Back to Journals » International Medical Case Reports Journal » Volume 16
Myocarditis Following Inactivated SARS-CoV-2 Vaccine (Vero Cell) in an Adult Female in Vietnam: A Case Report
Authors Tran H, Vu VH, Tran D, Pham QDD , Nguyen KD, Truong BQ
Received 10 May 2023
Accepted for publication 3 September 2023
Published 15 September 2023 Volume 2023:16 Pages 551—559
DOI https://doi.org/10.2147/IMCRJ.S410806
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Hoa Tran,1 Vu Hoang Vu,1 Duc Tran,2 Quang Dang Duy Pham,2 Khang Duong Nguyen,2 Binh Quang Truong1
1Department of Internal Medicine, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam; 2Cardiovascular Center, University Medical Center Ho Chi Minh City, Ho Chi Minh City, Vietnam
Correspondence: Quang Dang Duy Pham, Tel +84937134599, Email [email protected]
Abstract: During the Coronavirus disease 2019 (COVID-19) pandemic, vaccination against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has proven to be an important measure to help control disease spread and improve patient outcome. There are four distinct vaccine categories: inactivated viral vaccines, messenger RNA (mRNA) vaccines, adenovirus vector–based vaccines, and adjuvanted protein vaccines. In 2021, increased cases of myocarditis and pericarditis were reported after mRNA and adenovirus vector–based COVID-19 vaccination. A similar reporting pattern has not been observed after receipt of inactivated virus vaccines. Here, we present a case of clinically suspected acute myocarditis in a 26-year-old female, occurring 11 days after the administration of Sinopharm Vero Cell, an inactivated virus COVID-19 vaccine. This event led to acute heart failure, with marked clinical resolution observed within 34 days.
Keywords: COVID-19 vaccine-associated myocarditis, inactivated virus COVID-19 vaccine, Vero Cell, SARS-CoV-2
Introduction
Coronavirus disease 2019 (COVID-19), caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), adversely affects multiple human body systems, including the cardiovascular system. Thromboembolism, acute coronary syndrome, arrhythmia, and myocarditis are among the most common cardiovascular complications. Interestingly, these adverse events are also observed following SARS-CoV-2 vaccination.1 There are four distinct COVID-19 vaccine categories: inactivated virus vaccines, messenger RNA (mRNA) vaccines, adenovirus vector-based vaccines, and adjuvanted protein vaccines.2 Cases of myocarditis in individuals recently administered mRNA-based SARS-CoV-2 vaccines, such as BNT162b2 (BioNTech/Pfizer, USA) and mRNA-1273 (Moderna, USA), have been commonly reported.3 A study by Martina Patone et al found increased risks of myocarditis associated with both adenovirus vector-based and mRNA COVID-19 vaccines.4 On May 7th, 2021, the inactivated COVID-19 vaccine, Sinopharm Vero Cell (Beijing Institute of Biological Products Co., Ltd., China National Biotec Group (CNBG)/Sinopharm, China), was listed for emergency use by the World Health Organization (WHO). When compared with the two aforementioned vaccine types, there are currently no exhaustive reports of myocarditis following the administration of inactivated COVID-19 viral vaccines. In this context, we present a clinical case of suspected acute myocarditis after the administration of Sinopharm Vero Cell, an inactivated virus COVID-19 vaccine.
Case Report
A 26-year-old female patient, working as an office worker with no previous history of cardiovascular or autoimmune disease, visited the emergency room because of dyspnea. 18 days before her presentation to the emergency room, she received her first dose of inactivated virus COVID-19 vaccine (Sinopharm Vero Cell). Eleven days after vaccination, she developed flu-like symptoms throughout the day: pyrexia, body aches, and fatigue. She had a dry cough, exertional dyspnea, paroxysmal nocturnal dyspnea, and rapidly progressive edema of the arms, legs, and genital area. After admission to a primary care hospital, her disease progressed unfavorably, and she was transferred to our tertiary hospital with signs and symptoms of acute heart failure. These included: orthopnea, elevated jugular venous pressure, gallop S3, pulmonary crackles, enlarged liver and peripheral edema. Upon arrival at the emergency department, her heart rate measured 110, while her blood pressure recorded as 140/80 mmHg, and her SpO2 registered at 94%. Arterial blood gas revealed moderate hypoxic respiratory failure and acute respiratory alkalosis with concomitant metabolic acidosis. Her electrocardiogram revealed a sinus tachycardia, with a poorly progressive R wave from the V1 to V3 leads, a negative T wave in lead 1, aVL (Figure 1). Chest radiographs showed interstitial lesions, and parenchymal consolidation in the lower two-thirds of the lungs, with a moderate right pleural effusion (Figure 2). A non-contrasted computed tomography (CT) scan revealed regular thickening of the interlobular septum, opacified glass lesions in both lungs, (predominantly in the upper lobes), scattered consolidation of the right lung, “tree-in-bud” lesions in the upper lobes, and right-sided pleural effusion (Figures 3 and 4). Echocardiography revealed a non-dilated left ventricle with a reduced left ventricular ejection fraction of 45% (Simpson’s method), left ventricular end-diastolic diameter of 45.3 mm, left ventricular end-diastolic volume of 86 mL, severe left ventricular diastolic dysfunction, average E/e’ of 15.6, reduced global longitudinal strain (GLS) of −10.3% with diffuse and uniform reductions between regions, and moderate pericardial effusion. This did not indicate any specific cardiac pathology. The patient’s initial blood work-up confirmed the diagnosis of acute heart failure and ruled out active SARS-CoV-2 infection (Table 1).
 |
Table 1 Blood Test Results |
 |
Figure 1 Admission electrocardiogram showed sinus tachycardia, poorly progressive R wave from V1 to V3 leads, negative T wave in lead 1, aVL. |
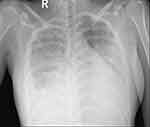 |
Figure 2 Admission chest radiograph demonstrating interstitial lesions, parenchymal consolidation of the lower two thirds of the lungs, and moderate right pleural effusion. |
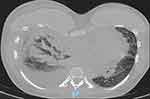 |
Figure 3 Chest CT scan with “tree-in-bud” lesions on the upper right lobe. |
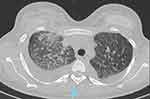 |
Figure 4 Contrast-enhanced chest computed tomography illustrating “crazy paving” pattern in the right lower lobe of the lung and bilateral pleural effusions. |
Cardiovascular magnetic resonance imaging (CMR) was performed. This revealed reduced left ventricular systolic function (left ventricular ejection fraction of 26.8%), basal and middle interventricular septal dyskinesia, hypokinesis of the remaining walls, reduced right ventricular systolic function (right ventricular ejection fraction of 31.5%), and right ventricular free wall akinesia. Late gadolinium enhancement in the mid myocardial and subepicardial left ventricular walls was observed in the septal, lateral, and basal inferior regions, suggestive of non-ischemic cardiomyopathy. T2-weighted images demonstrated a global increase in signal intensity, suggesting myocardial edema. These CMR findings fulfilled the 2018 updated Lake Louise Criteria for acute myocardial inflammation (Figures 5–7).5
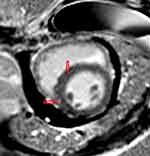 |
Figure 5 Streaky late gadolium mid-myocardial enhancement in the septal region (red arrows). |
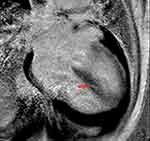 |
Figure 6 Streaky late gadolinium mid-myocardial enhancement in the septal wall, with small patchy late gadolium mid-myocardial enhancement in the inferior basal wall (red arrow). |
 |
Figure 7 Streaky late gadolium mid-myocardial enhancement in the septal region (red arrow). |
The provisional diagnosis at the time was clinically suspected myocarditis thought to be linked to inactivated virus COVID-19 vaccination (Vero Cell, Sinopharm, China). The patient was administered nasal oxygen, intravenous furosemide, nitroglycerin, and a short course of intravenous antibiotics for a suspected community-acquired pneumonia before negative blood and sputum culture results were obtained. Her congestion symptoms were relieved after 4 days of treatment, diuretics were weaned and ultimately stopped. Guideline-directed medical therapy for heart failure which included an angiotensin receptor-neprilysin inhibitor (sacubitril/valsartan 200 mg per day), a beta-blocker (metoprolol succinate 12.5 mg per day), and an aldosterone antagonist (spironolactone 50 mg per day) was initiated. On the 11th day of treatment, her symptoms improved markedly. NT-proBNP level decreased from 48,951 to 15,767 ng/mL, chest radiographs showed improvement (Figure 8), and the patient was discharged after 13 days.
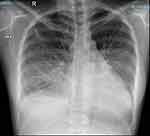 |
Figure 8 Chest radiograph before discharge, demonstrating enlargement of the cardiac silhouette. |
At the follow-up visit, 14 days after discharge, the patient’s exercise capacity was reported to have improved significantly, and the NT-proBNP level continued to decrease to 2485 ng/L. Echocardiography demonstrated improved systolic function with an ejection fraction of 59% and no regional wall motion abnormalities. The adjusted doses of standard heart failure medications: sacubitril/valsartan 200 mg daily, metoprolol succinate 50 mg daily, and spironolactone 50 mg daily, were well tolerated by the patient.
Discussion
COVID-19 has become a global pandemic. Vaccination against SARS-CoV-2 has proven to be an important measure to limit the spread of the disease and to improve patient outcomes. However, vaccines also cause rare but severe adverse reactions such as myocarditis.1
The first reports of myocarditis following COVID-19 vaccination, which is of the mRNA vaccine type (BNT162b2 vaccine, BioNTech/Pfizer, USA), were published in Israel in April 2021.6 The Vaccine Adverse Event Reporting System (VAERS) has reported 1226 cases of acute myocarditis and pericarditis associated with BNT162b2 (BioNTech/Pfizer, USA) and mRNA-1273 (Moderna, USA) vaccines out of 300 million vaccinated people as of June 11th, 2021.3,7 In May 2021, the European Medicines Agency highlighted the risk of post-vaccination myocarditis following BNT162b2 (BioNTech/Pfizer, USA) vaccination.3 A similar reporting pattern has not been observed after receipt of inactivated COVID-19 vaccines.8,9
Our case describes a young patient with no previous cardiovascular disease who presented with acute heart failure and marked clinical resolution within 34 days. Although endomyocardial biopsy was not performed, the acute clinical presentation, abnormal electrocardiogram, elevated troponin T level, new and reversible left ventricular structure and functional abnormalities recorded on echocardiography, and the characteristic cardiovascular magnetic resonance findings fulfilled the diagnostic criteria for clinically suspected myocarditis according to the 2013 position statement of the ESC Working Group on Myocardial and Pericardial Diseases.10 This case is notable because the symptoms developed 11 days after inactivated COVID-19 virus vaccination, which had no similar reports at the time of writing. Unfortunately, we did not rule out other viral pathogen that may cause myocarditis, such as adenovirus, parvovirus B-19, cytomegalovirus, human herpes virus-6, or Epstein-Barr virus. Other common acute myocarditis etiologies, including active SARS-CoV-2 infection, were ruled out.
In a November 2022 clinical consensus paper by ESC focusing on myocarditis following COVID-19 vaccine,11 primarily involving adenovirus-vectored and mRNA vaccines, myocarditis symptoms generally emerge within days after the second SARS-CoV-2 vaccine dose. The highest vulnerability is observed among young males. Symptoms, notably chest discomfort, breathlessness, palpitations, malaise, weakness, fatigue, and varying temperatures, are broadly mild and nonspecific. Elevated troponin levels, peaking 48 to 72 hours post-symptom onset, are common. Inflammatory markers, like C-reactive protein, may increase, particularly in cases of concurrent pericarditis. CMR often reveals cardiac inflammation among the majority, with sporadic minor pericardial effusion but rare instances of significant effusion. ECG changes, subtly non-specific, encompass mild ST-segment alterations, PQ segment depressions, or non-specific ST-segment changes akin to classic myocarditis or myopericarditis. In instances of vaccine-associated myocarditis and pericarditis, most cases manifest mild disease devoid of heart failure symptoms or lethal arrhythmias. Outcomes are generally positive; however, a few cases report severe chest pain, heart failure signs, and hemodynamic instability.11 In our case, the clinical trajectory exhibited an atypical course, possibly influenced by the distinct vaccine type employed. Specifically, the patient was administered only the first dose of the inactivated COVID-19 vaccine, after which symptoms manifested 11 days subsequent to vaccination. Remarkably, the progression of symptoms demonstrated heightened aggressiveness in contrast to the majority of documented instances involving myocarditis following administration of adenovirus-vectored and mRNA vaccines. Despite this heightened progression, the ultimate clinical outcome remained favorable.
Case reports regarding myocarditis following inactivated COVID-19 vaccination were limited. In January 2022, Huanglin Cui et al documented two instances of fulminant myocarditis subsequent to Vero Cell vaccination.12 Both cases exhibited symptom onset four days after vaccination, progressing to cardiogenic shock necessitating intra-aortic balloon pump support. High doses of corticosteroids and intravenous immunoglobulin were administered. In both instances, outcomes were favorable, enabling discharge after 9 to 10 days of hospitalization. When compared to reported cases of myocarditis linked to mRNA vaccines, these two cases bear greater resemblance to our presented case. In these instances, the disease course manifested heightened aggressiveness, yet yielded the same favorable outcome.
While the mechanism underlying the association between myocarditis and any of the COVID-19 vaccines remains unclear, an elevated level of suspicion should be employed to quickly identify this potentially dangerous complication in younger patients. However, it should be emphasized that these adverse effects are rare, and we cannot deny the critical role of vaccines, especially in elderly subjects, in preventing COVID-19 and its associated complications, including COVID-19 related myocarditis.
Conclusions
This case suggests that acute myocarditis may be a possible adverse effect of the inactivated COVID-19 virus (Vero Cell, Beijing Institute of Biological Products Co., Ltd., China National Biotec Group (CNBG)/Sinopharm, China) vaccine. This adverse effect was more frequently reported following mRNA-based COVID-19 vaccines. Acute myocarditis should be considered in patients who develop heart failure symptoms or chest pain following recent COVID-19 vaccination. However, it should be emphasized that these adverse effects are rare, and we cannot deny the crucial role of vaccines, especially in the elderly, in preventing COVID-19 and its associated complications, including COVID-19 related myocarditis.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper, including publication of any accompanying images. Institutional approval was not required to publish the case details.
Acknowledgments
We would like to express our gratitude to the interventional cardiology department for providing a high standard of patient care and tremendous assistance with our work in the publishing of this paper.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study received no external funding.
Disclosure
Prof. Dr. Binh Quang Truong reports personal fees from Novartis and Servier, outside the submitted work. The authors declare no other conflicts of interest in this work.
References
1. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471–484. doi:10.1161/CIRCULATIONAHA.121.056135
2. Barouch DH. Covid-19 vaccines — immunity, variants, boosters. N Engl J Med. 2022;387:1011–1020. doi:10.1056/NEJMra2206573
3. Das BB, Moskowitz WB, Taylor MB, Palmer A. Myocarditis and pericarditis following mRNA COVID-19 vaccination: what do we know so far? Children. 2021;8(7):607. doi:10.3390/children8070607
4. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28(2):410–422. doi:10.1038/s41591-021-01630-0
5. Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–3176. doi:10.1016/j.jacc.2018.09.072
6. Reuters. Israel examining heart inflammation cases in people who received Pfizer COVID shot; 2021.
7. Kim HW, Jenista ER, Wendell DC, et al. Patients with acute myocarditis following mRNA COVID-19 Vaccination. JAMA Cardiol. 2021;6(10):1196–1201. doi:10.1001/jamacardio.2021.2828
8. Lai FTT, Li X, Peng K, et al. Carditis After COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine: a case-control study. Ann Intern Med. 2022;175(3):362–370. doi:10.7326/M21-3700
9. Hromić-Jahjefendić A, Sezer A, Aljabali AAA, et al. COVID-19 vaccines and myocarditis: an overview of current evidence. Biomedicines. 2023;11(5):1469. doi:10.3390/biomedicines11051469
10. Caforio ALP, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur Heart J. 2013;34(33):2636–2648. doi:10.1093/eurheartj/eht210
11. Heidecker B, Dagan N, Balicer R, et al. Myocarditis following COVID-19 vaccine: incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC working group on myocardial and pericardial diseases. Eur J Heart Fail. 2022;24(11):2000–2018. doi:10.1002/ejhf.2669
12. Cui G, Li R, Zhao C, Wang DW. Case Report: COVID-19 vaccination associated fulminant myocarditis. Front Cardiovasc Med. 2022;2022:8.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
