Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 14
Musculoskeletal Disorders and Associated Factors Among Patients with Chronic Kidney Disease Attending at Saint Paul Hospital, Addis Ababa, Ethiopia
Authors Deme S , Fisseha B, Kahsay G , Melese H , Alamer A , Ayhualem S
Received 13 May 2021
Accepted for publication 22 July 2021
Published 4 August 2021 Volume 2021:14 Pages 291—300
DOI https://doi.org/10.2147/IJNRD.S319991
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Sisay Deme,1 Berihu Fisseha,1 Gebreslassie Kahsay,1 Haimanot Melese,1 Abayneh Alamer,1 Sileshi Ayhualem2
1Department of Physiotherapy, School of Medicine, College of Health Sciences, Mekelle University, Mekelle, Ethiopia; 2Department of human Anatomy, School of Medicine, College of Medicine and Health science, University of Gondar, Gondar, Ethiopia
Correspondence: Sisay Deme
Mekelle University, P.O. Box: 1871, Mekelle, Ethiopia
Tel +251 923272360
Email [email protected]
Background: Musculoskeletal disorders contributed from chronic kidney disease are increasing worldwide. Musculoskeletal disorders had a significant health burden and are leading causes of co-morbidities, disability and low productivity, which potentially affect individual’s functional status and quality of life.
Purpose: The aim of this study was to assess the prevalence of musculoskeletal disorders and its associated factors among patients with chronic kidney attending in Saint Paul Hospital, Addis Ababa, Ethiopia.
Patients and Methods: An institution-based cross-sectional study was conducted on 302 enrolled study participants through systematic random sampling techniques. Face-to-face interview, physical examination and chart reviews were used to collect data using semi-structured questionnaire adapted from a standard Nordic Musculoskeletal Questionnaire and other literatures. Data were entered into Epi Info version 7 and exported to SPSS version 23 for analysis. Bivariate logistic regression analysis was employed with a p-value less than 0.25. Finally, those variables having a p-value less than 0.05 with 95% CI in multivariate analysis were taken as statistically significant.
Results: The prevalence of musculoskeletal disorders among CKD individuals was found to be 58.6% (95% CI; 53.0, 64.1). Being female (AOR = 0.49; 95% CI 0.26, 0.94), age between 40 and 49 (AOR = 3.34; 95% CI 1.07, 10.44), stage III (AOR = 0.24; 95% CI 0.06, 0.89) and stage IV (AOR = 0.24; 95% CI 0.06, 0.89) chronic kidney disease, having HTN (AOR = 7.47; 95% CI 3.47, 16.06), parathyroid hormone level ≥ 100 pg/mL (AOR = 0.43; 95% CI 0.21, 0.87), calcium level < 8.4 mg/dl (AOR = 5.89; 95% CI 2.66, 13.56) and serum 25 hydroxy vitamin D level < 20 ng/mL (AOR = 3.91; 95% CI 1.32, 11.56) were significantly associated with musculoskeletal disorders.
Conclusion: MSDs were shown to be moderately common in CKD patients. Female gender, age between 40 and 49 yrs, stage III and stage IV CKD, hypertension, higher PTH level, lower calcium level and lower vitamin D level were statistically significant in their association with musculoskeletal disorders.
Keywords: musculoskeletal disorders, chronic kidney disease, prevalence, associated factors
Introduction
Chronic kidney disease (CKD) is defined as the presence of proteinuria or a decreased estimated glomerular filtration rate (GFR) of ≤90 mL/min per 1.73 meter square (m2) in two separate measurements within an interval of 3 months. There are five stages of chronic kidney disease. Stage 1 is Kidney damage with a normal Glomerular filtration rate (GFR) or GFR ≥90, stage 2: Kidney damage with mild decrease in GFR or GFR 60–89, stage 3: Moderate decrease GFR or GFR 30–59, stage 4: Severe decrease in GFR or 15–29 and stage 5: Kidney failure (dialysis) or end stage renal failure <15 mL/min per 1.73 m2 respectively.1,2
The number of patients with kidney disease is increasing worldwide, and also is an alarming to health problems.2,3 Globally, the prevalence of CKD accounts 11–13%, and most of the patients were in stage 3 CKD.4 The prevalence of CKD in the general adult population living in the western, middle, eastern, southern and northern Africa were 19.8%, 16.0%, 14.4%, 10.4%, 6.1%, respectively.5
Musculoskeletal disorders (MSDs) are injuries or dysfunctions of muscles, bones, nerves, tendons, ligaments, joints, cartilages, and spinal discs.6
Musculoskeletal disorders in patients with CKD are resulted from abnormal mineral metabolism and extra skeletal calcification.7,8
Individuals with CKD had various musculoskeletal manifestation such as joint pain, carpal tunnel syndrome, muscle cramp, fibromyalgia, flexor tenosynovitis, bone cyst, pathological fracture, joint infection, joint effusion and swelling in the extremities.9,10
The estimated worldwide prevalence of musculoskeletal disorders in patients with CKD accounts 8%–73%. Musculoskeletal pain was the commonest disorder and accounts for around 60% of the CKD patients.10,13 Dialysis related musculoskeletal disorders are observed at high frequency; which accounts 78% and 70% of these conditions lead to serious disabilities and functional restrictions.14
These musculoskeletal problems may contribute increased pain threshold, mobility restriction, impaired functional status, and loss of productivity that enormously affecting quality of life on subjects which undertook longer duration of hemodialysis.11,12,15
Moreover, it may also have tremendous burden on mental health problems like having an increased risk of mood disturbance, anxiety and drug or substance use disorders.16,17
Previous studies in Ethiopia have only focused on causes of CKD like hypertension (HTN) and diabetes mellitus (DM).18,19 Hence, it is failing to address CKD related musculoskeletal disorders and its associated factors. Besides, there is a significant inconsistency and controversial evidence among previous studies in terms of magnitude and associated factors. Additionally, most of researches had been conducted in developed countries, and to the limit of search result, the prevalence and associated factors of musculoskeletal disorders among patients with CKD remain unclear in Ethiopia. Therefore, this study attempts to fill this gap by identifying the burden and contributing factors for the development of musculoskeletal disorders among patients with CKD. Therefore, the aim of this study was to determine the prevalence and identify associated factors of musculoskeletal disorders among chronic kidney disease patients.
Patients and Methods
Study Area and Period
The study was carried out in Addis Ababa, Ethiopia’s capital. The city has a population of 2,739,551 people, according to the 2007 census. Addis Ababa is a grassland biome at an elevation of 2355 meters (7726 feet) and is located at 9°1′48′′N 38°44′24′′E. There are 12 state-run hospitals in the city, as well as several private hospitals. Saint Paul Hospital Millennium Medical College is one of the biggest referral governmental hospital; which had More than 2800 clinical, academic, administrative, and support staff members to provide medical specialist services to patients referred from all across the country. A dialysis unit and a national kidney transplant center are currently available at the hospital for patients with chronic renal disease.20,21 The study was conducted from January 15, 2020 to July 7, 2020 and the study period was March 10 to May 30, 2020.
Study Design and Population
An institutional-based cross-sectional study design was conducted. All patients with chronic kidney disease attending the Nephrology Unit of Saint Paul Hospital were included. Chronic kidney disease patients having severe psychiatric problem, upper extremity and lower extremity arthroplasty, upper extremity and lower extremity amputation, history of trauma and surgery with less than 90 days were excluded based on predetermined criteria.
Sample Size Determination, Sampling Technique and Procedure
The sample size was determined by Epi Info statcalc version 7 using a single population ratio formula,22,23 with the assumption of prevalence of musculoskeletal disorders among CKD was 60.4% in the study conducted in Egypt.11 Five percent marginal error, 95% significance level, with an estimated population of 1404. The overall sample size was 302 when the 10% non-response rate was taken into account.
A systematic random sampling technique was used for selecting study participants. To select the first participant simple random sampling technique (lottery method) was performed, and then each kth range was followed until the predetermined sample size was obtained. The sampling fraction (K) was stated as K= (1404/323) =4.34≈5.
Data Collection Procedures and Data Quality Control Issues
The data were collected by means of a semi-structured questionnaire developed from a standardized Nordic musculoskeletal questionnaire [51] and different literature. 11,13,14,24 Musculoskeletal problems, sociodemographic factors, behavioral issues, and comorbids are all included in the questionnaire. To ensure consistency, the questionnaires were translated into Amharic and then back into English. Three BSc nurses, two BSc physiotherapists, and two BSc physiotherapy supervisors gathered the data. Face-to-face interviews, physical exams, and file reviews were used to gather information. Height measurements were performed using Specification Adhesive Tape (RT-123) in the upright position. The weight was measured using a ground weight scale (TIANSHA = 2003A) with participants standing without shoes. A physiotherapist conducted a physical examination for carpal tunnel syndrome and flexor tenosynovitis. The Phalen test was used to examine for carpal tunnel syndrome, and the Finkelstein test was used to check for flexor tenosynovitis. The review of records was utilized to look for musculoskeletal diseases and clinical variables in the medical records of the study participants. To ensure data quality, data collectors and supervisors were trained for a day on how to approach study participants and implement the questionnaire. Supervisors conducted routine checks for completeness and consistency of data. The questionnaire was pre-tested using a sample of 5% (17 participants) of the total sample size of patients with next chronic kidney disease in a nephrology unit at Zewditu Memorial Hospital, which has similar characteristics with the population selected for the actual study to check for the accuracy of responses, language clarity, and appropriateness of the questionnaire before a week of the actual data collection period. The Principal Investigator and supervisors checked the completeness, accuracy and clarity of the data prior to data entry. Cross-checking of the data was performed prior to the analysis.
Data Processing and Analysis
The data were entered into Epi Info 7 and exported and analyzed using the Statistical Package for the Social Sciences (SPSS) version 23 software. Descriptive statistics were produced for all variables in the study using statistical measurement frequency tables, graphs, percentages, means and standard deviations. The relationship between musculoskeletal disorders and related factors was established through logistic regression analysis. Bivariate logistic regression analysis was used to verify musculoskeletal disorders associated with each independent variable and variables with P values below 0.25 were considered to be potential candidates in the final multivariate logistic regression analysis. In multivariate logistic regression analysis, the variables with a P-value <0.05 were considered statistically significant.
The quality of the model was tested using the Hosmer-Lemeshow goodness-of-fit test and Multi-collinearity was verified using the variance inflation factor (VIF) with a cut-off point of less than 10. Finally, AOR with 95% of confidence interval at p value of <0.05 was reported.
Operational Definitions
Musculoskeletal disorder: In this study, musculoskeletal disorder means having two or more than two of the following symptoms, pain in one or more than one part of the body, osteopenia, muscle pain, joint swelling, soft tissue swelling, CTS, flexor tenosynovitis, fracture, fibromyalgia, muscle cramp, osteoarthritis and septic arthritis.
Alcohol intake: Having more than three drinks of 12-ounce bottle of beer, one glass of wine (5 ounces), or one shot (1.5 ounces) of “hard liquor in a day or more than seven per week for women, and more than four drinks in a day or more than 14 per week for men.25
Cigarette Smoking: It is the habit of smoking if they ever smoked daily for a month or more at list one sticks of cigarette per day.26
Comorbidities: Is the presence of one or more additional confirmed diseases or disorders co-occurring with CKD: (hypertension, diabetes mellitus, heart diseases, COPD and Hyperuricemia).27
Physical exercise: any kinds of moderate to intensive exercise done at least 150 minutes per week.28
Results
Socio-Demographic and Behavioral Characteristics of Patients with Chronic Kidney Disease
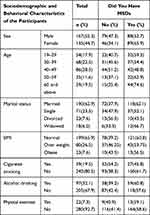
A total of 302 people responded to the study, giving it a 93.5% response rate. The mean age of the participants in the study was 44±15.03 years. More than half of the participants 167 (55.3%) were male, while the majority of the participants 190 (62.9%) were married. A total of 199 people (65.5%) had a normal BMI. Of a total 302 respondents most of the participants 243 (80.5%) did not smoke cigarette. Aside from alcohol use, 97 (32.1%) of the total 302 respondents drank alcohol, and 280 (92.7%) of the respondents did not engage in regular physical activity (Table 1).
Disease and Treatment Related Characteristics of Patients with Chronic Kidney Diseases
The majority of the subjects 81 (26.8%) had stage one CKD. Around 265 (87.7%) of those who took part in the study did not use hemodialysis. The majority of stage 5 CKD patients, 19 (51.4%), had been on hemodialysis for less than three years. Hypertension was the most prevalent co-morbid condition, accounting for 114 (37.7%). Around 77 (25.5%) of the individuals had diabetes, 49 (16.2%) had heart disease, 36 (11.9%) had Hyperuricemia, and COPD was the list of 13 (4.3%) comorbid conditions among the participants (Table 2).
Laboratory Findings of Patients with Chronic Kidney Diseases
A total of 145 (48.0%) of the 302 study participants had a parathyroid hormone level of 100 pg/mL or more, while 210 (69.5%) had a calcium content of less than 8.4 mg/dl. Out of a total of 302 study participants, 155 (51.3%) had a serum 25 hydroxy vitamin D level of 20–29 ng/mL, 145 (48.0%) had a serum 25 hydroxy vitamin D level of 100 pg/mL, 269 (89.1%) had a CRP level of >5 mg/l, and roughly 261 (86.4%) had a neutrophil level of less than 1500mm3 (Table 3).
 |
Table 3 Prevalence of Musculoskeletal Disorders Among Patients with Chronic Kidney Diseases Attending in Saint Paul Hospital, Addis Ababa, Ethiopia Based on Laboratory Findings (n=302) |
Prevalence of Musculoskeletal Disorders Among Chronic Kidney Disease Patients
Over the last year, the overall prevalence of musculoskeletal disorders in patients with chronic kidney disease visiting Saint Paul Hospital was 177 (58.6%), with [95% CI; (53%, 64.1%)]. The 7 day prevalence of musculoskeletal disorders was 159 (52.6%). Muscle pain 123 (69.5%) was the most commonly reported musculoskeletal complaint among persons with MSDs. Muscle pain, joint swelling, and soft tissue swelling were the most often reported musculoskeletal symptoms among patients with MSDs, with 123 (69.5%), 120 (67.7%), and 111 (62.7%), respectively. The list recorded was fracture 18 (5%) compared to other musculoskeletal disorders assessed in this study.
Among those who had MSDs, 65.5% were female and the prevalence of MSDs was higher among patients with the age group of 60 and above 44 (74.6%), and patients who were married 118 (62.1%). From a total of 177 participants who had MSDs 164 (58.6%) of them were not participating in physical exercises. The prevalence of MSDs was higher among study participants who had hypertension 96 (84.2%) as a comorbidity condition, and also in stage IV CKD patients 31 (81.6%). Among 177 participants who had MSDs 148 (70.6%) of the participants were scored <8.4 mg/dl in their calcium level.
Factors Associated with Musculoskeletal Disorders
In bivariate logistic regression analysis, MSDs were significantly associated with sex, age, marital status, cigarette smoking, physical activity, stage of the diseases, hemodialysis duration, comorbidities of hypertension, heart diseases and Hyperuricemia, level of parathyroid hormone, calcium level, CRP level, neutrophils and serum 25 hydroxy vitamin D levels. In multivariate logistic regression analysis, MSDs was significantly associated with female sex [AOR=0.49; 95% CI (0.26, 0.94)], stage III CKD [AOR=0.24; 95% CI (0.06, 0.89)], stage IV CKD [AOR=0.24; 95% CI (0.06, 0.89)], having hypertension [AOR=7.47; 95% CI (3.47, 16.06)], parathyroid hormone level ≥100 pg/mL [AOR=0.43; 95% CI (0.21, 0.87)], calcium level <8.4mg/dl [AOR=5.89; 95% CI (2.66, 13.56)] and serum 25 hydroxy vitamin D level <20ng/mL [AOR=3.91; 95% CI (1.32, 11.56)]. (Table 4)
 |
Table 4 Factors Associated with MSDs Among Patients with Chronic Kidney Diseases Attending in Saint Paul Hospital, Addis Ababa, Ethiopia (n=302) |
Discussion
The purpose of this study was to determine the prevalence of musculoskeletal disorders and their related factors among chronic kidney disease patients at Saint Paul’s Hospital’s Nephrology Unit in Addis Ababa, Ethiopia. The overall prevalence of musculoskeletal disorders was found to be 58.6% in this study, with variables such as being female, older age, stage of disease, comorbidity of hypertension, parathyroid level, serum 25 hydroxy vitamin D, and calcium level showing a significant association with musculoskeletal disorders.
According to this study, the prevalence of MSDs in the past 12 months among CKD patients attending at Saint Paul’s hospital was 58.6% (95% CI; 53.0, 64.1). The result of this study was relatively higher than the studies done in Spain 38.0%29 and Canada 26.7%.30 This disparity can be explained by differences in MSD inclusion; the current study investigated more musculoskeletal disorders, whereas the study in Spain assessed only musculoskeletal pain, and the study in Canada assessed only fracture prevalence. Differences in study design, eligibility criteria, and the use of a small sample size in both investigations compared to the current study could be another factor. However, the result of the current study showed relative similarity with the study done in Turkey Ankara 60.4%.25 This resemblance could be due to similarities in musculoskeletal disorder definitions, data collection methods, study design, and included musculoskeletal disorders.
On the other hand, the results of this study were lower than a study done in Beirut, Lebanon, which found a prevalence of MSDs, of 76.4%.31 This observed difference could be related to be difference in eligibility criteria; in the Lebanon study, all of the participants were stage V chronic kidney disease patients, which has been a positive correlation with MSDs.32 This discrepancy could be due to the adoption of differing definitions for musculoskeletal illnesses; in the Lebanon study, MSDs were classified according to predetermined diagnostic criteria. The difference in data collection methods could explain the discrepancy; they used a rheumatologist and additional radiographic evidence, whereas the current study used a standard Nordic musculoskeletal disorders questionnaire and chart revision from a patient’s medical record, which could explain the discrepancy.
The prevalence of current study showed comparable similarity with the study done in Taiwan 53.3%.33 This can be explained by the similarities in study design, data collection method, and eligibility criteria, as well as the fact that musculoskeletal problems are treated in similar clinical and hospital settings. Similarly, the findings of this research are similar to those of a study conducted in Zagazig, Egypt 60.4%.11 The possible explanation might be similarity in exclusion criteria, study design, method of data collection and analysis and the possibility of similar hospital setups. However, the result of the current study showed that the prevalence of MSDs was relatively higher with another study done in Egypt 51.9%.34 This difference can be explained by differences in the included MSDs the current study included more musculoskeletal disorders, while study in Egypt limited to assess only about musculoskeletal pain. The other possible reason might be the small sample size used in the study, compared to the current study that might be underestimating the prevalence.
This study found that there was a negative significant association between MSDs and female sex (AOR=0.49 P=0.03). Being female, approximately 50.6% less likely to develop MSDs than male. This result is supported by a study done in Egypt.24 In their study, MSDs were frequently observed in the male sex. On the contrary, the result of this study was not similar to the studies done in Spain and Canada.29,30 In Spanish study, male sex were 54% less likely to develop MSDs than female (AOR=0.46; P<0.00). This difference might be sample size difference of both studies with the current study, and also such differences might be due to the genetic or social differences between our community and those developed countries.
In the present study, participants between the ages of 40–49 years were 3.3 times more likely to develop MSDs than participants with age between 19 and 29 years (AOR=3.34; P=0.04). This result is supported by studies done in Lebanon (AOR=1.04; P=0.04), Spain (AOR=1.31; P<0.00) and also study in Canada.29–31 This similarity can be explained by with ageing musculoskeletal tissue had increased risk of bone fragility, a decrease in ligament elasticity, a decreased muscular strength and function and a loss in cartilage resilience. This will affect the tissue not to perform their normal function.35
According to this study, stage of CKD and MSDs showed a negative significant association. Participants who were in stage III CKD, 76.1%, stage IV CKD, 76.3% less likely to develop MSDs than participants in stage I CKD (AOR=0.24; P=0.03), (AOR=0.24; P=0.03), respectively. The finding of this study was different from the studies done in Taiwan and Canada.30,33 This disparity could be due to a difference in sample size and a higher number of participants in stage I of this investigation. Another variation could be the use of a prospective study design and eligibility criteria that differ.
This study indicates that participants having HTN as a co-morbidity were approximately 7.5 times more likely to develop MSDs than those who have not (AOR= 7.47; P=0.00). This result had shown difference with the study done in Taiwan, Lebanon and Ankara turkey.25,33,36 This difference might be the number of patients having MSDs and HTN can be varied in the studies, sample size difference and difference in eligibility criteria might be the potential reasons.
In the current study, parathyroid level shows a significant association with MSDs. Study participants having parathyroid level ≥100 pg/mL 57.3% less likely to develop MSDs than study participants having a parathyroid level ≤65 pg/mL (AOR=0.43; P=0.02). The result of this was shown difference with the study done in Egypt.34 Reported that higher level of parathyroid level was associated with MSDs. This difference might be because of differences in sample size and eligibility criteria. They used smaller sample size and they only included Stage V CKD patients, while the current study included participants from all stages and the possible reason could be that they obtained blood samples during data collection but in this study chart revision was made.
According to this study, participants with their calcium level below 8.4mg/dl were approximately 6.0 times more likely to develop MSDs than those with ≥8.4 mg/dl (AOR=5.89; P=0.00). This result showed similarity with the study done in Egypt P<0.00.34 This similarity might be lower calcium level and may play a role in the development of endothelial dysfunction and a disrupted mineral metabolism can be associated with different musculoskeletal problems.37
The finding of this study also showed that participants having Serum 25 hydroxy vitamin D level <20 ng/mL, approximately 4.0 times more likely to have MSDs than those with ≥30 ng/mL (AOR=3.91; P=0.01). This finding shows similarity with the studies done in Egypt and Canada.30,34 This similarity explained by lower level of Serum 25 hydroxy vitamin D leads to secondary hyperparathyroidism that causes increased bone loss, osteopenia, myopathy, muscle weakness and affect the muscle contraction function and muscle metabolism.38
The risk of recall bias while assessing some musculoskeletal problems was one of the study’s weaknesses, as was the use of secondary data. The participants’ alcohol use and cigarette smoking were subject to social desirability bias. Furthermore, this study did not assess pain intensity or define the severity of musculoskeletal disorders. To address musculoskeletal issues in chronic renal disease patients, it is advised that preventive initiatives, policies, and treatment guidelines be developed. It is suggested that a referral mechanism be established between the nephrology unit and the physiotherapy department. To address this health problem among people with CKD, physiotherapists must take the lead in raising awareness and developing a screening program. Further rigorous empirical studies are warranted.
Conclusion
The prevalence of musculoskeletal problems was moderate, according to this study. Patients with chronic renal disease face a substantial number of musculoskeletal issues. MSDs were significantly associated with female gender, age between 40 and 49 yrs, stage III and stage IV CKD, hypertension, higher PTH level, lower calcium level and lower vitamin D level.
Data Sharing Statement
Since this is a funded work, the data sets used and/or analyzed during the current study are available from the corresponding author on reasonable formal request.
Ethics and Consent
Ethical approval was obtained from Mekelle University, College of Health Sciences research and community service, ethical review committee. The aims of the study were explained to the participants of the study and a written informed consent was obtained from the study subjects. The involvement of the participants in the study was on a voluntary basis; participants who were unwilling to participate in the study and those who wished to quit their participation at any stage were informed to do so without any restrictions confidentiality was maintained at all level of the study. This study was conducted in accordance with the Declaration of Helsinki. Consent for publish: not applicable.
Acknowledgments
The authors gratefully acknowledge and appreciate the financial support provided by Mekelle University’s College of Health Science, School of Medicine, and Department of Physiotherapy for this project. The authors also want to express their gratitude to all of the study participants, data collectors, and supervisors for their invaluable and fruitful contributions to this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was fully funded by Mekelle University. The funder has no role in the design of the study, data collection, and analysis, interpretation of data and in writing the manuscript.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Levey AS, Eckardt K-U, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. 2005;67(6):2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
2. Kazancioğlu R. Risk factors for chronic kidney disease: an update. Kidney Int Suppl. 2013;3(4):368–371. doi:10.1038/kisup.2013.79
3. Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi:10.1016/S0140-6736(13)60687-X
4. Control CFD. And prevention, chronic kidney disease in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease; 2019.
5. Kaze AD, Ilori T, Jaar BG, et al. Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol. 2018;19(1):125. doi:10.1186/s12882-018-0930-5
6. da Costa BR, Vieira ER. Risk factors for work‐related musculoskeletal disorders: a systematic review of recent longitudinal studies. Am J Ind Med. 2010;53(3):285–323.
7. Alexander AJ, Jahangir D, Lazarus M, et al. Imaging in chronic kidney disease‐metabolic bone disease. Semin Dial. 2017;30:361–368. doi:10.1111/sdi.12598.
8. Qunibi WY, Henrich WL. Overview of chronic kidney disease-mineral and bone disorder (CKD-MBD); 2018.
9. Lim C, Ong K. Various musculoskeletal manifestations of chronic renal insufficiency. Clin Radiol. 2013;68(7):E397–e411. doi:10.1016/j.crad.2013.01.025
10. Kay J, Bardin T. Osteoarticular disorders of renal origin: disease-related and iatrogenic. Best Pract Res Clin Rheumatol. 2000;14(2):285–305. doi:10.1053/berh.2000.0066
11. El-Najjar AR, Amar HA, El Wahab Selim HA, et al. Musculoskeletal disorders in hemodialysis patients and its impact on physical function (Zagazig University Nephrology Unit, Egypt). Egypt Rheumatol Rehabil. 2014;41(4):152. doi:10.4103/1110-161X.147356
12. Pham P-CT, Toscano E, Pham PM, et al. Pain management in patients with chronic kidney disease. NDT Plus. 2009;2(2):111–118.
13. Cohen SD, Patel SS, Khetpal P, et al. Pain, sleep disturbance, and quality of life in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2007;2(5):919–925. doi:10.2215/CJN.00820207
14. Ballestas IP, Santos AM, Angarita I, et al. Cross-cultural adaptation of the community oriented program for the control of rheumatic diseases (COPCORD) in a Colombian population. Revista Colombiana De Reumatología (English Edition). 2019;26(2):88–96. doi:10.1016/j.rcreue.2019.01.010
15. Brennan-Olsen SL, Cook S, Leech MT, et al. Prevalence of arthritis according to age, sex and socioeconomic status in six low and middle income countries: analysis of data from the World Health Organization study on global ageing and adult health (SAGE) wave 1. BMC Musculoskelet Disord. 2017;18(1):271. doi:10.1186/s12891-017-1624-z
16. Fries JF, Spitz P, Kraines RG, et al. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23(2):137–145. doi:10.1002/art.1780230202
17. Ren Q, Shi Q, Ma T, et al. Quality of life, symptoms, and sleep quality of elderly with end-stage renal disease receiving conservative management: a systematic review. Health Qual Life Outcomes. 2019;17(1):78. doi:10.1186/s12955-019-1146-5
18. Fiseha T, Kassim M, Yemane T. Prevalence of chronic kidney disease and associated risk factors among diabetic patients in southern Ethiopia. Am J Health Res. 2014;2(4):216–221. doi:10.11648/j.ajhr.20140204.28
19. Kore C, Tadesse A, Teshome B, et al. The magnitude of chronic kidney disease and its risk factors at Zewditu Memorial Hospital, Addis Ababa, Ethiopia. J Nephrol Ther. 2018;8(3):313. doi:10.4172/2161-0959.1000313
20. Assefa B, Duko B, Ayano G, et al. Prevalence and factors associated with depressive symptoms among patient with chronic kidney disease (CKD) in Black Lion Specialized Hospital and Saint Paulo’s Hospital Millennium Medical College, Addis Ababa, Ethiopia: cross sectional study. J Psychiatry. 2016;19(390):2. doi:10.4172/2378-5756.1000390
21. Collage SPHMM. About saint Paul's hospital millennium medical collage; 2010. Available from: https://sphmmc.edu.et/.
22. Degu G, Tessema F. Lecture notes for health science students. Biostatistics. Carter Center; EPHTI, Ethiopia Ministry of Health; Ethiopia Ministry of Education; 2005. Available from: https://www.cartercenter.org/resources/pdfs/health/ephti/library/lecture_notes/env_health_science_students/ln_biostat_hss_final.pdf. Accessed August 2, 2021.
23. Daniel WW, Cross CL. Biostatistics: A Foundation for Analysis in the Health Sciences. Wiley; 2018.
24. Haroon MM, Sayed S, Al-ghitany A, et al. Rheumatic and musculoskeletal manifestations in renal hemodialysis patients. Int J Clin Rheumtol. 2018;13(5):263. doi:10.4172/1758-42721000196
25. Fidan F, Alkan BM, Tosun A, et al. Quality of life and correlation with musculoskeletal problems, hand disability and depression in patients with hemodialysis. Int J Rheum Dis. 2016;19(2):159–166. doi:10.1111/1756-185X.12171
26. Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164(20):2206–2216. doi:10.1001/archinte.164.20.2206
27. Tonelli M, Wiebe N, Guthrie B, et al. Comorbidity as a driver of adverse outcomes in people with chronic kidney disease. Kidney Int. 2015;88(4):859–866. doi:10.1038/ki.2015.228
28. Global recommendations on physical activity for health. World Health Organization; 2010. Available from: https://www.who.int/dietphysicalactivity/global-PA-recs-2010.pdf. Accessed August 2, 2021.
29. Caravaca F, Gonzales B, Bayo MÁ, et al. Musculoskeletal pain in patients with chronic kidney disease. Nefrología (English Edition). 2016;36(4):433–440. doi:10.1016/j.nefroe.2016.10.005
30. West SL, Lok CE, Langsetmo L, et al. Bone mineral density predicts fractures in chronic kidney disease. J Bone Mineral Res. 2015;30(5):913–919. doi:10.1002/jbmr.2406
31. Hage S, Hage V, El-Khoury N, et al. Musculoskeletal disorders in hemodialysis patients: different disease clustering according to age and dialysis vintage. Clin Rheumatol. 2019;39:533.
32. Akasbi N, Houssaini TS, Tahiri L, et al. Rheumatic complications of long term treatment with hemodialysis. Rheumatol Int. 2012;32(5):1161–1163. doi:10.1007/s00296-010-1756-z
33. Hsu H-J, Wu I-W, Hsu K-H, et al. The association between chronic musculoskeletal pain and clinical outcome in chronic kidney disease patients: a prospective cohort study. Ren Fail. 2019;41(1):257–266. doi:10.1080/0886022X.2019.1596817
34. Ghonemy TA, Allam HM, Elokely AM, et al. Chronic pain in hemodialysis patients: role of bone mineral metabolism. Alexandria j Med. 2016;52(4):337–342. doi:10.1016/j.ajme.2015.12.002
35. Gheno R, Cepparo JM, Rosca CE, Cotten A. Musculoskeletal disorders in the elderly. J Clin Imaging Sci. 2012;2:39. doi:10.4103/2156-7514.99151
36. Hage S, Hage V, el-Khoury N, et al. Musculoskeletal disorders in hemodialysis patients: different disease clustering according to age and dialysis vintage. Clin Rheumatol. 2020;39(2):533–539. doi:10.1007/s10067-019-04786-w
37. Pazianas M, Miller PD. Current understanding of mineral and bone disorders of chronic kidney disease and the scientific grounds on the use of exogenous parathyroid hormone in its management. J Bone Metab. 2020;27(1):1–13. doi:10.11005/jbm.2020.27.1.1
38. Molina P, Carrero JJ, Bover J, et al. Vitamin D, a modulator of musculoskeletal health in chronic kidney disease. J Cachexia Sarcopenia Muscle. 2017;8(5):686–701. doi:10.1002/jcsm.12218
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.