Allergy. 2010;65(6):698-711. | ||
Kemenv L, Koreck A, Kis K, et al. Endogenous phospholipid metabolite containing topical product inhibits ultraviolet light-induced inflammation and DNA damage in human skin. Skin Pharmacol Physiol. 2007; 20(3):155-161. | ||
Bíró T, Tóth BI, Haskó G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30(8):411-420. | ||
Ozawa M, Tsuchiyama K, Gomi R, Kurosaki F, Kawamoto Y, Aiba S. Neuroselective transcutaneous electric stimulation reveals body area-specific differences in itch perception. J Am Acad Dermatol. 2006;55(6): 996-1002. | ||
Kobayashi H, Kikuchi K, Tsubono Y, Tagami H. Measurement of electrical current perception threshold of sensory nerves for pruritus in atopic dermatitis patients and normal individuals with various degrees of mild damage to the stratum corneum. Dermatology. 2003;206(3): 204-211. | ||
Mori T, Ishida K, Mukumoto S, et al. Comparison of skin barrier function and sensory nerve electric current perception threshold between IgE-high extrinsic and IgE-normal intrinsic types of atopic dermatitis. Br J Dermatol. 2010;162(1):83-90. | ||
Koga K, Furue H, Rashid M, Takaki A, Katafuchi T, Yoshimura M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain. 2005;25:1-13. | ||
Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86(4):1309-1379. | ||
Proksch E, Lachapelle JM. The management of dry skin with topical emollients – recent perspectives. J Dtsch Dermatol Ges. 2005;3(10): 768-774. | ||
Lo Verme J, La Rana G, Russo R, Calignano A, Piomelli D. The search for the palmitoylethanolamide receptor. Life Sci. 2005;77(14): 1685-1698. | ||
Hesselink JM. Evolution in pharmacologic thinking around the natural analgesic palmitoylethanolamide: from nonspecific resistance to PPAR-α agonist and effective nutraceutical. J Pain Res. 2013;6: 625-634. | ||
Costa B, Comelli F, Bettoni I, Colleoni M, Giagnoni G. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB (1), TRPV1 and PPARgamma receptors and neurotrophic factors. Pain. 2008;139(3):541-550. | ||
Szepietowski JC, Szepietowski T, Reich A. Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: a preliminary study. Acta Dermatovenerol Croat. 2005;13(2):97-103. | ||
Dalle Carbonare M, Del Giudice E, Stecca A, et al. A saturated N-acylethanolamine other than N-palmitoyl ethanolamine with anti-inflammatory properties: a neglected story. J Neuroendocrinol. 2008; 20 Suppl 1:26-34. | ||
Cerrato S, Brazis P, della Valle MF, Miolo A, Puigdemont A. Effects of palmitoylethanolamide on immunologically induced histamine, PGD2 and TNFalpha release from canine skin mast cells. Vet Immunol Immunopathol. 2010;133(1):9-15. | ||
Norman RA. Xerosis and pruritus in the elderly: recognition and management. Dermatol Ther. 2003;16(3):254-259. | ||
Elias PM, Choi EH. Interactions among stratum corneum defensive functions. Exp Dermatol. 2005;14(10):719-726. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.