Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Mycosis Fungoides Exhibits the Features of Skin Tags: A Case Report
Received 21 April 2023
Accepted for publication 27 June 2023
Published 7 July 2023 Volume 2023:16 Pages 1765—1768
DOI https://doi.org/10.2147/CCID.S411041
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
XiaoYan Zhang, Ping Wang
Department of Dermatology, Hangzhou Third People’s Hospital, Hangzhou, People’s Republic of China
Correspondence: Ping Wang, Department of Dermatology, Hangzhou Third People’s Hospital, Hangzhou, People’s Republic of China, Tel +86-13588812862, Email [email protected]
Abstract: We present the case of a 37-year-old male diagnosed with Mycosis fungoides (MF) after gradually developing multiple skin tags and brownish lichenoid papules. The patient had pre-existing erythema over his entire body, especially his face, upper extremities, and trunk, for over 1.5 years. Microscopic examination of the papule and the skin tag (ST) exhibited similar features mainly characterized by superficial dense band-like lymphoid infiltrates and epidermotropism of atypical lymphocytes (Pautrier’s micro-abscesses). Immunohistochemistry further revealed the lymphoid infiltrates predominantly expressed LCA, CD3, CD4, and CD45RO but lacked CD7, CD8, CD30, CD20, and CD79a. The finding of this study that reports MF characterized by unusual STs suggests that some causes and effects have not been previously described in MF.
Keywords: skin tags, lymphoma, T-Cell, cutaneous, mycosis fungoides
Mycosis fungoides (MF) is the most common subtype of Cutaneous T cell lymphoma (CTCL), a heterogeneous group of lymphoproliferative disorders characterized by the clonal accumulation of neoplastic T lymphocytes in the skin.1 Clinically, classical MF may manifest as patches, plaques, or tumors. However, its manifestation is highly variable, presenting unfamiliar symptoms clinically and in histopathology, thus posing a diagnostic challenge.2 This report describes a case of MF exhibiting features of multiple STs and lichenoid papules. Of note, MF has been described in acrochordon, but its involvement in multiple STs is yet to be reported in the literature.
Case Report
This case report is of a 37-year-old Chinese male with a 1.5-year history of acrochordons and brownish lichenoid papules with pre-existing erythema of the entire body. Four years later, the patient initially developed erythema, dry skin, and skin desquamation and pruritus, affecting his face, upper extremities, and trunk. These skin conditions eventually spread to the entire body with scattered skin tags and lichenoid papules. Intermittent treatment with topical corticosteroids and oral antihistamine drugs had failed to improve his clinical condition. Physical examination of the patient revealed erythemas, lichenoid papules, and scaly patches on his face, upper extremities, and trunk (Figure 1a and b). Scattered STs ranging between 3×2 and 10×8mm were mainly located on his upper extremities and trunk (Figure 1b–d). One superficial lymph node on the posterior neck was palpable and indurated and could move freely over the bridge.
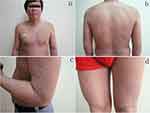 |
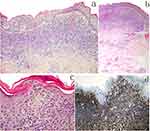
Figure 1 Erythemas, lichenoid papules, scaly patches on his face, upper extremities and trunk (a and b). The scattered skin tags were mainly located on his upper extremities and trunk (b–d). |
Two biopsy specimens displaying similar histopathologic features of dense, band-like lymphoid infiltrate with areas of epidermotropism were obtained from different lesions on the upper breast (the ST and brownish lichenoid papule). Notably, dermal and intraepidermal lymphocytes were predominantly small to medium size, with round or cerebriform nuclei. Atypical lymphocytic infiltrate was also observed in the perivascular and skin appendages (Figure 2a–c). Immunohistochemical studies revealed that the atypical lymphocytes predominantly expressed CD4, CD45RO, and LCA (Figure 2d). However, they did not express CD30 and CD20. A polymerase chain reaction analysis of the skin lesions revealed a monoclonal rearrangement of the T-cell receptor. The histopathologic, immunohistochemical, and PCR findings confirmed the patient had MF.
Of note, the patient’s blood, urine, and stool tests and the liver and kidney function test results were normal. The patient also had normal chest X-ray, echocardiography, electrocardiogram, and ultrasonography results.
The patient was subsequently treated with a combination of NB-UVB irradiation and interferon-alpha (IFN-α) injection therapy three times weekly following the diagnosis. There was a gradual improvement of the lesions containing erythema and desquamation. Most skin tags and brownish lichen papules gradually shrank and disappeared within six months of treatment. The patient still undergoes follow-up examination to date.
Discussion
MF accounts for almost half of all primary lymphoproliferative disorders of the skin. It encompasses many morphological variants, such as erythrodermic, follicular, poikilodermic, syringotropic, papular, granulomatous, hyperpigmented, ichthyosiform, and pigmented purpura-like lesions.3 Previously, ST and acrochordon-like lesions have been largely described in malignancies, such as metastatic malignant melanoma and lipomatous tumors.4,5 Only one similar case describing a 77-year-old male undergoing treatment for MF revealed acrochordon-like lesions despite the histopathologic examination being consistent with MF.6 However, MF involving multiple STs over the whole body has not been previously reported.
Notably, the pathogenesis of ST remains unclear. A study by Fu et al showed that cigarette smoking, alcohol consumption, and genetics might be significant factors in ST development.7 Previous studies postulate that lesion development is also associated with obesity and various endocrinal disorders, especially those involving serum leptin and atherogenic lipid profiles.8 It is reported that mast cells, TNF-α, and TNF-related apoptosis-inducing ligand (TRAIL) play a role in the pathogenesis of STs.9 Abdou et al demonstrated the possible role of mast cells in promoting fibrosis and the development of skin tags that attract eosinophils to synergistically work in inducing more fibrosis in ST development.10
Contrary to the report by McClain CM,6 the patient was the first time diagnosed with MF. The presence of identical clonal T-cell populations of two biopsy specimens and the immunohistochemistry results helped confirm the diagnosis of MF. Notably, the patient’s blood test and the level of serum sex hormone were in the normal range. The lipid profile and body mass index were also normal, suggesting a lack of obesity or hypertriglyceridemia. Staining of the mast cells obtained from the two skin biopsy specimens showed no difference and could not provide enough evidence to prove the role of mast cells in the development of the ST. However, microscopy revealed that the ST was characterized by dense, band-like lymphoid infiltrate with areas of epidermotropism. Most of the STs gradually disappeared within six months of treatment, a phenomenon that might involve the regulation of TNF-α and TRAIL.9
Currently, there lacks enough literature to clearly describe the formation and regression of the skin tag in CTCL or MF. In this case study, the similarities of the histopathologic features of the two different lesions forming the skin tags were consistent with MF diagnosis. The findings of this study suggest that some causes and effects were not previously described in CTCL or MF.
Consent Statements
Written informed consent was provided by the patient to have the case details and any accompanying images published. Institutional approval was not required for this case study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350(19):1978–1988. doi:10.1056/NEJMra032810
2. Furmanczyk PS, Wolgamot GM, Kussick SJ, Sabath DE, Olerud JE, Argenyi ZB. Diagnosis of mycosis fungoides with different algorithmic approaches. J Cutan Pathol. 2010;37:8. doi:10.1111/j.1600-0560.2009.01289.x
3. Hodak E, Amitay-Laish I. Mycosis fungoides: a great imitator. Clin Dermatol. 2019;37(3):255–267. doi:10.1016/j.clindermatol.2019.01.004
4. Kollitz KM, Tcheung WJ, Scheri RP, Selim MA, Nelson KC. An acrochordon-like melanoma metastasis. Arch Dermatol. 2012;148:136. doi:10.1001/archdermatol.2011.1105
5. Paredes BE, Mentzel T. Atypical lipomatous tumor/ “well-differentiated liposarcoma” of the skin clinically presenting as a skin tag: clinicopathologic, immunohistochemical, and molecular analysis of 2 cases. Am J Dermatopathol. 2011;33:603. doi:10.1097/DAD.0b013e3181f1b226
6. McClain CM, Cole MB, Robbins JB, Kantrow SM. Mycosis fungoides involving an acrochordon: a case report. J Cutan Pathol. 2012;39:1131–1134. doi:10.1111/cup.12009
7. Fu Z, Shrubsole MJ, Li G. Interaction of cigarette smoking and carcinogen-metabolizing polymorphisms in the risk of colorectal polyps. Carcinogenesis. 2013;34:779–786. doi:10.1093/carcin/bgs410
8. Idris S, Sunitha S. Assessment of BMI, serum leptin levels and lipid profile in patients with skin tags. J Clin Diagn Res. 2014;8(9):CC01–CC03. doi:10.7860/JCDR/2014/10350.4853
9. El Safoury OS, Fawzy MM, Hay RM. The possible role of trauma in skin tags through the release of mast cell mediators. Indian J Dermatol. 2011;56(6):641–646. doi:10.4103/0019-5154.91819
10. Abdou AG, Maraee AH, Antar AG, Fareed S. Role of mast cells in skin tag development: an immunohistochemical study. Anal Quant Cytol Histol. 2014;36(4):222–230.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.