Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Myxedema Psychosis: Systematic Review and Pooled Analysis
Authors Mohamed MFH , Danjuma M , Mohammed M, Mohamed S, Siepmann M, Barlinn K, Suwileh S, Abdalla L, Al-Mohanadi D, Silva Godínez JC, Elzouki AN , Siepmann T
Received 4 May 2021
Accepted for publication 5 August 2021
Published 18 August 2021 Volume 2021:17 Pages 2713—2728
DOI https://doi.org/10.2147/NDT.S318651
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Mouhand FH Mohamed,1,2 Mohammed Danjuma,1,3 Mohammed Mohammed,4 Samreen Mohamed,4 Martin Siepmann,5 Kristian Barlinn,6 Salah Suwileh,1 Lina Abdalla,1 Dabia Al-Mohanadi,7 Juan Carlos Silva Godínez,8,9 Abdel-Naser Elzouki,1,3 Timo Siepmann2,6
1Department of Medicine, Hamad Medical Corporation, Doha, Qatar; 2Department of Health Care Sciences, Center for Clinical Research and Management Education, Dresden International University, Dresden, Germany; 3College of Medicine, Qatar University, Doha, Qatar; 4Department of Psychiatry, Hamad Medical Corporation, Doha, Qatar; 5Department of Psychotherapy and Psychosomatic Medicine, University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany; 6Department of Neurology, University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany; 7Department of Endocrinology, Hamad Medical Corporation, Doha, Qatar; 8Department of Surgery, National Medical Center Siglo XXI, Mexican Social Security Institute, Mexico City, Mexico; 9Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA
Correspondence: Mouhand FH Mohamed Email [email protected]
Background and Objective: The term myxedema psychosis (MP) was introduced to describe the occurrence of psychotic symptoms in patients with untreated hypothyroidism, but the optimal assessment and treatment of this condition are unclear. We aimed to synthesize data from the literature to characterize the clinical presentation and management of MP.
Methods: We performed a systematic review according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines in PubMed (Medline), Embase, Google Scholar, and Cochrane databases, including observational studies, case series, and case reports published from 1/1/1980 to 31/12/2019 in the English language. Descriptive statistics along with univariate and multivariate analysis were used for data synthesis.
Results: Out of 1583 articles screened, 71 case reports met our inclusion criteria providing data on 75 MP cases. The median age at diagnosis was 42 years [32– 56]. About 53% had no prior hypothyroidism diagnosis. Delusions occurred in 91%, with a predominance of persecutory ideas (84%), while hallucinations occurred in 78%. Physical symptoms and signs of hypothyroidism were absent in 37% and 26%, respectively. If symptoms occurred, nonspecific fatigue was seen most frequently (63%). The median thyroid-stimulating hormone value was 93 mIU/L [60– 139]. Thyroid peroxidase antibodies were found positive in 75% (23/33) of reported cases. Creatinine kinase was reported abnormal in seven cases. Cranial imaging (CT or MRI) and electroencephalogram were normal in 89%, 75%, and 73% of the cases reported. The majority of patients were treated orally with thyroxine in combination with short-term antipsychotics. More than 90% of them showed complete recovery. Univariate analysis revealed a trend towards a shorter duration of psychosis with IV thyroid hormone therapy (p= 0.0502), but the effect was not consistent in a multivariate analysis.
Conclusion: While we identified a substantial lack of published research on MP, our pooled analysis of case observations suggests that the condition presents a broad spectrum of psychiatric and physical symptoms lending support to the value of screening for thyroid dysfunction in patients with first-ever psychosis.
Prospero Registration Number: CRD42020160310.
Keywords: psychosis, hypothyroidism, madness, myxedema, depression, neuropsychiatric
Background
Hypothyroidism is a common disease with an estimated global prevalence of 0.1–3.6%.1–4 The Committee on Myxedema of the Clinical Society of London issued the first report that described the development of delusions and hallucinations in almost half of hypothyroid patients (109 patients).5 Sixty years later, in 1949, Asher et al reexamined this relationship in fourteen patients who had psychosis and clinical evidence of hypothyroidism. The patients received thyroid hormones supplements, with nine patients achieving full recovery.6 He labeled this association “myxedema madness,” which later was renamed “myxedema psychosis” (MP).6 MP is a secondary psychotic disorder resulting from other medical conditions according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).7
The underlying pathophysiology is poorly understood. Previous research linked hypothyroidism to changes in neurometabolic activity that might contribute to MP, including;8 tyrosine hydroxylase imbalance in the anterior locus coeruleus;9 abundance of T3 receptors in the amygdala and the hippocampus;10 altered serotonin-mediated neurotransmission11,12 and attenuation of cerebral regional blood flow and glucose metabolism.13,14
Diagnostic discrimination between MP and other secondary psychoses is clinically relevant as the management differs according to the exact etiology. An important differential diagnosis of psychosis in hypothyroidism patients is Hashimotos’ encephalopathy (HE), also called steroids responsive encephalopathy with autoimmune thyroiditis.15 While the pathophysiological mechanism underlying MP is related to brain neurochemical alterations accompanying thyroid hormones deficiency, neuropsychiatric changes in HE are caused by an autoimmune response not directly linked to hypothyroidism. This explains the excellent response to steroids in most HE cases.15
Seventy years have elapsed since Asher’s description, yet, little is known about MP, likely due to the paucity of available literature.8 Thus, we aimed to review the literature and synthesize data on its clinical symptomatology, diagnosis, management strategies, and clinical outcomes.
Methods
This systematic review complied with preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.16 The review protocol was registered at PROSPERO (registration number: CRD42020160310) and was published.17
Eligibility Criteria
Observational studies, case series, and case reports providing data on patients diagnosed with MP were eligible for inclusion. We only included reports on adult patients (18 years or older) with confirmed hypothyroidism (thyroid stimulating hormone> normal range plus low thyroid hormones, or clinical evidence of hypothyroidism) and psychotic features meeting the DSM-5 criteria of psychosis due to a general medical condition in whom myxedema psychosis was the likely diagnosis as per the treating physician. We excluded cases of Hashimoto’s encephalopathy (HE), thyroxine-induced mania, subclinical hypothyroidism, secondary hypothyroidism (due to possible confounding by other hormonal imbalances or mass effect), or cases with alternative diagnoses more likely than MP.
Search Strategy and Information Source
We conducted a comprehensive search in the following databases; PubMed, Medline, EMBASE, Google Scholar (first 300 hits), and Cochrane databases for studies published from 1/1/1980 to 31/12/2019. We included articles labeled “letter to the editor” if they were, in fact, case reports. We limited our search to articles written in the English language only. We used combinations of free text, keywords, Emtree, and MesH-terms, including psychosis, psycho, madness, psychiatric, hypothyroid, myxedema, myxoedema. Search strings used in each database are detailed in the supplement. (Supplementary 1) We also performed a snowball search in bibliographies of identified full‐text articles and relevant review articles.
Screening, Data Extraction, and Quality Assessment
Two independent reviewers (MFHM) and (SS) performed the literature search and screening. First, the titles and abstracts were screened. Subsequently, the full text of potentially eligible articles was reviewed and assessed for inclusion. At each step, the two reviewers discussed discrepancies noted, and if consensus could not be reached, a third reviewer (MD) settled the discrepancy per protocol. We used a web-based literature screening application (Rayvan; http://rayyan.qcri.org) to conduct article screening and duplicate removals.18
We extracted general data on included publications such as type, author, year, and journal as well as demographic data of the patients reported such as sex, age, gender, history of psychosis, history of hypothyroidism, and causes of hypothyroidism. Moreover, data on clinical presentation data was extracted, including psychiatric presentation, duration of psychosis, hypothyroidism symptoms or signs, associated rhabdomyolysis, cranial imaging finding; electroencephalography (EEG) findings; thyroid stimulating hormone level; thyroid hormone levels; anti-thyroid peroxidase status; creatinine kinase levels. We used the tool proposed by Murad et al to adjudicate the quality of included case reports and series.19 The tool comprises eight questions assessing four domains (selection, ascertainment, causality, and reporting). We generated an overall score, and we then graded the quality as either good (> 5), fair (4–5), or poor (< 3).
Statistical Analysis
We used the Jamovi 1.1.9 software for statistical analysis.20 Descriptive statistics were applied to summarize data using the median (IQR) for continuous variables and frequencies for categorical variables. We used (n/N) and percentage values for presenting numbers of cases with a specific characteristic amongst cases that reported either the presence or the absence of this characteristic. Acknowledging the subjective nature of reporting in case studies, the two reviewers had to agree on whether a specific characteristic was present in any given case before inclusion in the final analysis. Exploratory multivariate logistic regression including potentially clinically relevant variables (gender, age, symptoms duration, TSH level, FreeT4, antipsychotic drugs duration, IV thyroid hormone therapy, and starting thyroxine dose) associated with recovery (resolution of psychosis) or rapid recovery (less than 2 weeks) was also performed.
Results
The initial search retrieved 2733 articles; 50 additional articles were identified through other means, of which 71 references describing 75 cases were included for the final analysis.21–91 The PRISMA flow diagram is shown in Figure 1. All included studies were case reports due to the absence of other forms of evidence (Table 1). Quality assessment utilizing the methodological quality and synthesis of case series and case reports tool revealed fair to good quality of most of the included cases (Supplement 2).
 |  |  |  |  |  |
Table 1 Summary of Included Case Studies |
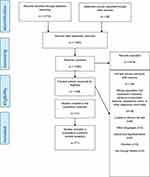 |
Figure 1 PRISMA flow diagram.Note: PRISMA figure adapted from Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. Creative Commons.93 |
Baseline Characteristics
The female-to-male ratio was 2:1. The median age was 42 [32–56] years, with the oldest case reported aged 90 years. The majority of cases were Caucasians, 44%, followed by Asians, 36%. 53% of patients had no prior history of hypothyroidism, and 82% had no prior psychosis history. Autoimmune thyroiditis was the most common reported cause of hypothyroidism 51%.
Clinical Features
The median duration of psychotic symptoms was 14.5 [7–82.5] days ranging from two days to three years. Delusions were present in 91%. The most common form of delusions was paranoid/persecutory 84%. Hallucinations were present in 77.5%, with auditory hallucinations being the most prevalent 77.6%. Manic symptoms accompanied psychosis more than depressive symptoms, 52% and 36%, respectively (Table 2). Hypothyroidism symptoms and signs were not always reported, and when presented, often lacking sufficient details. Hypothyroidism symptoms were present in 63% (26/41) of the cases. Only 22 cases described the nature of hypothyroidism symptoms. Fatigue occurred in 63% (14/22), weight gain 36%, cold intolerance 36% (7/22), and hoarse voice was seen in 18% of the cases (4/22). Hypothyroidism signs were present in 75% of the cases (39/52). The most common abnormal findings were dry skin 60% (23/43), facial or pretibial edema 52% (20/43), delayed relaxation, or diminished deep tendon reflexes (DTR) 47% (18/38), while hoarseness of voice occurred 26% (10/38).
 |
Table 2 Summary of Baseline Characteristics, Clinical Features, and Diagnostic Workup of Included Cases |
Laboratory Testing
The median thyroid-stimulating hormone median value was 93 mIU/L [60–93]. The median-free T4 was 0.2 ng/dl [0.13–0.39]. The median thyroid peroxidase value was 138 IU/L [82.5–323]. In 7 of the reported cases, creatinine kinase was observed abnormal with a median value of 4490 [1767–9485] IU/L. Lumbar puncture was reported in six patients. Analysis of cerebrospinal fluid revealed mild protein elation in two cases (33%) and was found normal in four cases (67%).
Diagnostic Imaging
Cranial magnetic resonance imaging was normal in 75% (12/16) of the cases and showed structural brain changes in four patients, including crescent-shaped foci of T2 hyperintensity visualized as slight effusion below the dura matter (n=1), nonspecific white matter changes (n=1), and age-related atrophic changes (n=1). Similarly, patients who underwent cranial computed tomography displayed normal brain scans in 89% of cases (24/27). The electroencephalogram was normal in 73% (11/15) and showed generalized slowing without a focal change in 27% of cases (4/15) (Supplementary 3).
Antipsychotic Medications
Antipsychotic medications were utilized in the treatment of 92% (n=55/60) of the cases. The median duration of antipsychotic use was 1.8 [0.6–8] weeks. The longest antipsychotic treatment duration was 39 weeks.
Thyroid Hormone Supplementation
The median initiating and maintenance dose of thyroxine was 100 mcg [50–141]. Intravenous thyroid hormone therapy was administered in 10% of the patients (5/50). In 85% (44/52) of the cases, no thyroxine loading was given. Triiodothyronine was administered to 8% (n=5/58) of the cases.
Steroids
Steroids were administered in 3% of the cases (2/67). The median dose used was equivalent to 50 mg of prednisolone that was used for periods of three days and two weeks, respectively.
Outcome and Follow-Up
Clinical outcome data were reported for 96% of the cases (72/75). The majority of patients, 97% (66/68), required hospitalization, and 93% demonstrated remission. However, two patients (3%) showed no improvement or residual psychosis,23,75 while another three cases (4%) displayed recovery of psychosis with persisting residual deficits in cognition, memory, orientation, attention.31,66 The duration-to outcome occurrence was reported in 83% (62/75) of the cases. The median duration to the outcome (recovery) was 1.93 [0.8–2] weeks. The use of intravenous thyroid hormone supplementation (4/46) was associated with faster recovery compared to oral administration (0.55 [0.5–0.85] weeks vs 2.0 [1–2.85] weeks (p= 0.022). The univariate analysis also revealed a trend towards a shorter duration of psychosis (p= 0.0502) with IV thyroid hormone therapy. However, this effect could not be confirmed in the multivariate analysis (Table 3). Recovery duration did not differ between patients who received triiodothyronine and those who did not 0.5 [0.37–1.95] weeks vs 2 [1–2] weeks, p= 0.2). In the multivariate analysis (Table 3), gender (p= 0.33), age (p=0.46), symptoms duration (p=0.98) TSH level (p=0.29), FreeT4 (p=0.32), antipsychotic drugs duration (p=0.22), IV thyroid hormone therapy, and starting thyroxine dose (0.52) were not associated with shorter duration of psychosis (<2 weeks).
 |
Table 3 Table Summarizing the Result of the Multivariate Analysis |
Discussion
The major finding of this systematic review is that evidence on the pathophysiology as well as the clinical course and management of MP is limited to case reports. Descriptive pooling of extracted data from these reports and exploratory analysis indicates that MP can manifest with a wide range of psychiatric and physical symptoms and is commonly treated with antipsychotics and thyroid hormone supplementation. Although the vast majority of patients needed to be hospitalized, very few displayed persisting residual deficits after treatment. Prospective research is urgently needed to improve our understanding of MP and identify factors that may modulate clinical outcomes in order to design standardized diagnostic and therapeutic regimens.
The prevalence of MP was not primarily studied. Therefore, it can only be indirectly estimated from studies evaluating psychotic symptoms in patients with hypothyroidism. Based on the Committee on Myxedema of the Clinical Society of London report, the prevalence of psychotic symptoms was around 50% in hypothyroid patients in the late nineteenth century,5 and was less than 2% in 1965 based on a study of four-hundred hypothyroid patients in which 2% of patients were described to have hypothyroidism associated mental changes (this study did not provide details about the nature of psychic changes, which could be non-psychotic or hypothyroidism unrelated).92
In this review, we identified reports of MP in all adult age groups with a slightly higher proportion of reports on younger patients. Symptoms of hypothyroidism were observed in half of the cases, indicating that the absence of a prior history of hypothyroidism does not rule out MP. The majority of the cases did not have a personal nor family history of psychosis, supporting the direct link between thyroid pathology and psychosis. Although the pooled data from reported cases do not constitute a representative population, we observed a possible pattern in the clinical manifestation of the predominance of paranoid/persecutory delusions and auditory hallucinations. Manic symptoms tended to accompany MP more often than symptoms of depression. Fatigue was the most frequent somatic symptom; that is, however, nonspecific and can be seen in both primary and secondary psychiatric disorders. Although interesting, the predominance of manic symptoms and fatigue can be due to under-reporting as many clinical features were reported in few cases only. Interestingly, almost 40% of the cases did not have any physical hypothyroidism symptoms, while TSH was elevated in all cases with low thyroid hormones supporting the value of screening for thyroid dysfunction in patients with first-ever psychosis. Cranial imaging data did not suggest any relevant changes in brain structure related to MP. Almost all patients needed hospital admission. Antipsychotic medications were given to most of the patients (92%) with remission following short-term administration. Whether this indicates that termination of antipsychotic therapy after remission of psychotic symptoms is safe in patients with MP remains to be answered by prospective research.
Our pooled data of MP cases showed that administration of thyroxin was performed either intravenously or orally. Explorative comparison of synthesized data did not show the superiority of one administration route over the other, although unadjusted comparison showed a possible trend toward faster recovery after IV administration. However, this observation is solely based on cumulative case reports and needs to be viewed in conjunction with the potential increased risk of arrhythmias associated with IV thyroxin.94,95 Administration of triiodothyronine in MP was reported small fraction of the cases and should be investigated in prospective research. In most cases, steroids were not needed, questioning the value of immunosuppressive medication in MP, contrary to myxedema coma and HE. Most cases recovered completely, and only a few cases were left with residual psychosis or cognitive deficits. The etiology of partial improvement or residual cognitive deficits could be either a chronic and irreversible metabolic effect induced by hypothyroidism, as hypothesized by Asher et al or a primary psychiatric illness precipitated by hypothyroidism. All cases that recovered improved within two weeks on average, not exceeding six weeks at most. However, not all cases had long-term follow-up details to study recurrence of psychosis in the presence or absence of dysthyroid status.
Our review excluded two reports of patients with mania that did not exert psychotic features.96,97 Although the reported cases would be classified under the initial term “Myxedema Madness,” we have excluded them as they would not fit within the term “Myxedema psychosis.”
Prior systematic reviews examining HE,98–100 showed that only 20–25% of patients had clinical hypothyroidism, and most of them were euthyroid. It has been stated that psychosis occurs in around a third or less of the patients (26–36%) and is usually accompanied by other features such as; seizures in up to two-thirds (59–66%) and myoclonus (36–42%). These features were not seen in our patient population and their presence may help distinguish HE from MP, especially if the patients are euthyroid. Thyroperoxidase antibodies were found positive in almost all HE cases (86–100%), while our review revealed that 50% of MP cases had autoimmune thyroiditis. This may support the diagnostic value of a negative, not a positive, thyroperoxidase antibody test to differentiate MP from HE. Elevated protein in the cerebrospinal fluid is another characteristic feature of HE occurring in 71–78%, while electroencephalogram is usually abnormal (80–98%).15 These may also help differentiate it from MP as shown in our review, abnormal CSF and or EEG are not common in MP cases. Moreover, the small number of MP cases with abnormal CSF or EEG as depicted by our review could have been simply misdiagnosed HE cases.
Our systematic review has strengths, including a comprehensive literature search following PRISMA guidelines after a priori registration and publication of the predefined review protocol. We were able to pool data from a wide variety of sources identified in the published literature. We provided data on the demographics, diagnosis, and management of myxedema psychosis. Our pooled analysis is solely based on case reports constituting a non-representative population. However, our work clearly shows a substantial research gap, substantiating an urgent need for prospective well-designed research to characterize the clinical course and characteristics of myxedema and to test tailored diagnostic and therapeutic strategies. Publication and reporting bias cannot be ruled since cases considered not interesting or those with adverse outcomes could have been under-reported. Moreover, incomplete reporting of many clinical symptoms or details’ reporting within individual cases may have biased the final conclusion with regards to background data, outcomes, and follow-up duration. Furthermore, the diagnosis of MP was a clinical diagnosis as deemed likely by the treating physicians. We limited our search to case reports starting from 1980. By doing this, we may have missed including a small number of cases; however, considering that the differential diagnosis of HE was first described in 1966,101 we intended to collect comparable cases in order to differentiate between both conditions. Another reason for limiting our search to the time period since 1980 was changes in presentation and symptomatology of hypothyroidism due to improved diagnosis and disease management over time.
Conclusion
Our systematic review and pooled analysis identified a substantial lack of published research on MP. Available case observations indicate that patients with MP present with a broad spectrum of psychiatric and physical symptoms lending support to the value of screening for thyroid dysfunction in patients with first-ever psychosis.
Ethical Approval
Ethical approval is not required for this article as it is a secondary synthesis of publicly available data.
Acknowledgments
This work is the publication of a Master’s thesis of the Master’s program in Clinical Research, Center for Clinical Research and Management Education, Division of Health Care Sciences, Dresden International University, Dresden, Germany.
Disclosure
Dr. Timo Siepmann reports being a editorial board member at Neuropsychiatric Disease and Treatment. He received grants from Michael J. Fox Foundation, the German Federal Ministry of Health, Kurt Goldstein Institute and the German Parkinson Association that were not related to this work. He received royalties from Dresden International University for serving as teacher and program director of the Master’s Program in Clinical Research (MPCR) and from AstraZeneca for serving as advisor. Dr. Barlinn and Dr. Siepmann are editorial board members of Neuropsychiatric Disease and Treatment. The authors report no other conflicts of interest in this work.
References
1. Hunter I, Greene SA, MacDonald TM, Morris AD. Prevalence and aetiology of hypothyroidism in the young. Arch Dis Child. 2000;83(3):207–210. doi:10.1136/adc.83.3.207
2. Hoogendoorn EH, Hermus AR, De Vegt F, et al. Thyroid function and prevalence of anti-thyroperoxidase antibodies in a population with borderline sufficient iodine intake: influences of age and sex. Clin Chem. 2006;52(1):104–111. doi:10.1373/clinchem.2005.055194
3. Aoki Y, Belin RM, Clickner R, et al. Total T 4 in the United States population and their association with participant characteristics: national health and nutrition examination survey (NHANES 1999–2002). Thyroid. 2007;17(12):1211–1223. doi:10.1089/thy.2006.0235
4. Mehran L, Amouzegar A, Rahimabad PK, Tohidi M, Tahmasebinejad Z, Azizi F. Thyroid function and metabolic syndrome: a population-based thyroid study. Horm Metab Res. 2017;49(3):192–200. doi:10.1055/s-0042-117279
5. Report of a committee of the Clinical Society Of London. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1288618/.
6. Asher R. Myxoedematous madness. Br Med J. 1949;2(4627):555–562. doi:10.1136/bmj.2.4627.555
7. Regier DA, Kuhl EA, Kupfer DJ, The DSM-5. Classification and criteria changes. World Psychiatry. 2013;12(2):92–98. doi:10.1002/wps.20050
8. Sardar S, Habib M-B, Sukik A, et al. Myxedema psychosis: neuropsychiatric manifestations and rhabdomyolysis unmasking hypothyroidism. Case Rep Psychiatry. 2020;2020:7801953. doi:10.1155/2020/7801953
9. Crocker AD, Overstreet DH, Crocker JM. Hypothyroidism leads to increased dopamine receptor sensitivity and concentration. Pharmacol Biochem Behav. 1986;24(6):1593–1597. doi:10.1016/0091-3057(86)90491-0
10. Claustre J, Balende C, Pujol JF. Influence of the thyroid hormone status on tyrosine hydroxylase in central and peripheral catecholaminergic structures. Neurochem Int. 1996;28(3):277–281. doi:10.1016/0197-0186(95)00088-7
11. Ruel J, Faure R, Dussault JH. Regional distribution of nuclear T3 receptors in rat brain and evidence for preferential localization in neurons1. J Endocrinol Investig off J Ital Soc Endocrinol. 1985;8(4):343–348. doi:10.1007/BF03348511
12. Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Mol Psychiatry. 2002;7(2):140–156. doi:10.1038/sj.mp.4000963
13. Bauer M, Silverman DHS, Schlagenhauf F, et al. Brain glucose metabolism in hypothyroidism: a positron emission tomography study before and after thyroid hormone replacement therapy. J Clin Endocrinol Metab. 2009;94(8):2922–2929. doi:10.1210/jc.2008-2235
14. Constant EL, de Volder AG, Ivanoiu A, et al. Cerebral blood flow and glucose metabolism in hypothyroidism: a positron emission tomography study. J Clin Endocrinol Metab. 2001;86(8):3864–3870. doi:10.1210/jcem.86.8.7749
15. Mocellin R, Walterfang M, Velakoulis D. Hashimoto’s encephalopathy: epidemiology, pathogenesis and management. CNS Drugs. 2007;21(10):799–811. doi:10.2165/00023210-200721100-00002
16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(7716):332–336. doi:10.1136/bmj.b2535
17. Mohamed MFH, Siepmann T, Suwileh S, et al. Myxedema psychosis: a protocol for a systematic review and a pooled analysis. Medicine (Baltimore). 2020;99(26):e20778. doi:10.1097/MD.0000000000020778
18. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi:10.1186/s13643-016-0384-4
19. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evidence-Based Med. 2018;23(2):60–63. doi:10.1136/BMJEBM-2017-110853
20. Available from: https://www.jamovi.org/download.html.
21. Mohamed MFH, Mahgoub AB, Sardar S, Elzouki AN. Acute psychosis and concurrent rhabdomyolysis unveiling diagnosis of hypothyroidism. BMJ Case Rep. 2019;12:10. doi:10.1136/bcr-2019-231579
22. Neal JM, Yuhico RJO. “Myxedema madness” associated with newly diagnosed hypothyroidism and obstructive sleep apnea. J Clin Sleep Med. 2012;8(6):717–718. doi:10.5664/jcsm.2274
23. Ueno S, Tsuboi S, Fujimaki M, et al. Acute psychosis as an initial manifestation of hypothyroidism: a case report. J Med Case Rep. 2015;9(1):1–4. doi:10.1186/s13256-015-0744-z
24. Mavroson MM, Patel N, Akker E. Myxedema psychosis in a patient with undiagnosed Hashimoto thyroiditis. J Am Osteopath Assoc. 2017;117(1):50–54. doi:10.7556/jaoa.2017.007
25. Nathan R, Rix K, Kent J. Myxoedematous madness and grievous bodily harm. J Clin Forensic Med. 1997;4(2):85–90. doi:10.1016/S1353-1131(97)90079-1
26. Hines A, Stewart JT, Catalano G. A case of capgras syndrome related to hypothyroidism. J Psychiatr Pract. 2015;21(6):445–448. doi:10.1097/PRA.0000000000000108
27. Bel Feki M, Derouiche S, Kammoun R, Mziou O, Mnif L, Melki W. Myxœdema madness: case report. Eur Psychiatry. 2015;30:1869. doi:10.1016/s0924-9338(15)31433-4
28. Lazaro PCF, Loureiro JC, Banzato CEM. Psychosis associated with methimazoleinduced hypothyroidism: a case report. J Bras Psiquiatr. 2013;62(2):171–173. doi:10.1590/S0047-20852013000200012
29. AmdouniI F, Abid Y, Ben Khedher M, Ghachem R. Acute psychosis in the setting of severe hypothyroidism. Eur Psychiatry. 2015;30:1270. doi:10.1016/s0924-9338(15)30994-9
30. Shlykov MA, Rath S, Badger A, Winder GS. “Myxoedema madness” with Capgras syndrome and catatonic features responsive to combination olanzapine and levothyroxine. BMJ Case Rep. 2016;2016:bcr2016215957. doi:10.1136/bcr-2016-215957.
31. Darko DF, Krull A, Dickinson M, Gillin JC, Risch SC. The diagnostic dilemma of myxedema and madness, axis I and axis II: a longitudinal case report. Int J Psychiatry Med. 1988;18(3):263–270. doi:10.2190/y6ym-9f5w-d24l-34ak
32. Berkowitz MR. Resolution of hypothyroidism after correction of somatovisceral reflex dysfunction by refusion of the cervical spine. J Am Osteopath Assoc. 2015;115(1):46–49. doi:10.7556/jaoa.2015.007
33. Tor PC, Lee HY, Fones CSL. Late-onset mania with psychosis associated with hypothyroidism in an elderly Chinese lady. Singapore Med J. 2007;48(4):354–357.
34. Er C, Anil Sule A. Late onset radioiodine-induced hypothyroidism presenting with psychosis 14 years after treatment: a rare case. Oxford Med Case Rep. 2016;2016(4):68–70. doi:10.1093/omcr/omw020
35. Hynicka LM. Myxedema madness: a case for short-term antipsychotics? Ann Pharmacother. 2015;49(5):607–608. doi:10.1177/1060028015570089
36. Manea M, Rusanu V, Patrichi BE, et al. P03-563 - Psychotic disorder after radioactive iodine treatment in a 42-year-old woman thyroidectomized for papillary thyroid carcinoma: case report. Eur Psychiatry. 2011;26:1733. doi:10.1016/s0924-9338(11)73437-x
37. Gupta A, Bastiampillai T, Disha TI, Lam DH. Rapid response to loading dose levothyroxine in myxedema psychosis. Prim Care Companion CNS Disord. 2017;19(1). doi:10.4088/PCC.16l01974
38. Moeller KE, Goswami R, Larsen LM. Myxedema madness rapidly reversed with levothyroxine. J Clin Psychiatry. 2009;70(11):1607–1608. doi:10.4088/JCP.08l04958yel
39. Nazou M, Parlapani E, Nazlidou EI, Athanasis P, Bozikas VP. Psychotic episode due to Hashimoto’s thyroiditis. Psychiatrike. 2016;27(2):144–147. doi:10.22365/jpsych.2016.272.144
40. Madakasira S, Hall TB. Capgras syndrome in a patient with myxedema. Am J Psychiatry. 1981;138(11):1506–1508. doi:10.1176/ajp.138.11.1506
41. Santiago JM, Stoker DL, Beigel A, et al. Capgras’ syndrome in a myxedema patient. Hosp Community Psychiatry. 1987;38(2):199–201.
42. Lin CL, Yang SN, Shiah IS. Acute mania in a patient with hypothyroidism resulting from Hashimoto’s Thyroiditis. Gen Hosp Psychiatry. 2013;35(6):
43. Stowell CP, Barnhill JW. Acute mania in the setting of severe hypothyroidism. Psychosomatics. 2005;46(3):259–261. doi:10.1176/appi.psy.46.3.259
44. Cook DM, Boyle PJ. Rapid reversal of myxedema madness with triiodothyronine. Ann Intern Med. 1986;104(6):893–894. doi:10.7326/0003-4819-104-6-893_2
45. Larouche V, Snell L, Morris DV. Iatrogenic myxoedema madness following radioactive iodine ablation for Graves’ disease, with a concurrent diagnosis of primary hyperaldosteronism. Endocrinol Diabetes Metab Case Rep. 2015;2015. doi:10.1530/edm-15-0087.
46. Khemka D, Ali JA, Koch CA. Primary hypothyroidism associated with acute mania: case series and literature review. Exp Clin Endocrinol Diabetes. 2011;119(8):513–517. doi:10.1055/s-0031-1277137
47. Juneja V, Nance M. Treatment of hypothyroidism and psychosis. Aust N Z J Psychiatry. 2014;48(8):780. doi:10.1177/0004867414526285
48. Samuel W, Maniam T. Paranoid psychosis in myxoedema: a case report. Med J Malaysia. 1984;39(2):156–158.
49. Morgado P, Mendonça-Gonçalves M. Acute mania induced by hypothyroidism in a male patient after thyroidectomy. J Neuropsychiatry Clin Neurosci. 2016;28(1):e21–e22. doi:10.1176/appi.neuropsych.15090219
50. Khaldi S, Dan B, Basiaux P, De Nutte N, Kornreich C. Manic episode precipitated by withdrawal of hormone replacement therapy in severe hypothyroidism. J Psychiatr Pract. 2006;12(6):409–410. doi:10.1097/00131746-200611000-00010
51. Hall RCW, Popkin MK, Devaul R, Hall AK, Gardner ER, Beresford TP. Psychiatric manifestations of Hashimoto’s thyroiditis. Psychosomatics. 1982;23(4):337–342. doi:10.1016/S0033-3182(82)73397-3
52. Reddy KS, Rao GP. Hypothyroidism presenting as acute mania-rare case report. Indian J Psychiatry. 2019;61(9):S527–S529.
53. Pearce MJ, Walbridge DG. Myxoedema madness: a case report. Int J Geriatr Psychiatry. 1991;6(3):189–190. doi:10.1002/gps.930060313
54. Selvaraj V, Padala PR. Thyroid myopathy with rhabdomyolysis presenting as agitation: a case report. Prim Care Companion J Clin Psychiatry. 2008;10(4):328. doi:10.4088/pcc.v10n0411a
55. Fernandes S, Safeekh A, Shetty S, Chandini S. Hypothyroidism presenting as hallucinosis: a clinical masquerade. Arch Med Heal Sci. 2019;7(1):90. doi:10.4103/amhs.amhs_65_19
56. Dastjerdi G. Delusional type psychosis associated with hypothyroidism: a case report. Procedia - Soc Behav Sci. 2013;84:1050–1052. doi:10.1016/j.sbspro.2013.06.697
57. Hyams C, Joshi P, Foster P, Katz J. Acute psychosis caused by hypothyroidism following radioactive iodine treatment of Graves’ disease. JRSM Short Rep. 2013;4(4):1–4. doi:10.1177/2042533313476858
58. Fernandes VC. Severe hypothyroidism presenting with acute mania and psychosis: a case report and literature review. Bipolar Disord. 2017;3:116. doi:10.4172/2472-1077.1000116
59. Mehta R, Nd S, Ry M. A case report of psychosis in a patient of myxedema: myxedema madness. Available from: www.scholarena.com.
60. Todorov L, Boudaoud AA, De Raykeer RP, et al. A case of violent suicide attempt in a context of myxedema psychosis following radioiodine treatment in a patient with graves’ disease. Case Rep Psychiatry. 2019;2019. doi:10.1155/2019/4972760.
61. Westphal SA. Unusual presentations of hypothyroidism. Am J Med Sci. 1997;314(5):333–337. doi:10.1097/00000441-199711000-00011
62. Rao AC, Bhat VK, Kini S. Myxoedema presenting with psychosis. Indian J Psychiatry. 1990;32(3):287–289.
63. Morosán Allo YJ, Rosmarin M, Urrutia A, et al. Myxedema madness complicating postoperative follow-up of thyroid cancer. Arch Endocrinol Metab. 2015;59(4):359–364. doi:10.1590/2359-3997000000090
64. Parikh N, Sharma P, Parmar C. A case report on myxedema madness: curable psychosis. Indian J Psychol Med. 2014;36(1):80–81. doi:10.4103/0253-7176.127260
65. Nielsen JM. I see dead people. Hypothyroid myopathy and hypothyroid psychosis. J Miss State Med Assoc. 2010;51(5):135–136.
66. Kandukuri RC, Khan MA, Soltys SM. Nonadherence to medication in hypothyroidism: a case report. Prim Care Companion J Clin Psychiatry. 2010;12(3). doi:10.4088/PCC.09m00863gre
67. Natarajan MN. Hypothyroidism presenting as psychosis - a case report n m natarajan. Univ J Med Med Specialities. 2020. Available from: http://ejournal-tnmgrmu.ac.in/index.php/medicine/article/view/11655.
68. Martell T, Matuszak J. Mania and psychosis in a receiving interferon. Psychiatr Ann. 2012;42(9):312–313. doi:10.3928/00485713-20120906-02
69. Das S, Doval N, Moun V. Autoimmune thyroiditis presenting as psychosis. Shanghai Arch Psychiatry. 2017;29(3):174–176. doi:10.11919/j.issn.1002-0829.216059
70. Weston SN, Weston C. The mysterious case of the lost pituitary: amiodarone-induced hypothyroidism. Hosp Med. 2000;61(1):64–65. doi:10.12968/hosp.2000.61.1.1869
71. MNM Natarajan. Hypothyroidism presenting as psychosis: a case report. Indian J Psychiatry. 2013;55(S99).
72. O’Hanlon S, Kingston T. That sneaking suspicion, a case of “myxoedema madness. Ir J Med Sci. 2017;186:171–280. doi:10.1007/s11845-017-1629-5
73. Shaw E, Halper J, Yi PE, Asch S. Diagnosis of “myxedema madness”. Am J Psychiatry. 1985;142(5):655. doi:10.1176/ajp.142.5.655b
74. Sathya A, Radhika R, Mahadevan S, Sriram U. Mania as a presentation of primary hypothyroidism. Singapore Med J. 2009;50(2):e65–e67.
75. Azzopardi L, Murfin C, Sharda A, De Silva N. Myxoedema madness. BMJ Case Rep. 2010;2010(sep16 1):bcr0320102841–bcr0320102841. doi:10.1136/bcr.03.2010.2841
76. Singh G, Choudhary S, Agarwal S, Yadav A. Severe hypothyroidism playing a major cause of psychiatric illness. Indian J Psychiatry. 2019;61(9):S554–S555.
77. Philip D, Bauman AJ, Comi RJ. Reversible mania: an unusual presentation of hypothyroidism. Endocr Rev. 2017;38(3).
78. Rizvi A, Shaan F, Fatima N. Hashimoto’s thyroiditis presenting as acute psychosis with religious delusion: a case report. Sri Lanka J Psychiatry. 2017;8(2):29. doi:10.4038/sljpsyc.v8i2.8158
79. Agachanli R, Balaban OD, Sila M, Eradamlar N. Psychosis related with Hashimoto thyroiditis: a case report. Dusunen Adam. 2016;29(2):181–186. doi:10.5350/DAJPN2016290212
80. Psychotic depression due to Hashimoto thyroiditis. Klin Psikofarmakol Bul. 2014;24:S290–S291.
81. Islam L, Selle V, Masu A, Gambini O. Latrogenic hypothyroidism leading to an acute psychotic episode. Int Clin Psychopharmacol. 2011;26:e153. doi:10.1097/01.yic.0000405898.34742.e5
82. Hypothyroidism induced psychosis: a case report. Klin Psikofarmakol Bul. 2013;23:S128–S129.
83. Klinik psikofarmakoloji bülteni-bulletin of clinical psychopharmacology; 2016. doi:10.1080/10177833.2013.11790875
84. Leung JG. Severe hypothyroidism presenting as psychosis: a case of myxedema madness. J Pharm Pract. 2011;24(2):249. doi:10.1177/0897190011403437
85. Kumar A, Ramaraju D, Veera RL. “Seeing things with hypothyroidism”-a case report of amiodarone induced hypothyroidism presenting with hallucinations. J Am Geriatr Soc. 2011;59:S117. doi:10.1111/j.1532-5415.2011.03416.x
86. Post-partum thyroiditis with myxedema madness. Thyroid. 2009;19:S85. doi:10.1089/thy.2009.1589
87. Heinrich TW, Grahm G. Hypothyroidism presenting as psychosis. Prim Care Companion J Clin Psychiatry. 2003;05(06):260–266. doi:10.4088/pcc.v05n0603
88. Benvenga S, Lapa D, Trimarchi F. Don’t forget the thyroid in the etiology of psychoses. Am J Med. 2003;115(2):159–160. doi:10.1016/S0002-9343(03)00298-5
89. Chari S. The lesson from a yellow psychotic patient. Hosp Med. 2002;63(6):370–371. doi:10.12968/hosp.2002.63.6.2010
90. Ward A, Bergmann K. Myxoedematous madness - or not?. Int J Geriatr Psychiatry. 1994;9(5):419–420.
91. Davis AT. Psychotic states associated with disorders of thyroid function. Int J Psychiatry Med. 1989;19(1):47–56.
92. Evans TC, Hodges RE. Myxedema: a study of 400 cases. Arch Intern Med. 1965;116(2):183–190. doi:10.1001/archinte.1965.03870020023008
93. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
94. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331(19):1249–1252. doi:10.1056/NEJM199411103311901
95. Biondi B, Fazio S, Carella C, et al. Cardiac effects of long term thyrotropin-suppressive therapy with levothyroxine. J Clin Endocrinol Metab. 1993;77(2):334–338. doi:10.1210/jcem.77.2.8345037
96. Alex C, Kumar R. An interesting case of myxedema mania-a case report. RGUHS J Med Sci. 2015;5:27–29
97. Levitte SS. Coexistent hypomania and severe hypothyroidism. Psychosomatics. 1993;34(1):96–97. doi:10.1016/S0033-3182(93)71934-9
98. Chong JY, Rowland LP, Utiger RD. Hashimoto encephalopathy: syndrome or myth? Arch Neurol. 2003;60(2):164–171. doi:10.1001/archneur.60.2.164
99. Menon V, Subramanian K, Thamizh JS. Psychiatric presentations heralding Hashimoto’s encephalopathy: a systematic review and analysis of cases reported in literature. J Neurosci Rural Pract. 2017;8(2):261–267. doi:10.4103/jnrp.jnrp_440_16
100. de Holanda NCP, de Lima DD, Cavalcanti TB, Lucena CS, Bandeira F. Hashimoto’s encephalopathy: systematic review of the literature and an additional case. J Neuropsychiatry Clin Neurosci. 2011;23(4):384–390. doi:10.1176/jnp.23.4.jnp384
101. Brain L. Hashimoto’s disease and encephalopathy. Lancet. 1966;288(7462):512–514. doi:10.1016/S0140-6736(66)92876-5
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
