Back to Journals » OncoTargets and Therapy » Volume 13
Myeloid Blast Crisis of Chronic Myeloid Leukemia Followed by Lineage Switch to B-Lymphoblastic Leukemia: A Case Report
Authors Liu J, Zhou Y, Yuan Q, Xiao M
Received 25 February 2020
Accepted for publication 2 April 2020
Published 17 April 2020 Volume 2020:13 Pages 3259—3264
DOI https://doi.org/10.2147/OTT.S251214
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Jiduo Liu,* Yingchun Zhou,* Qing Yuan, Mingfeng Xiao
Department of Clinical Laboratory, The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, Guangdong 510405, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mingfeng Xiao Email [email protected]
Abstract: Lineage switch is very rare in blastic crisis of chronic myeloid leukemia (CML-BC). Here, we report a case of CML-BC in which the blast lineage switched from myeloid to B-lymphoid. A 35-year-old male was initially admitted to our hospital because of abdominal distention for over a year and dizziness for one week. Prior to presentation at our hospital, he visited a local hospital because of abdominal distention where his white blood cell count and bone marrow (BM) smear indicated CML. Results from peripheral blood (PB) counts, bone marrow analysis, immunophenotyping by flow cytometry, and the detection of the Philadelphia chromosome were consistent with a diagnosis of myeloid blast crisis from CML. The patient received chemotherapy with imatinib for induction, which diminished the number of blasts. However, after three months, the blasts were increased in the PB and BM. The BM study and immunophenotyping by flow cytometry revealed B-lymphoblastic leukemia. In accordance with his first admission, a chromosome study revealed a karyotype of 46, XY, t(9; 22)(q34; q11) in all 20 cells analyzed, and B-lymphoblastic transformation from CML was diagnosed. Despite three months of treatment with DVCP (daunorubicin, vincristine, cyclophosphamide and prednisone) chemotherapy in combination with dasatinib, the patient did not achieve complete remission. The patient decided to stop treatment and was discharged from the hospital for financial reasons. This case implicates the Philadelphia chromosome with p210 BCR-ABL1 fusion proteins as a key molecule in CML-BC. Further research is needed to assess the frequency, treatment, and prognosis of CML-BC patients with lineage switch.
Keywords: CML, myeloid blastic crisis, B-lymphoblastic transformation, Philadelphia chromosome, BCR-ABL1 fusion gene
Introduction
Chronic myeloid leukemia (CML) is a common myeloproliferative disease characterized by the BCR-ABL fusion gene from the chromosomal translocation t(9; 22)(q34;q11), which is referred to as the Philadelphia (Ph) chromosome.1–3 There are three stages in the progression of CML: the chronic phase (CP), the accelerated phase (AP), and the blastic phase (BP). The CP can be followed by the AP, or direct progression to the final BP, which occurs more frequently in the cells of myeloid lineage than in those of lymphoid lineage.4 Patients with CML are treated with tyrosine kinase inhibitor (TKI) therapy according to the blast phenotype.5,6 Here, we report a rare case in which the blast lineage switched from myeloid to B-lymphoid.
Case Report
A 35-year-old male was admitted to our hospital in December 2015 because of abdominal distention for more than 1 year and dizziness for 1 week. Prior to his presentation at our hospital, the patient visited a local hospital because of abdominal distention where he was found to have a white blood cell (WBC) count and bone marrow (BM) smear that indicated CML; however, these reports were not available for review. The patient was then transferred to our hospital for further symptomatic treatment. Upon admission, the patient’s vital signs were within normal limits although was pale and had splenomegaly and anemic face on examination.
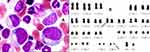
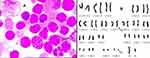
His initial complete blood count (CBC) showed a hemoglobin level of 71 g/L, a platelet count of 43,000/µL, and a WBC count of 573,090/µL with 17% blasts. A BM study (Figure 1) revealed hypercellular marrow with 25% blasts (Table 1). The neutrophil alkaline phosphatase (NAP) activity was low. A chromosome study revealed a karyotype of 46, XY, t(9; 22)(q34; q11) in all 20 cells analyzed (Figure 1). Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis revealed the presence of genes encoding p210 BCR/ABL1 fusion proteins (Table 1). Immunophenotyping by flow cytometry suggested that the blasts were positive for cytoplasmic myeloperoxidase (MPO), CD4, CD11b, CD15, CD16, CD33, CD34, CD38, CD56, and CD71 (Figure 2). A BM biopsy showed CML in myeloid blast crisis and fibrosis. A diagnosis of myeloid blast crisis from CML was made, and the patient was treated with imatinib (600 mg daily) and hydroxyurea (2000 mg daily). One month after the initiation of therapy, routine laboratory tests revealed a WBC count of 4920/µL with 2% blast cells in both the PB and BM. A treatment in combination with DVCP chemotherapy was recommended to the patient; however, treatment was delayed, and the patient was discharged due to financial reasons and the original treatment plan was maintained. Three months later, the patient was re-admitted to the hospital again for extensive cervical subcutaneous nodules, and PB tests indicated a high leukocyte count with 4% blasts. His BM showed hypercellularity with 62.5% blasts (Table 2). Similar to the first admission, a chromosome study revealed a karyotype of 46, XY, t(9; 22)(q34; q11) in all 20 cells analyzed (Figure 3). RT-PCR analysis revealed the presence of genes encoding p210 BCR/ABL1 fusion proteins (Table 2). Immunophenotyping by flow cytometry suggested that the blasts were positive for cytoplasmic CD22, cytoplasmic CD79a, and CD10, CD19, CD20, CD33, CD38, CD117, HLA-DR with membrane-expressed (Figure 4). Pathological biopsy of the cervical subcutaneous nodules revealed diffuse B-lymphoblastic lymphoma and B-lymphoblastic transformation from CML was diagnosed. Despite chemotherapy with DVCP (daunorubicin, vincristine, cyclophosphamide and prednisone) in combination with dasatinib for three months, the patient did not achieve complete remission. The routine laboratory tests revealed a WBC count of 4020/µL with 6% blast cells in the PB and 20% blast cells in the BM. The patient decided to stop treatment and was discharged from the hospital due to financial reasons.
 |
Table 1 Laboratory Data on First Admission |
 |
Table 2 Laboratory Data on Second Admission |
 |
Figure 2 Immunophenotyping by flow cytometry of the blasts at acute myeloid leukemia phase. |
 |
Figure 4 Immunophenotyping by flow cytometry of the blasts at acute B-lymphoid leukemia phase. |
Discussion
There are several previous reports on lineage switch that in Ph chromosome-positive acute leukemia.7–14 Blast lineage switch has been shown to be related to clonal selection and transformation of multipotent progenitor cells during chemotherapy;8 however, lineage switch is rare in CML-BC. Lineage switch often occurs during conventional chemotherapy, although in this case, the switch occurred during treatment with imatinib. Previous studies on the blast lineage switch suggested that the origin was multipotent progenitor cells.11,15 Our case also suggests that blasts in CML-BC have the potential to change phenotype, which may be related to factors such as gene expression and treatment modality.
The case presented here provides interesting insights into the mechanism of CML-BC. Our findings indicate that the chromosomal translocation t(9; 22)(q34; q11) with p210 BCR-ABL1 fusion gene that exists in multipotent progenitor cells is the primary molecular event in this case. The blastic crisis occurred in the presence of the Ph chromosome in the different multipotent progenitor cells. In our case, it is also possible that blastic crisis arose independently from two different Ph chromosome-positive clones. At the first admission, the blasts were successfully diminished by maintenance therapy with TKI imatinib, suggesting that imatinib may be an effective treatment. At the second admission, TKI dasatinib combined with chemotherapy was ineffective, suggesting that the different Ph chromosome-positive clones were resistant to a combined treatment of dasatinib and DVCP, resulting in B-lymphoid blastic crisis.
This case indicates that the Ph chromosome with p210 BCR-ABL1 fusion gene is a key molecule in CML-BC. However, the Ph chromosome also occurs as a specific primary chromosomal change in acute myeloid leukemia. The mechanisms underlying CML-BC are complex, involving changes to many aspects of the molecular pathology, including the interaction of oncogenes, anti-oncogenes, and other abnormal genes2,5,16 in addition to the BCR-ABL1 gene. Further research is needed to assess the frequency, treatment, and prognosis of CML-BC patients with lineage switch.
Ethics and Consent Statement
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations, and it does not need institutional approval to publish the case details. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Informed Consent
The authors state that they have obtained verbal and written informed consent from the patient for the inclusion of their medical and treatment history within this case report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Goldman JM, Melo JV. BCR-ABL in chronic myelogenous leukaemia—how does it work ? Acta Haematol. 2008;119(4):212–217. doi:10.1159/000140633
2. Negi V, Vishwakarma BA, Chu S, et al. Hoxa9 and Hoxa10 induce CML myeloid blast crisis development through activation of Myb expression. Oncotarget. 2017;8(58):98853–98864. doi:10.18632/oncotarget.22008
3. Qing X, Qing A, Ji P, et al. Mixed phenotype (T/B/myeloid) extramedullary blast crisis as an initial presentation of chronic myelogenous leukemia. Exp Mol Pathol. 2018;104(2):130–133. doi:10.1016/j.yexmp.2018.02.005
4. Hernandez JM, Gonzalez-Sarmiento R, Martin C, et al. Immunophenotypic, genomic and clinical characteristics of blast crisis of chronic myelogenous leukaemia. Br J Haematol. 1991;79(3):408–414. doi:10.1111/j.1365-2141.1991.tb08048.x
5. Marum JE, Yeung DT, Purins L, et al. ASXL1 and BIM germ line variants predict response and identify CML patients with the greatest risk of imatinib failure. Blood Adv. 2017;1(18):1369–1381. doi:10.1182/bloodadvances.2017006825
6. Goldman JM, Marin D, Olavarria E, Apperley JF. Clinical decisions for chronic myeloid leukemia in the imatinib era. Semin Hematol. 2003;40(2 Suppl 2):
7. Lee HR, Kang SH, Kang HJ, et al. Lineage switch from acute myeloid leukemia to biphenotypic acute leukemia with acquisition of philadelphia chromosome. Cancer Genet Cytogenet. 2008;184(2):124–126. doi:10.1016/j.cancergencyto.2008.04.002
8. Stass S, Mirro J, Melvin S, et al. Lineage switch in acute leukemia. Blood. 1984;64(3):701–706.
9. Lounici A, Cony-Makhoul P, Dubus P, et al. Lineage switch from acute myeloid leukemia to acute lymphoblastic leukemia: report of an adult case and review of the literature. Am J Hematol. 2000;65(4):319–321. doi:10.1002/1096-8652(200012)65:4<319::AID-AJH13>3.0.CO;2-1
10. Cuneo A, Balboni M, Piva N, et al. Lineage switch and multilineage involvement in two cases of ph chromosome-positive acute leukemia: evidence for a stem cell disease. Haematologica. 1994;79(1):76–82.
11. Monma F, Nishii K, Ezuki S, et al. Molecular and phenotypic analysis of philadelphia chromosome-positive bilineage leukemia: possibility of a lineage switch from t-lymphoid leukemic progenitor to myeloid cells. Cancer Genet Cytogenet. 2006;164(2):118–121. doi:10.1016/j.cancergencyto.2005.06.021
12. Pane F, Frigeri F, Camera A, et al. Complete phenotypic and genotypic lineage switch in a philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 1996;10(4):741–745.
13. Reardon DA, Hanson CA, Roth MS, Castle VP. Lineage switch in philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 1994;73(5):1526–1532. doi:10.1002/1097-0142(19940301)73:5<1526::AID-CNCR2820730534>3.0.CO;2-E
14. Saso R, Zomas A, Hamblin M, et al. Sequential development of myelodysplasia and acute myeloid leukemia but with no karyotypic evolution after autografting in a patient with philadelphia positive acute lymphoblastic leukemia. Leuk Lymphoma. 1997;26(5–6):625–628. doi:10.3109/10428199709050900
15. Oh SH, Park TS, Kim HR, et al. Chronic myelogenous leukemia showing biphenotypic blast crisis followed by lineage switch to B lymphoblastic leukemia. Leuk Res. 2009;33(11):e195–e198. doi:10.1016/j.leukres.2009.04.026
16. WANG YUAN‑YUAN, DING WEN-JING, JIANG FENG, et al. Coexistence of p210 BCR‑ABL and CBF β-MYH11 fusion genes in myeloid leukemia: A report of 4 cases. Oncol Lett. 2017;14:5171–5178. doi:10.3892/ol.2017.6812
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.