Back to Journals » OncoTargets and Therapy » Volume 12
Multiple myeloma with dural mater involvement
Authors Liu S , Li X, Li Y , Li D, Wang Y , Tian C
Received 23 January 2019
Accepted for publication 12 March 2019
Published 3 May 2019 Volume 2019:12 Pages 3373—3375
DOI https://doi.org/10.2147/OTT.S202662
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Su Liu,1 Xubin Li,2 Yueyang Li,1 Dongying Li,1 Yafei Wang,1 Chen Tian1
1Department of Hematology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin 300060, People’s Republic of China; 2Imaging Department, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin 300060, People’s Republic of China
Abstract: Multiple myeloma is an incurable clonal B-cell malignancy which may present with neoplastic monoclonal plasma cells in either bone or soft tissues. Central nervous system (CNS) involvement such as dural myeloma or intraparenchymal infiltration, or with diffuse leptomeningeal involvement, is uncommon. Dural involvement of myeloma without parenchymal or leptomeningeal disease is an even rarer occurrence; therefore there are no established treatment guidelines for CNS myelomatosis. Here we reported a refractory MM patient progressed to dura mater involvement after the induction therapy but showed good response to lenalidomide treatment.
Keywords: multiple myeloma, dura mater involvement, lenalidomide
Multiple myeloma (MM) is a malignant neoplasm of plasma cells, generally located in the bone marrow (BM).1 Extramedullary plasmacytoma (EMP) can arise at any time during the course of the disease,2 with the most frequent involved metastases being the head and neck region (sinuses, naso-, and oropharynx), gastrointestinal tract, and lungs.3,4 Intracranial and central nervous system (CNS) involvement are rare, accounting for approximately 1% of MM patients, while dura mater involvement is even rarer.5,6 Here, we present a case of refractory MM patient progressed to dura mater involvement after the induction therapy.
A 49-year-old woman presented with a 4-month history of right rib pain. 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)–computed tomography (CT) showed increased FDG uptake in multiple bones with a soft tissue mass on the right fifth rib. The laboratory findings were as follows: serum immunofixation electrophoresis (SIFE) revealed IgD-λ type monoclonal immunoglobulin; serum-free λ light chain 645 mg/L, κ/λ ratio 0.0108; CBC counts revealed hemoglobin (Hb) 119 g/L, white blood cell (WBC) count 4.43×109/L, platelet count 210×109/L; beta-2 microglobulin 3.52 mg/L, LDH 188U/L. Flow cytometry of the BM showed 8.15% plasma cells with the immunophenotype CD138+, CD38+, cLambda+, CD200+, CD19−, CD20−, CD45−, CD56−, and cKappa−. BM biopsy showed 80% lambda-restricted, CD138 and CD38 double-positive plasma cells. The patient was diagnosed as IgD-λ MM in June 2018 (Durie–Salmon stage ⅢA, ISS stageⅡ). After two cycles of PAD (bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11; liposomal doxorubicin 30 mg/m2 on day 1; dexamethasone 20 mg on days 1–2, 4–5, 8–9, and 11–12) treatment, she achieved partial response (PR). Another two cycles of PAD regimen was given to her.
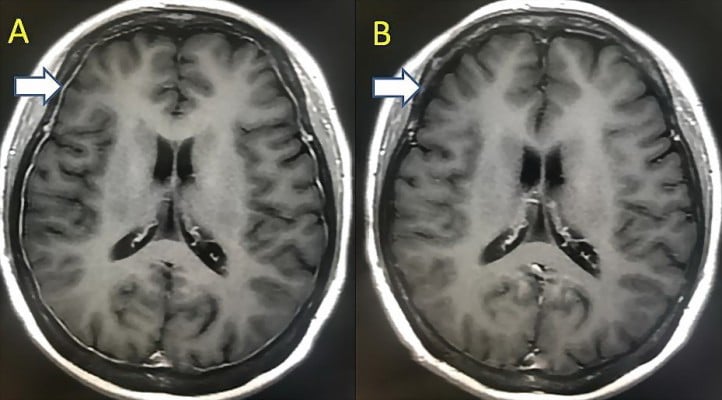
But after the induction treatment, she complained of progressive jaw numbness and mild dysphagia. Laboratory tests were as follows: CBC counts revealed Hb 83 g/L, WBC count 4.81×109/L, platelet count 40×109/L; SIFE revealed IgD-λ-type monoclonal immunoglobulin; serum-free λ light chain 267.5 mg/L, κ/λ ratio 0.0239. BM aspirate showed no abnormal plasma cells. Flow cytometry of the BM showed 2.22% lambda-restricted, CD138- and CD38-positive plasma cells. She denied any history of head trauma. Enhanced magnetic resonance imaging (MRI) of the head revealed thickening of dural mater with enhanced signal indicating metastasis of malignant plasma cells, but the tissue biopsy was not attempted due to low platelet count (Figure 1A). Flow cytometry of the cerebrospinal fluid (CSF) showed no plasmacytosis. According to the results of the examination, her disease was progressed. Aggressive systemic therapy that crosses the blood–brain barrier was implemented, ie, lenalidomide 25 mg on days 1–21, cyclophosphamide 500 mg on days 1, 8, 15 and 21, and dexamethasone 30 mg on days 1–4 and 12–15 (RCD). After one cycle of RCD treatment, all the symptoms disappeared and the platelet count increased to normal. Serum-free λ light chain decreased to 51.6 mg/L. The enhanced MRI of the head revealed a considerable decrease in the size of the dural masses with a significant weak signal (Figure 1B).
 | Figure 1 (A) The MRI of the patient when neurological symptoms appeared. (B) The MRI of the patient after treatment.Abbreviation: MRI, magnetic resonance imaging. |
CNS involvement of myeloma is rare, generally located in brain parenchyma, pia, and dura mater. Amyloid deposition may be seen in dura-associated PCM. It is seldom that dura mater is the only involved area, with few cases reported; most were women with IgG-κ isotype.7–9 The case we present is the only one with IgD-λ isotype, and with only dura mater involvement without cranial nerve or subdural hemorrhage complications.
The prognosis of MM patients with CNS involvement is poor.10,12 All the cases reported previously died soon after meningeal metastases, with a median survival time 0.1–3 months, except one case reported by Roddiehad.12 The patient he reported had a favorable response to combined chemotherapy (methotrexate, idarubicin, and dexamethasone) and cranial radiotherapy.13
Because of the low incidence, there is no treatment guideline for patients with CNS involvement. The appropriate treatment may include intrathecal chemotherapy and systemic chemotherapy that cross the blood–brain barrier, with or without cranial irradiation.5 Although prognosis is generally poor, the survival of previously untreated patients and patients with favorable cytogenetic profile might be prolonged due to systemic treatment and/or radiotherapy,14 and new agents plus SCT appear to represent optimal treatment.15 There are some new potentionally effective drugs like pomalidomide, marizomib, and daratumumab.16–18 In this case, the patient was resistant to bortezomib and liposomal doxorubicin, and her platelet count was very low, indicating she was unable to endure the standard chemotherapy. So lenalidomide, cyclophosphamide, and dexamethasone were given to her. The neurologic symptoms disappeared after one cycle treatment; however, her response to RCD regimen need further evaluation.
Ethics approval and consent to participate
The patient has given her written informed consent to publish the case (including publication of images). The study protocol was approved by the institution committee on human research of the Tianjin Medical University Cancer Institute and Hospital.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Availability of data and materials
The material supporting the conclusion of this case has been included within the article.
Acknowledgments
This work was supported by grants 81670104 from the National Natural Science Foundation of China (NSFC).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi:10.3322/caac.21387
2. Cerase A, Tarantino A, Gozzetti A, et al. Intracranial involvement in plasmacytomas and multiple myeloma: a pictorial essay. Neuroradiology. 2008;50(8):665–674. doi:10.1007/s00234-008-0390-x
3. Nahi H, Genell A, Walinder G, et al. Incidence, characteristics, and outcome of solitary plasmacytoma and plasma cell leukemia. Population-based data from the swedish myeloma register. Eur J Haematol. 2017;99:216–222. doi:10.1111/ejh.12907
4. Creach KM, Foote RL, Neben-Wittich MA, et al. Radiotherapy for extramedullary plasmacytoma of the head and neck. Int J Radiat Oncol Biol Phys. 2009;73(3):789–794. doi:10.1016/j.ijrobp.2008.04.077
5. Lee D, Kalff A, Low M, et al. Central nervous system multiple myeloma—potential roles for intrathecal therapy and measurement of cerebrospinal fluid light chains. Br J Haematol. 2013;162:371–375. doi:10.1111/bjh.12404
6. Kusano Y, Terui Y, Nishimura N, et al. Myelomatous meningitis: acase report. Int J Hematol. 2016;104:149–150. doi:10.1007/s12185-016-2024-0
7. Wang AR, Bogusz AM. Extreme amyloid depositions in the calvarium and dura associated with plasma cell myeloma. Int J Surg Pathol. 2017;25(2):163–164. doi:10.1177/1066896916668991
8. Méndez CE, Hwang BJ, Destian S, et al. Intracranial multifocal dural involvement in multiple myeloma: case report and review of the literature. Clin Lymphoma Myeloma Leuk. 2010;10(3):220–223. doi:10.3816/CLML.2010.n.035
9. Gascón N, Pérez-Montero H, Guardado S, et al. Dural plasmacytoma with meningeal myelomatosis in a patient with multiple myeloma. Case Rep Hematol. 2018;2018:1–4. doi:10.1155/2018/6730567
10. Wilberger AC, Prayson RA. Intracranial involvement by plasma cell neoplasms. Am J Clin Pathol. 2016;146:156–162. doi:10.1093/ajcp/aqw058
11. Maghfoor I, Perry MC. Malignancy: meningeal myeloma: a case report and review of the literature. Hematology. 2000;5:47–52.
12. Kaito S, Muto H, Takebayashi S, et al. Extradural plasmacytoma with central nervous system involvement in newly diagnosed multiple myeloma. Int J Hematol. 2017;106:455–456. doi:10.1007/s12185-017-2277-2
13. Roddie P, Collie D, Johnson P. Myelomatous involvement of the dura mater: a rare complication of multiple myeloma. J Clin Pathol. 2000;53:398–399. doi:10.1136/jcp.53.5.398
14. Jurczyszyn N, Grzasko A, Gozzetti J, et al. Central nervous system involvement by multiple myeloma: a multi-institutional retrospective study of 172 patients in daily clinical practice. Am J Hematol. 2016;91(6):575–580. doi:10.1002/ajh.24351
15. Gozzetti A, Cerase F, Lotti F, et al. Extramedullary intracranial localization of multiple myeloma and treatment with novel agents: a retrospective survey of 50 patients. Cancer. 2012;118(6):1574–1584. doi:10.1002/cncr.26447
16. Mussetti A, Dalto S, Montefusco V. Effective treatment of pomalidomide in central nervous system myelomatosis. Leuk Lymphoma. 2013;54(4):864–866. doi:10.3109/10428194.2012.718343
17. Badros Z, Singh B, Dhakal B, et al. Marizomib for central nervous system-multiple myeloma. Br J Haematol. 2017;177(2):221–225. doi:10.1111/bjh.14498
18. Varga G, Mikala G, Gopcsa L, et al. Multiple myeloma of the central nervous system: 13 cases and review of the literature. J Oncol. 2018;2018:3970169. doi:10.1155/2018/3970169
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
