Back to Journals » International Journal of Nanomedicine » Volume 17
Multifunctional Targeting Liposomes of Epirubicin Plus Resveratrol Improved Therapeutic Effect on Brain Gliomas
Authors Kong D, Hong W , Yu M , Li Y , Zheng YX, Ying X
Received 30 October 2021
Accepted for publication 9 February 2022
Published 14 March 2022 Volume 2022:17 Pages 1087—1110
DOI https://doi.org/10.2147/IJN.S346948
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Dehua Kong,* Wenyu Hong,* Miao Yu, Yanxia Li, YaXin Zheng, Xue Ying
Collaborative Innovation Center of Sichuan for Elderly Care and Health & Key Laboratory of Sichuan Province for Specific Structure of Small Molecule Drugs, School of Pharmaceutical Sciences, Chengdu Medical College, Chengdu, 610500, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xue Ying; YaXin Zheng, School of Pharmaceutical Sciences, Chengdu Medical College, Chengdu, 610500, People’s Republic of China, Tel +86 135-7945-5890 ; +86 173-8187-6167, Email [email protected]; [email protected]
Introduction: The inability of many therapeutic molecules to cross the blood–brain barrier (BBB) combined with poor penetration into tumor tissue leads to difficult challenges for treatment of brain tumors. In order to settle these hurdles, we developed a novel multifunctional targeting carrier which enables drugs to transport across the BBB and targets brain tumor tissues.
Methods: In the multifunctional targeting liposomes, the natural compound resveratrol (RES) was incorporated into the lipid bilayer membranes of liposomes, while p-aminophenyl-α-D-manno-pyranoside (MAN) and wheat germ agglutinin (WGA) were conjugated to the liposomal surface. Epirubicin (EPI) as an anticancer drug was then loaded into liposomes. Then, liposomes were characterized by evaluation on particle size, zeta potential and apparent morphology. The epirubicin plus resveratrol liposomes modified with WGA and MAN were applied to glioma cells and BBB model in vitro and C6 glioma-bearing rats in vivo.
Results: The multifunctional targeting liposomes were round shaped with a smooth surface and uniform particle size. In consideration of the SRB results, the multifunctional targeting liposomes indicated a significant inhibitory effect, suggesting that MAN plus WGA generated robust drug delivery effects into the brain tumor cells. The glioma cells after administering epirubicin plus resveratrol liposomes modified with WGA and MAN displayed the most significant uptake and apoptosis conducted by flow cytometry. In the multifunctional targeting effects assay, the epirubicin plus resveratrol liposomes modified with WGA and MAN exhibited the strongest effects of crossing the BBB and then targeting brain tumor cells. In tumor-bearing rats after applying multifunctional targeting liposomes, the median survival time was evidently observed as being markedly longer than other controls.
Conclusion: The epirubicin plus resveratrol liposomes modified with WGA and MAN exhibited strong ability to improve epirubicin and resveratrol transporting across the BBB and therapeutic effect on brain glioma, showing multifunctional targeting capability.
Keywords: multifunctional targeting liposomes, blood–brain barrier, apoptosis, resveratrol, brain gliomas
Graphical Abstract:
Introduction
Cancer is a common and frequently-occurring disease which causes a serious threat to human health. The treatment of brain cancer is one of the most difficult challenges in oncology. Primary brain tumors are one of the 10 main causes of death by cancer.1,2 Of all primary brain tumors, malignant brain glioma remains a significant problem world-wide: invasive glioma cells rapidly infiltrate and disrupt normal tissue architecture, making complete surgical removal virtually impossible.3,4 Despite treatment combining surgical resection, radiotherapy and chemotherapy, the median survival span of patients (1 year) has not been significantly changed for 30 years.4 On the one hand, a majority of therapeutic drugs cannot be delivered to tumors in the brain directly5,6 because of the existence of blood–brain barrier (BBB) formed by a network of closely sealed endothelial cell monolayers.7,8 On the other hand, the therapeutic drugs are not always enriched in the tumor but also distributed to normal tissues, and cause serious side effects including therapeutic resistance leading to recurrence and metastasis. Therefore, it is urgent to develop a novel drug delivery system that can meet the requirement of efficient brain tumor therapy of transport across the BBB and glioma tissue-specific targeting. In this regard, we hypothesized that multifunctional targeting liposomes modified with ligands could transfer therapeutic agent through the BBB and subsequently target brain tumor cells. Thus, it can improve the anti-glioma effect and reduce the adverse effects to normal cells.
Epirubicin (EPI) is called adriamycin, and is an isomer of doxorubicin, namely a 4 ‘ hydroxyl from the sequence reversed.9 Epirubicin is a weak alkaline due to its primary amine and generally medicinal hydrochloride. Epirubicin hydrochloride, identified as one of the most potent anti-glioblastoma agents, is water-soluble with red fluorescence10 and significantly more effective than current clinical anti-glioblastoma drug TMZ.11 However, epirubicin can cause cardiotoxicity and often leads to serious side effects after systematic administration. The mechanism of epirubicin’s antitumor effect is due to its activity as an intercalating agent, embedding between DNA base pairs, interfering with transcription, therefore preventing the synthesis of mRNA and leading to cellular senescence.12,13
Resveratrol (RES) is a natural product which is widely found in plant species and belongs to flavonoid polyphenol family of compounds. Resveratrol has multiple functions of adjusting lipoprotein metabolism and lipid oxidation, inhibiting platelet aggregation, anti-inflammatory and anti-tumor effect.14 In recent years, resveratrol’s antitumor effect has caught the attention of many researchers. Resveratrol has been found to inhibit tumor cell proliferation, induce cell cycle arrest and apoptosis, and interfere with related signal transduction pathways. In vivo studies have also shown that resveratrol has little to no harmful side effects.15 Thus, due to its tumor suppressive nature, resveratrol is therefore an ideal natural chemotherapeutic agent.16 Nevertheless, the application of resveratrol in clinic is confined because of its high lipid solubility and low bioavailability.
The p-aminophenyl-α-D-manno-pyranoside (MAN) is considered to efficiently assist the drug delivery systems to pass through the BBB. Due to the specific affinity to the GLUT1,17,18 MAN is a kind of mannose analog and is incorporated onto the surface of liposomes in the present study. However, the MAN-modified liposome, the same as glucose, would be pumped out to bloodstream after entering CNS, thus preventing the concentration of chemotherapeutic agent in the brain because of the GLUT1 expressing on both the luminal and abluminal membranes on the endothelial cells of BBB.19 This indicated that other ways are needed to enhance the ability to cross the BBB and increase drug concentration in the brain.
Wheat germ agglutinin (WGA) is an additional candidate for drug carrier targeting to brain tissues because of its low cytotoxic effect and high affinity for cerebral capillary endothelium compared with other lectins.20 The propensity of malignant cells to exhibit high lectin agglutination has led to the development of lectins as tumor diagnostic markers. Some findings show that the surface conjugation of paclitaxel-loaded PLGA nanoparticles with WGA could potentiate the extent and selectivity of anticancer activity.21 Accordingly, WGA may have the potential ability to target the BBB and C6 glioma cells.22
Liposomes are a kind of carriers being used for the delivery of anticancer agents, and have attracted increasing interest not only for the enhancement of therapeutic efficacy but also for the reduction of systemic side effects.
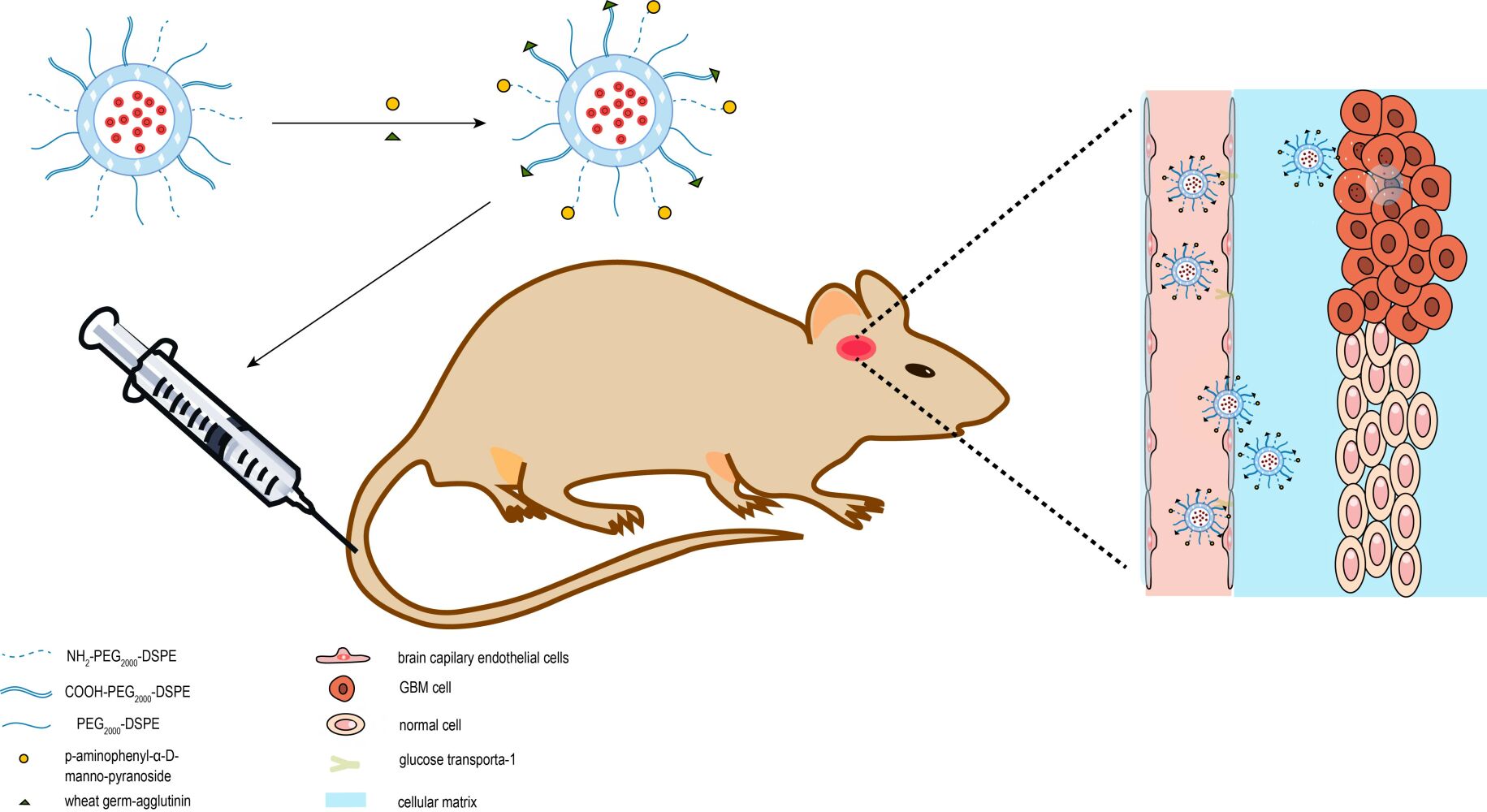
In the study reported here, we constructed a multifunctional targeting liposomal system. The multifunctional targeting liposomes, epirubicin plus resveratrol liposomes modified with MAN and WGA (as shown in Figure 1) could transport epirubicin and resveratrol across the BBB and subsequently target glioma cells. The multiple targeting efficacies were evaluated on the BBB model in vitro, C6 glioma cells in vitro, and C6 glioma-bearing rats through a systemic administration.
 |
Figure 1 Overall schematic representation for the preparation of epirubicin plus resveratrol liposomes modified with p-aminophenyl-α-D-manno-pyranoside (MAN) and wheat germ agglutinin (WGA). |
Materials and Methods
Preparation of Liposomes
Blank Liposomes
Blank liposomes were firstly prepared according to the following procedures. Egg phosphatidylcholine (EPC) (Germany Lipoid company, Shanghai local agent, China), cholesterol and polyethylene glycol distearoylphosphosphatidylethanolamine (PEG2000-DSPE, NOF Corporation, Japan) (52:43:5, mmol/mmol) was dissolved by methanol in a pear-shaped flask. The methanol was evaporated to dryness under vacuum with a rotary evaporator (EYELA-1000S, EYELA Rikakikai Corporation, Tokyo, Japan), then hydrated with 250 mM ammonium sulfate by sonication in a water bath for 5 min. Suspensions were treated with an ultrasonic cell disruptor (JY92 cell sonicator, Ningbo Chibio-Technology Co., Ltd., Ningbo, China) for 3 min (200 W), and successively extruded through polycarbonate membranes (Millipore, Bedford, MA, USA) with pore size of 400 nm and 200 nm for 3 times, respectively. After extrusion, the suspensions were further dialyzed (12,000–14,000 molecular mass cutoff) against the phosphate buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4 and 2 mM KH2PO4, pH 7.4) for 3 times (each time 8 h) to obtain the blank liposomes.
Epirubicin Liposomes
The epirubicin liposomes (EPI-L) were prepared with loading epirubicin into blank liposomes. Epirubicin (the Buddha Chemical Co., Ltd., Nanjing, China, batch number: 20120110) was mixed in blank liposomes, and then oscillated gently for 20 minutes. Unloaded epirubicin was removed by dialysis against PBS for 3 times (each time 8 h). Thus the epirubicin liposomes were obtained.
Epirubicin Plus Resveratrol Liposomes
The epirubicin plus resveratrol liposomes (EPI-RES-L) were prepared following the same procedure with loading epirubicin and resveratrol into blank liposomes. Firstly, resveratrol (Buddha Chemical Co., Ltd., Nanjing, China, batch number: 20120110) was added together with EPC, cholesterol and PEG2000-DSPE to prepare resveratrol liposomes (RES-L). After being dialyzed by PBS, unloaded resveratrol was removed by Sephadex G-50 (Beijing Biodee Biotechnology Co., Ltd. Beijing, China). Then, resveratrol liposomes were preheated at 60 °C in water bath pot, and epirubicin was mixed in, and then oscillated gently for 20 minutes. Unloaded epirubicin was removed by dialysis against PBS 3 times (each time 8 h). Thus the epirubicin plus resveratrol liposomes were obtained.
Epirubicin Plus Resveratrol Liposomes Modified with MAN
1.2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol) 2000] (NH2-PEG2000-DSPE, AvantiPolar Lipids, Alabaster, AL, USA) was added with EPC, cholesterol, PEG2000-DSPE and resveratrol to obtain the resveratrol liposomes with NH2.group. Blank liposomes modified with MAN were prepared by coupling blank liposomes with MAN according to the reported method. The procedures of resveratrol liposomes modified with MAN were the same as blank liposomes. Briefly, a volume of 4 mL resveratrol liposomes suspensions (15 mg lipid/mL) was mixed with 3 mg of MAN (Sigma-Aldrich Corporation, Beijing local agent, China). Excessive glutaraldehyde (200 μmol, 20 mg) was added slowly to the liposome suspensions, and further incubated for 5 min at room temperature. In this reaction, the NH2 group of resveratrol liposomes was coupled with MAN using glutaraldehyde as a coupling agent. Uncoupled MAN and glutaraldehyde were removed by dialysis against PBS 3 times (each time 8 h). After the resveratrol liposomes modified with MAN were prepared, epirubicin was loaded as above procedure to obtain the epirubicin plus resveratrol liposomes modified with MAN (MAN-EPI-RES-L).3
To evaluate the coupling efficiency of MAN, the uncoupled NH2 group of NH2-PEG2000-DSPE on the liposomes modified with MAN was measured by spectrophotometry after adding a visualization reagent, trinitrobenzene sulphonic acid (TNBS) (Biodee Biotechnology Co., Ltd. Beijing, China), as described previously. Briefly, 1 mL of 4% NaHCO3 and 1 mL of 10% sodium dodecyl sulphate (SDS; Beijing Biodee Biotechnology Co., Ltd. Beijing, China) were added to 1 mL of liposomal suspensions coupled (or uncoupled) with MAN. The suspensions were kept at 30 °C for 20 min, followed by adding 1 mL of 0.1% TNBS solution and leaving them to react for a further 2 h at 40 °C. The reaction was terminated by adding 0.5 mL of 1 M HCl. The absorbance of the final solution was read on ultraviolet–visible spectrophotometer (UV-2401PC, Shimadzu Technologies Inc., Cotati, CA, Japan) at 320 nm against blank control prepared as above with 1 mL of water instead of the liposome suspensions. The coupling efficiency of MAN on the liposomes was calculated with the formula: CEMAN=(1-Auncoupled/Atotal) × 100%, where CEMAN is the coupling efficiency of MAN, Auncoupled is the absorbance of uncoupled amino groups of NH2-PEG2000-DSPE on the liposomes after modifying with MAN, and Atotal is the absorbance of amino groups of NH2-PEG2000-DSPE on the liposomes prior to conjugation with MAN.
Epirubicin Plus Resveratrol Liposomes Modified with WGA
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy(polyethylene glycol) 2000] (COOH-PEG2000-DSPE, Avanti Polar Lipids, Alabaster, AL, USA) was added with EPC, cholesterol, PEG2000-DSPE and resveratrol to obtain the resveratrol liposomes with COOH group. The resveratrol liposomes modified with WGA (Sigma-Aldrich Corporation, Beijing local agent, China) was prepared with the following procedures: a volume of 2 mL resveratrol liposomes suspension (15 mg lipid/mL) was mixed with EDIC/NHS (5.99 mg/3.60 mg) solution of the PBS and activated for 1 h by constant temperature oscillation. Then WGA (5 mg) was added to the suspensions and incubation for 12 h by constant temperature oscillation. In this reaction, the COOH group of resveratrol liposomes was coupled with NH2 group of WGA using carbodiimide as a coupling agent. Uncoupled WGA and carbodiimide were removed by Sephadex G-50 (Beijing Biodee Biotechnology Co., Ltd. Beijing, China). Then epirubicin was loaded into resveratrol liposomes modified with WGA to obtain epirubicin plus resveratrol liposomes modified with WGA (WGA-EPI-RES-L).
The coupling efficiency of WGA was evaluated by measuring the uncoupled WGA on surface of the liposomes by coomassie brilliant blue (CBB) method. Briefly, 10.00 mg of coomassie brilliant blue G-250 was dissolved by 5 mL of 95% ethanol in 100 mL volumetric flask and a further 10 mL of 85% H3PO4 was added. The 2.00 mg of WGA was dissolved into 200 μg/mL of reservoir fluid by PBS. The reservoir fluid was used to prepare 1 mL of serial WGA standard solutions at the concentration of 40.0, 80.0, 120.0, 160.0, 200.0 μg/mL. Afterwards, 5.0 mL of CBB G-250 developer was mixed well and the reaction was kept at room temperature for 5 min. The absorbance of the final solution was read on ultraviolet–visible spectrophotometer at 595 nm against a blank prepared as above with 1 mL of water instead of the liposome suspensions. The coupling efficiency of WGA on the liposomes was calculated with the formula: CEWGA=(1-Auncoupled/ Atotal) × 100%, where CEWGA is the coupling efficiency of WGA, Auncoupled is the absorbance of uncoupled WGA on the liposomes after modifying with WGA, and Atotal is the absorbance of the initial amount of WGA on the liposomes prior to conjugation with WGA.
Epirubicin Plus Resveratrol Liposomes Modified with MAN and WGA
Epirubicin plus resveratrol liposomes modified with MAN and WGA (MAN-WGA-EPI-RES-L) were prepared based on resveratrol liposomes modified with MAN (MAN-RES-L), followed by coupling with WGA as the same rationale as above. Afterwards, epirubicin was loaded as above procedure to obtain the epirubicin plus resveratrol liposomes modified with MAN and WGA.
Characterization of Liposomes
Encapsulation Efficiency
Epirubicin and resveratrol were measured with C18 column (Phenomenex, 250×4.6 mm, 5μm) at 1.0 mL/min of flow rate by high performance liquid chromatography (HPLC) system with UV detector (Shimadzu Technologies Inc., Cotati, CA, Japan). The mobile phase consisted of methanol, acetonitrile and 0.4% phosphoric acid aqueous solution (16:21:63, v/v). The detected wavelength was set at 254 and 306 nm
Drug-loaded liposomes were dialyzed against 10-fold PBS (v/v) 3 times (each 12 h) to remove the unloaded epirubicin. And unloaded resveratrol was removed by Sephadex G-50. To measure the content of epirubicin and resveratrol, the liposome suspensions were destroyed by adding methanol. The encapsulation efficiency of epirubicin and resveratrol was estimated with the formula: EE = (Wdialysis/Wtotal) × 100%, where EE is the encapsulation efficiency of epirubicin or resveratrol, Wdialysis is the measured amount of epirubicin or resveratrol in the liposome suspensions after unloaded drug was removed, and Wtotal is the measured amount of epirubicin or resveratrol in the equal volume of liposome suspensions before dialysis.
Particle Size and Zeta Potential Measurements
Mean diameter and zeta potential of the liposomes was determined by a light-scattering particle size analyzer (Malvern Instruments, Ltd, UK). The wavelength of the beam was set to 633 nm, and the incident angle of the scattered light beam was set at 90 degrees. The liposomes were diluted with a 20-fold volume of PBS. Additionally, the samples were measured 10 times each testing cycle with a 300 KCps minimum count rate at room temperature.
Microscopic Characterization
The morphology of liposomal formulations was evaluated using atomic resolution analytical transmission electron microscopy (600×) (TEM; JEM-ARM200F, JEOL, Japan). The liposomes were diluted and stained in 3% phosphotungstic acid. Copper mesh coated with carbon films was used to absorb the liposome particles for 1 min in the liposome suspension and dried at room temperature. Then, the specimens were observed by TEM at 120 kV.
Drug Release
The release characteristics of liposomal suspensions in vitro were examined with PBS as the release medium by dynamic membrane dialysis method. A volume of 2 mL liposomal suspensions was sealed in the processed dialysis bag. The dialysis bag was followed by an immediate shaking at a speed of 150 times per minute in a constant temperature oscillator at 37±0.5 °C. A volume of 2 mL release sampling was taken at 0.5, 1, 2, 4, 6, 12, 16, 20, 24 h, and the same volume of fresh release medium was immediately supplemented. The samples were filtered over 0.45 μm microporous membrane. Afterwards, the content of epirubicin and resveratrol in the release medium was determined by HPLC as above. The samples were parallel-measured three times, and the cumulative release percentage was calculated at different time points (%), respectively. The released percentage of epirubicin or resveratrol was calculated according to the following formula: F=(Ct/Ctotal)×100%, where F is the drug release rate (%), Ct is the measured concentration of epirubicin and resveratrol at each time point in the dissolution medium, and Ctotal is the determined concentration of drug prior to dialysis.
Cell Culture
Two kinds of cell lines were used, including mouse brain microvascular endothelial cells (bEnd.3 cells) and C6 gliomas cells.
The bEnd.3 cells (Biaowu Biotechnology Co., Ltd. Beijing, China) were passaged in DMEM (high glucose, Gibco Biotech Co., Ltd., Beijing local agent, China) containing 20% fetal calf serum (Gibco Biotech Co., Ltd., Beijing local agent, China), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco Biotech Co., Ltd., Beijing local agent, China).
C6 gliomas cells (Institute of Sciences, Shanghai, China) were routinely grown in Dulbecco’s modified Eagle’s medium (DMEM, high glucose, Gibco Biotech Co., Ltd., Beijing local agent, China) supplemented with 10% fetal bovine serum (FBS) (Hangzhou Evergreen Company, Hangzhou, China), 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were maintained at 37 °C in the presence of 5% CO2.
Cytotoxicity Assay in vitro
The cytotoxicity of the liposomes was determined by sulforhodamine B (SRB; Sigma, CA, USA) assay. C6 cells were seeded into 96-well plates at a density of 3×103 cells/well and cultured for 24 h before analysis. Free epirubicin, epirubicin liposomes, epirubicin plus resveratrol liposomes, epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes modified with WGA, epirubicin plus resveratrol liposomes modified with WGA and MAN were added into 96-well culture plates to make the final epirubicin and resveratrol concentrations from 0 to 25 μM and 0 to 6 μM, respectively. After 48 h incubation, the cells were washed three times with PBS and treated by SRB staining assay. The absorbance at 540 nm was detected with a microplate reader (BIO-RAD Model 680, Bio-Rad Laboratories, Inc. Shanghai, China) and the cell viability was calculated using the following formula: Survival% = (A540 nm for the treated cells/A540 nm for the control cells) × 100%, where the A540 nm was the absorbance value. Each assay was repeated at least three times. IC50 values were calculated from the dose–effect curves.
To study the short-term cytotoxicity of the drug carriers at high concentration, bEnd.3 cells were seeded into 96-well culture plates at a density of 3×103 cells/well and cultured at 37 °C for 24 h. Then, the cells were treated with free epirubicin, epirubicin liposomes, epirubicin plus resveratrol liposomes, epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes modified with WGA, epirubicin plus resveratrol liposomes modified with WGA and MAN at the epirubicin concentration of 10 μM, respectively. To further observe the effects of MAN, bEnd.3 cells were incubated with free MAN for 30 min in advance. After incubation for 3 h at 37 °C, cell viability was determined by SRB staining assay.
Cellular Uptake
C6 cells were seeded into 6-well culture plates (2 × 105/well) and incubated for 24 h. After adding free epirubicin, epirubicin liposomes, epirubicin plus resveratrol liposomes, epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes modified with WGA, epirubicin plus resveratrol liposomes modified with WGA and MAN to the cells at an epirubicin concentration of 10 μM, respectively, cells were further incubated for 6 h, 12 h, 24 h each. Cells without any drugs were used as a blank control. Thereafter, the cells were trypsinized and collected under centrifuge (1200 rpm, 5 min) after being washed twice with cold PBS. The fluorescence intensity of EPI was measured by FAScan flow cytometer (Becton Dickinson FACSCalibur, Mountain View, CA, USA) using FL1-H filter (520±30 nm) and 560 nm as the emission wavelength for collection of fluorescence intensity. Then, the data were analyzed through FCS Express V3 software.
Apoptosis Assay
C6 cells were placed in 6-well culture plate (2 × 105/well). After incubation for about 24 h, blank liposomes, free epirubicin, epirubicin liposomes, epirubicin plus resveratrol liposomes, epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes modified with WGA, epirubicin plus resveratrol liposomes modified with WGA and MAN were added at an epirubicin concentration of 5 μM, respectively, and further incubated for 24 h. After that, the C6 gliomas cells were collected by centrifuge (1200 rpm, 5 min) after trypsinization without EDTA and washed twice with cold PBS. And staining of cells was performed using Annexin V-APC/7-AAD. Then, samples were analyzed using FAScan flow cytometer. The events collected were ten thousands and the data were analyzed through FCS Express V3 software.
Intracellular Localization Assay
Laser scanning confocal microscope (LSM 510, Zeiss, Germany) was used to detect the intracellular localization of epirubicin in different formulations in C6 cells. Cells were cultured in the chambered cover slips at a density of 1×105 cells/well for 12 h and then treated with free epirubicin, epirubicin liposomes, epirubicin plus resveratrol liposomes, epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes modified with WGA, epirubicin plus resveratrol liposomes modified with WGA and MAN at an epirubicin concentration of 10 μM for 2 h. Afterwards, cells were washed with cold PBS three times, fixed with 30% (v/v) paraformaldehyde and finally stained with Hoechst 33258 (Sigma-Aldrich Corporation, Beijing local agent, China).
The microscopic images of intracellular localization were captured through laser scanning confocal microscope with the excitation wavelength for 346 nm and 488 nm and the absorption wavelength for 460 nm and 560 nm, respectively. The data were analyzed with Aim Image Examiner software.
Transport Across BBB Model in vitro
In vitro BBB Model
BBB model was established using the method previously described.3 The bEnd.3 cells were seeded into the inserts at a density of 5×104 cells/well and cultured for 4 days whilst changing the medium every 2 days. The BBB model was viewed with scan electron microscope (SEM, JSM-5600 LV, JEOL, Japan) at instrumental magnification of 10,000 folds. And the BBB model was examined by 4 h of permeation assay and transendothelial electrical resistance instrument (Word Precision Instruments, Inc. Sarasota, FL, USA) to measure the TEER value. Only these plates with no medium permeation in 4 h together with TEER value over 250 Ωcm2 could be used for the experiments.
Transport Across BBB and Competition of MAN
The ability of the liposomes across the BBB model was evaluated. The liposomes including free epirubicin, epirubicin liposomes, epirubicin plus resveratrol liposomes, epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes modified with WGA, epirubicin plus resveratrol liposomes modified with WGA and MAN were added into the corresponding inserts at the epirubicin concentration of 20 μg/mL, respectively. For the competition assay, cells were incubated with 200 μg of MAN for 30 min and further treated with epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes modified with WGA and MAN. A volume of 500 μL solution was taken out from the acceptor compartments at 0, 2, 4, 6, 8 and 24 h, followed by 500 μL fresh medium added to the compartments immediately. The samples were determined by HPLC as above.
Effect on the Avascular C6 Glioma Spheroids
Avascular murine C6 glioma spheroids were grown in vitro using liquid overlay system as described previously.3 C6 glioma spheroids were incubated with serum-free F10 culture medium, free epirubicin, epirubicin liposomes, epirubicin plus resveratrol liposomes, epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes modified with WGA, epirubicin plus resveratrol liposomes modified with WGA and MAN, respectively. The concentration of epirubicin was 4 μM. Growth inhibition was monitored by measuring the size of C6 glioma spheroids using an inverted microscope at day 0, 1, 3, and 7, respectively. The major (dmax) and minor (dmin) diameters of each spheroid were measured and the spheroid volume was calculated using the following formula: V=(π×dmax×dmin)/6. The C6 glioma spheroid volume ratio was estimated with the formula: R=(Vday i/Vday 0)×100%, where Vday i is the C6 glioma spheroid volume at the ith day after applying the drug, and Vday 0 is the C6 glioma spheroid volume prior to administration.
Multifunctional Targeting Effects
The bEnd.3 cells/C6 cells co-culture model and bEnd.3/C6 glioma spheroid co-culture model were established to determine the multifunctional targeting effects as previously reported.3,23 The BBB model was established and transferred to the 24-well plates which were pre-coated with C6 cells at a density of 1000 cells/compartment or with C6 glioma spheroid, and further incubated for 5 days before the model was used for the experiments. After applying epirubicin concentration of 20 μg/mL of free epirubicin, epirubicin liposomes, epirubicin plus resveratrol liposomes, epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes were modified with WGA, epirubicin plus resveratrol liposomes modified with WGA and MAN into the donor inserts, respectively. C6 cells or C6 glioma spheroid were allowed to grow for another 48 h and treated by the SRB staining assay. The competition assay was done with the same procedures as described above.
In vivo Anti-Glioblastoma Study
Wistar rats (purchased from Urumqi of Xinjiang institute of endemic diseases at 9 weeks of age and initially weighing 200–300 g) were employed to explore anti-glioblastoma activity of the liposomes in vivo. The animals were housed under standard conditions with free access to food and water. All the experimental protocols were performed in accordance with the national guidelines of the principles for care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of Shihezi University (Approval No. A2018-173-01). A sagittal incision was made through the skin to expose the cranium. Approximately 5×105 C6 glioma cells/10 µL in serum-free F10 medium were stereotaxically implanted into the right forebrain of each rat by using the following coordinates: 0.4 mm anterior and 3.0 mm lateral from the bregma, and at a depth of 5.0 mm from the brain surface.24 Then, the rats were intramuscularly injected with penicillin 10000 U after the incision was sewn up and disinfected.
Survival Curves
On the 8th day after tumor inoculation, the rats were randomly divided into 7 groups (6 rats per group). Animals in blank control group were administered physiological saline. Animals in other 6 groups were treated with free epirubicin, epirubicin liposomes, epirubicin plus resveratrol liposomes, epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes modified with WGA, epirubicin plus resveratrol liposomes modified with WGA and MAN via tail vein at a dose of 5 mg/kg epirubicin, respectively. The survival time was calculated from the 0th day since tumor inoculation to the day of death. Kaplan–Meier survival curves were plotted for each group.
Hematoxylin-Eosin (HE) Staining Study
The treated animals were sacrificed at day 14 after being successfully inoculated. The brain tissues were then made to settle in 4% paraformaldehyde. After that, the tissues were dehydrated by graded ethanol, decolorized by xylene, and embedded by dipping into wax. Finally, the brain blocks obtained from the tumor were cut into 6 μm thick slices along the coronal suture. Then, the sections were stained by HE and examined under light microscopy.
Statistical Analysis
Data were presented as mean ± standard deviation. One-way analysis of variance (ANOVA) was used to determine significance among groups following the Bonferroni’s post-test. Survival data were presented as Kaplan–Meier plots and analyzed with a log rank test. A value of P < 0.05 was considered to be significant.
Results
Preparation and Characterization of the Liposomes
Liposome was a phospholipid bilayer into which lipophilic drug was loaded at the lipid layers and hydrophilic drug at the inner core. The multifunctional targeting liposomes, epirubicin plus resveratrol liposomes modified with WGA and MAN, were prepared as mentioned above with a stable structure (shown in Figure 1). The inner part was packaged epirubicin and the external part was connected with resveratrol. MAN was linked with NH2-PEG2000-DSPE, and WGA was linked with COOH-PEG2000-DSPE at the external part. PEG chains of PEG2000-DSPE and the PEG with functional groups were grafted on liposomes to reduce the toxicity and improve the biocompatibility of the liposome on account of the shielding effect for the positive charges on the liposomal exterior.
The atomic resolution analytical transmission electron microscopy was used to evaluate the morphology of liposomal formulations. The results of Figure 2 showed that the epirubicin plus resveratrol liposomes modified with WGA and MAN were rounded and had uniform particle size.
The light-scattering particle size analyzer was performed to characterize the size and zeta potential values and multi-dispersion coefficient of the resulting multiple targeting liposomes. For blank liposomes, epirubicin plus resveratrol liposomes, epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes modified with WGA, epirubicin plus resveratrol liposomes modified with WGA and MAN, the average particle sizes were 85.24 ± 0.33 nm, 94.30 ± 0.51 nm, 99.18 ± 0.61 nm, 100.21 ± 0.26 nm, and 103.77 ± 0.65 nm, respectively. The particle size distributions (polydispersity index, PDI) were 0.210 ± 0.002, 0.160 ± 0.009, 0.178 ± 0.008, 0.225 ± 0.009, and 0.159 ± 0.007, respectively. The charge values were close to a neutral state with slightly negative charges distributed around the liposomal vesicles (−3.91 ± 0.08 mV for blank liposomes, −8.30 ± 0.45 mV for epirubicin plus resveratrol liposomes, −13.43 ± 0.31 mV for epirubicin plus resveratrol liposomes modified with MAN, −12.07 ± 0.59 mV for epirubicin plus resveratrol liposomes modified with WGA, and −14.40 ± 0.70 mV for epirubicin plus resveratrol liposomes modified with WGA and MAN).
The drug encapsulation efficiency was measured by the high performance liquid chromatography system with UV detector. For epirubicin plus resveratrol liposomes, epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes modified with WGA, epirubicin plus resveratrol liposomes modified with WGA and MAN, the results showed that all encapsulation efficiencies of epirubicin and resveratrol were ≥ 90% and ≥ 65%, respectively. And the concentrations of epirubicin and resveratrol in the liposome suspensions were 1.13 mg/mL and 0.30 mg/mL, respectively.
The coupling efficiencies of MAN in epirubicin plus resveratrol liposomes modified with MAN, and epirubicin plus resveratrol liposomes modified with WGA and MAN were 37.83 ± 1.04%, and 36.59 ± 0.42, respectively. The coupling efficiencies of WGA in epirubicin plus resveratrol liposomes modified with WGA, and epirubicin plus resveratrol liposomes modified with WGA and MAN were 21.46 ± 0.62% and 19.37 ± 0.46%, respectively.
To explore the release parameters of the liposomes in vitro, the release profiles were conducted at PBS containing 4% of Tween-80 by dynamic dialysis bag. The release rates of epirubicin during 48 h were 95.07 ± 0.09% for free epirubicin, 27.45 ± 0.06% for epirubicin plus resveratrol liposomes, 31.46 ± 0.06% for epirubicin plus resveratrol liposomes modified with MAN, 43.96 ± 0.06% for epirubicin plus resveratrol liposomes modified with WGA, and 33.96 ± 0.11% for epirubicin plus resveratrol liposomes modified with WGA and MAN, respectively. And the release rates of resveratrol during 48 h were 87.58 ± 0.30% for free resveratrol, 13.65 ± 0.08% for epirubicin plus resveratrol liposomes, 13.47 ± 0.044% for epirubicin plus resveratrol liposomes modified with MAN, 15.82 ± 0.17% for epirubicin plus resveratrol liposomes modified with WGA, and 16.77 ± 0.15% for epirubicin plus resveratrol liposomes modified with MAN and WGA, respectively. Most of epirubicin and resveratrol in all kinds of liposomes was released within the first 12 h.
Cytotoxicity Assay
The results of Figure 3 showed the inhibition effect of epirubicin plus resveratrol liposomes modified with MAN and WGA on C6 glioma cells after 48 h incubation with different liposomes. The results from SRB assay showed that epirubicin plus resveratrol liposomes modified with MAN and WGA at various concentrations exhibited the strongest inhibitory effect on the proliferation of C6 glioma cells among various formulations, and indicated that the antiproliferative effect of the drug-loaded liposomes was markedly elevated by dual-modifying with MAN and WGA.
 |
Figure 3 Antiproliferative effect against C6 glioma cells by different epirubicin plus resveratrol liposomes (Mean ± SD). |
Cellular Uptake
Among all five kinds of liposomes, the internalization of epirubicin plus resveratrol liposomes modified with MAN and WGA by the C6 glioma cells was the most evident (Figure 4). Data from the C6 glioma cellular uptake assay with the flow cytometry showed that the C6 glioma cellular uptake ratios were 55.22 ± 1.99% for free epirubicin (A), 44.88 ± 3.23% for epirubicin liposomes (B), 53.92 ± 2.57% for epirubicin plus resveratrol liposomes (C), 61.63 ± 2.86% for epirubicin plus resveratrol liposomes modified with MAN (D), 63.70 ± 3.78% for epirubicin plus resveratrol liposomes modified with WGA (E), and 70.39 ± 2.99% for epirubicin plus resveratrol liposomes modified with MAN and WGA (F), respectively. Furthermore, this experiment demonstrated that the endocytosis of epirubicin plus resveratrol liposomes modified with MAN and WGA was the most significant among all five kinds of liposomes.
 |
Figure 4 Drug uptake in C6 glioma cells following applying epirubicin formulations by FAScan flow cytometry assay. *P < 0.05; **P < 0.01; n.s.: no statistical significance. |
Apoptosis Assay
For further assessment of the cytotoxicity of epirubicin formulations, apoptosis assay was conducted by FAScan flow cytometer and cells without any drugs were used as controls. As shown in Figure 5, with the same incubation time and the concentrations of epirubicin or resveratrol, different formulations of C6 cells apoptosis rates were in the order of epirubicin plus resveratrol liposomes modified with WGA and MAN > epirubicin plus resveratrol liposomes modified with WGA > epirubicin plus resveratrol liposomes modified with MAN > epirubicin plus resveratrol liposomes> epirubicin liposomes > free epirubicin, with the apoptosis rates of 35.67 ± 3.51% for epirubicin plus resveratrol liposomes modified with WGA and MAN, 28.90 ± 0.67% for epirubicin plus resveratrol liposomes modified with WGA, 23.68 ± 3.58% for epirubicin plus resveratrol liposomes modified with MAN, 17.49 ± 1.22% for epirubicin plus resveratrol liposomes, 9.61 ± 0.94% for epirubicin liposomes, 7.15 ± 0.18% for free epirubicin, respectively. The results showed that the multifunctional targeting liposomes displayed the more obvious function of expediting C6 glioma cells apoptosis than other liposomes after C6 glioma cells being treated with different liposomes as mentioned above.
Cellular Localization Experiment
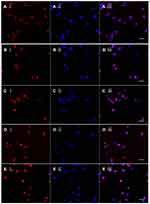
To evaluate the targeting effect of MAN and WGA, laser scanning confocal microscope (LSCM) was employed to quantify the uptake and cellular location of the epirubicin. The results in Figure 6 show the cellular distribution of epirubicin in different formulations after 2 h incubation with C6 cells. The confocal microscopy images reveal that epirubicin was mainly distributed in the nuclei after being internalized by the C6 glioma cells. Also, the epirubicin plus resveratrol liposomes modified with WGA and MAN displayed stronger fluorescence than others, which indicated that the functional targeting liposomes can mostly improve the endocytosis of epirubicin by C6 glioma cells compared with other groups of liposomes; this result was consistent with the results of cellular uptake.
In vitro BBB Transport Competition Assay of MAN
TEER value of bEnd.3 cells was higher than 250 Ωcm2. The permeability of HRP on the BBB model in vitro was less than 7% during 24 h. Figure 7A represents the transport ratios across the BBB in vitro over a period of 24 h. The transport ratios were 4.96 ± 0.14% for free epirubicin, 7.47 ± 0.24% for epirubicin liposomes, 8.97 ± 0.34% for epirubicin plus resveratrol liposomes, 23.71 ± 0.55% for epirubicin plus resveratrol liposomes modified with MAN, 15.47 ± 0.47% for epirubicin plus resveratrol liposomes modified with WGA, and 29.91 ± 0.81% for epirubicin plus resveratrol liposomes modified with MAN and WGA, respectively. The results exhibited that the transport of epirubicin across the BBB was evidently increased when epirubicin plus resveratrol liposomes were coupled with both MAN and WGA.
In the competition assay, when MAN was added in advance, the transport ratios of epirubicin plus resveratrol liposomes modified with MAN, and epirubicin plus resveratrol liposomes modified with MAN and WGA were significantly decreased due to free MAN competitively binding with GLUT1 (Figure 7B). It demonstrated that the MAN plays a major role in transporting the liposomes across the BBB and the transport of the liposomes across the BBB could be significantly increased after being modified with MAN and WGA.
Inhibitory Effect on the Avascular C6 Glioma Spheroids
The results in Figure 8 represent the C6 glioma spheroid volume appearance after applying free epirubicin, epirubicin liposomes, epirubicin plus resveratrol liposomes, epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes modified with WGA, epirubicin plus resveratrol liposomes modified with WGA and MAN, respectively. Compared with blank control, free epirubicin, epirubicin liposomes, epirubicin plus resveratrol liposomes, epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes modified with WGA, epirubicin plus resveratrol liposomes modified with WGA and MAN could significantly inhibit the growth of the C6 glioma spheroids. And the inhibitory effects were obviously observed after applying all kinds of liposomes, respectively. In detail, the size of tumor spheroids was reduced, and the surface of tumor cells was lysed. In contrast, epirubicin plus resveratrol liposomes modified with WGA and MAN produced the most significant reduction in the size and volume of tumor spheroids. After treatment with the targeting liposomes (epirubicin plus resveratrol liposomes modified with WGA and MAN), the density of spheroids was decreased, and the degree of cleavage increased. In Figure 9, the C6 glioma spheroid volume ratios at the 5th day were 0.61 ± 0.09 for free epirubicin, 0.64 ± 0.12 for epirubicin liposomes, 0.62 ± 0.08 for epirubicin plus resveratrol liposomes, 0.59 ± 0.07 for epirubicin plus resveratrol liposomes modified with MAN, and 0.57 ± 0.07 for epirubicin plus resveratrol liposomes modified with WGA, and 0.46 ± 0.05 for epirubicin plus resveratrol liposomes modified with WGA and MAN, respectively. Epirubicin plus resveratrol liposomes modified with WGA and MAN exhibited the ability to reduce the volume and inhibit the growth on the C6 glioma spheroid.
 |
Figure 8 Morphological changes of C6 glioma spheroid volume after treatment of epirubicin formulations (×100). |
Multifunctional Targeting Effects
The Inhibitory Study in a Co-Culture Model of BBB/C6 Glioma Cells
In order to analyze the potentially multifunctional targeting effect of epirubicin–resveratrol liposomes modified with MAN and WGA in mimicking the condition in vivo, a bEnd.3/C6 glioma co-culture model was established and used for evaluation (Figure 10). The rank of the inhibitory rates (%) to C6 glioma cells after crossing the BBB was epirubicin plus resveratrol liposomes modified with MAN and WGA (64.48 ± 0.59%) > epirubicin plus resveratrol liposomes modified with MAN (58.88 ± 0.84%) > epirubicin plus resveratrol liposomes modified with WGA (39.95 ± 0.83%) > epirubicin plus resveratrol liposomes (29.11 ± 0.56%) > epirubicin liposomes (27.02.3 ± 0.23%) > free epirubicin (17.23 ± 0.39%), demonstrating that the epirubicin plus resveratrol liposomes modified with MAN and WGA exhibited a significant “multiple targeting effect” (P < 0.01), ie, transporting across the BBB and targeting the C6 glioma cells.
The Inhibitory Study in a Co-Culture Model of BBB/C6 Glioma Spheroid
In order to analyze the potentially multifunctional targeting effects of epirubicin plus resveratrol liposomes modified with MAN and WGA in mimicking the condition in vivo, a bEnd.3/C6 glioma spheroid co-culture model was established and used for evaluation (Figure 11). The rank of the volume rates (%) to C6 glioma spheroid after crossing the BBB was epirubicin plus resveratrol liposomes modified with MAN and WGA (0.72 ± 0.04) < epirubicin plus resveratrol liposomes modified with MAN (0.82 ± 0.03) < epirubicin plus resveratrol liposomes modified with WGA (0.86 ± 0.05) < free epirubicin (0.94 ± 0.03) < epirubicin plus resveratrol liposomes (0.98 ± 0.05) < epirubicin liposomes (1.06 ± 0.14) <, demonstrating that the epirubicin plus resveratrol liposomes modified with MAN and WGA exhibited a significant “multifunctional targeting effect” (P < 0.01), ie transporting across the BBB and targeting the C6 glioma cells. The volume ratio of epirubicin plus resveratrol liposomes modified with MAN was lower than epirubicin plus resveratrol liposomes modified with WGA; it is possible that MAN exhibits stronger cross BBB and also shows tumor targeting ability. The volume of the tumor spheroid was reduced, and the degree of the cracking on the spheroid surface was deepened. The results showed that the functional targeting liposomes display the ability to transport through the blood–brain barrier, then to target C6 glioma spheroid after WGA modification, thus effectively inhibiting the growth of C6 glioma spheroid.
In vivo Anti-Glioblastoma Study
The tumor inhibitory ratios on the 14th day were 10.71 ± 1.27% for free epirubicin, 14.64 ± 1.57% for epirubicin liposomes, 17.02 ±1.41% for epirubicin plus resveratrol liposomes, 35.71 ± 1.28% for epirubicin plus resveratrol liposomes modified with MAN, 25.92 ± 1.33% for epirubicin plus resveratrol liposomes modified with WGA, and 46.06 ± 2.46% for epirubicin plus resveratrol liposomes modified with WGA and MAN, respectively. Comparatively, the animals treated with epirubicin plus resveratrol liposomes modified with WGA and MAN showed the most significant reduction in tumor volume among all groups. On the contrary, physiological saline did not exhibit any inhibitory effect on the tumor (Figure 12).
Regarding the results of HE staining, Figure 13 shows the images of pathological section staining by HE which were obtained by stereoscope. Figure 13A displays the complete brain pathology. The gliomas infiltrated the brain tissues with hemorrhage and necrotic areas on the right side, and the invasive brain tumor tissue in irregular shape replaced and compressed the normal tissue without a membrane and a distinct boundary.
From Figure 13B and C, there were no obvious effects in the brain tumor in the groups of free epirubicin, epirubicin liposomes, and epirubicin plus resveratrol liposomes, and gliomas continued to rapidly proliferate the same as those in the physiological saline group. The gliomas and glioma clusters were mostly oval shaped in rapidly dividing and proliferating around the blood vessels, and scattered at the border of the brain gliomas and normal brain tissues (in Figure 13C). Large portions of necrosis and hemorrhage were apparent at the largest tumor area. Microvessels full of red blood cells were abundant and were surrounded by the necrotic area (in Figure 13D). However, the gliomas were obviously inhibited by treating with epirubicin plus resveratrol liposomes modified with MAN, epirubicin plus resveratrol liposomes modified with WGA, and epirubicin plus resveratrol liposomes modified with WGA and MAN. Furthermore, significant therapeutic effects were exhibited in the group of epirubicin plus resveratrol liposomes modified with WGA and MAN in comparison with other groups. The gliomas were shrunken and generated abnormal gliomas with regions of numerous vacuoles with scattered red cells to a certain extent (in Figure 13E). Especially, in the section of the animal with the longest survival time, the pathology showed the hollow hole in the site of tumor which might be the result of the therapy effect, which scattered the few recurring or residual tumor cell clusters (in Figure 13F).
The survival curves were drawn with Kaplan–Meier survival method by SPSS software. The results of Figure 14 represent Kaplan–Meier survival curves. After one week treatment, the median survival time of rats treated with epirubicin plus resveratrol liposomes modified with WGA and MAN (22.83 days) was significantly longer than that of rats treated with physiological saline (10.50 days, P < 0.001), free epirubicin (11.83 days, P <0.001), epirubicin liposomes (14.33 days, P = 0.001) epirubicin plus resveratrol liposomes (13.50 days, P < 0.001), epirubicin plus resveratrol liposomes modified with MAN (16.00 days, P = 0.007), and epirubicin plus resveratrol liposomes modified with WGA (17.67 days, P = 0.038), respectively. The results indicated that epirubicin plus resveratrol liposomes modified with WGA and MAN significantly improved the efficiency of anti-glioblastoma in vivo which was markedly superior to other resulting liposomes.
Discussion
The blood–brain barrier (BBB) is a dynamic barrier protecting the brain against invading organisms and undesired substances. It is also the primary barrier impeding drug transport into the brain via blood circulation.25,26 Such a difficulty in delivering therapeutic molecules to the brain can only be overcome by focusing on various transport receptors at the BBB and available delivery technologies.27,28 In the present study, we have conceived modern approaches using ligand-conjugation and nanotechnology to target the BBB via adsorptive or receptor-mediated transport of drug molecules. As drug delivery carriers, liposomes have gained popularity in many fields and extensively been used to increase the transport of drugs across the BBB through binding between endogenous transporters localized on the BBB and specific ligands modified on the surface of the delivery system.29 Mechanisms for drug targeting of brain tumors involve two key aspects: passing “through” the BBB, then specific targeting to the tumor. In this study, we proposed a novel type of multifunctional targeting liposomal carrier by conjugating with MAN and WGA with the purposes of delivering anticancer agent through the BBB to target brain tumor cells.
PEGylated liposomes30 loaded with epirubicin and resveratrol were included for consideration to construct the dual-targeting nanocarriers. Our previous studies showed that such carriers were more stable in both physicochemical and biological conditions.3 This was associated with steric stability31,32 and with the bulky polyethylene glycol (PEG) head group,33,34 which inhibits the rapid uptake of reticuloendothelial system (RES).35 It was reported that drug carriers with a size less than 100 nm would transport through the BBB efficiently36,37 and could avoid the fast elimination of drugs by the kidney to maintain better permeation and accumulation in tumor tissues, which is a phenomenon called the “enhanced permeability and retention (EPR) effect”.38 In our study, the multifunctional targeting liposomes were consistent and uniform suggesting their potential ability to transport across the BBB and circulate longer in vivo, which were proven in experiment results.
The cytotoxicity of free drug (epirubicin or resveratrol) and liposomes with drugs were examined by using C6 glioma cells. Utilizing the SRB assay, the results demonstrated that free EPI resulted in expected inhibitory effects compared to free resveratrol in C6 glioma cells following the same incubation, thus proving the anticancer effects on such brain tissues.39 Resveratrol was an ancillary drug, and could play the role of synergies together. The multifunctional targeting liposomes exhibited higher cytotoxicity efficacy than free epirubicin or resveratrol while liposomes conjugated with MAN or WGA were consistent with results from cellular uptake by flow cytometry. The data from measurements showed that multifunctional targeting liposomes displayed a comparatively higher cell uptake property than others. This may be owing to the ligands of MAN and WGA in the system. In addition, there was a significant difference in the inhibitory effect between epirubicin plus resveratrol liposomes modified with WGA and epirubicin plus resveratrol liposomes. This could be explained by the high affinity of WGA to C6 glioma cells, while common PEGylated liposomes would hinder the contact of drugs with tumor cells in vitro. Such a phenomenon was also expounded upon in other reports.31 More significantly, epirubicin plus resveratrol liposomes modified with WGA and MAN exhibited the strongest inhibitory effects, suggesting that MAN plus WGA contributes to a stronger drug delivering effect into C6 glioma cells. And, because GLUT1 is also overexpressed on the tumor of brain,40 it may lead to being not obviously different between the epirubicin plus resveratrol liposomes modified with MAN and epirubicin plus resveratrol liposomes modified with WGA in cellular uptake.
A laser scanning confocal microscope was used to observe cellular uptake and distribution of carriers in C6 cells. The fluorescence intensity from confocal images further proved a targeting effect of MAN and WGA, similar with that from flow cytometry assay. Fluorescence was mainly observed in cell nuclei, denoting that EPI could be transported to nuclei by endocytosis. The confocal images of epirubicin plus resveratrol liposomes modified with WGA and MAN revealed the strongest fluorescence intensity, indicating that the ligands of MAN and WGA could improve the ability to transport EPI to nuclei.
To build a BBB in vitro model, mouse brain microvascular endothelial cells (bEnd.3) were cultured on the upper side of the insert. This model had been characterized by displaying BBB characteristics and linking the barrier junction with its transendothelial electrical resistance (TEER) value.
FAScan flow cytometer was conducted to investigate the apoptosis of the liposomes to C6 glioma cells. From the results we could see that cell apoptosis parent of blank control group was 0.5%, indicating only a small part of the cell apoptosis, and blank liposome group was 3.8%, demonstrating that liposome shows almost no promoting effect of cell apoptosis. However, apoptosis of the groups with EPI significantly increased, thus further proving that the epirubicin played a main role in the drug delivery system, which was consistent with the experiment results of cytotoxicity. Among all groups, the apoptosis parent of the multifunctional targeting liposomes was up to 37.44%, thus showing that the combination of MAN and WGA could significantly promote cell apoptosis. The possible mechanisms could be deduced as follows: (1) the WGA has a higher affinity with tumor cells and then has the characteristic of targeting C6 cells,41,42 then, according to the cellular receptor mediated-swallowing function, the fact that more drug loading liposomes can enter cells plays a role; (2) as a result of GLUT1 in C6 cells also being high, MAN mediated by GLUT143 can “bind” the compact, so that more drug liposomes enter cells; (3) MAN plus WGA can promote epirubicin into cell nucleus (positioning results). Epirubicin after entering the nucleus can be directly embedded between DNA bases, interfere with the transcription process, prevent the formation of the mRNA, and inhibit the synthesis of DNA and RNA44 so as to promote cell apoptosis.
To understand the multifunctional targeting effect in vitro, epirubicin plus resveratrol liposomes modified with WGA and MAN were passed through the BBB model on the insert first and then reached the tumor cells in the culture plate tank. The inhibitory effect on the C6 cells further proved the results obtained from drug transport across the BBB.45
To further verify these results in vivo, C6 glioma-bearing animal model was established and treated with the dual-targeting liposomes through a systemic administration. The epirubicin plus resveratrol liposomes modified with WGA and MAN leads to the most significant dual-targeting effect. The results could be evidently observed as this combination leads to a significantly improved chemotherapy in the overall survival of the brain glioma-bearing animals, compared with free epirubicin or resveratrol or with other liposomes.
In conclusion, the multifunctional targeting liposomes modified with MAN and WGA exhibited strong ability to improve epirubicin and resveratrol transport across the BBB and then to target the brain tumor cells to improve the treatment effect of brain glioma, showing dual-targeting effects.
Conclusions
The epirubicin plus resveratrol liposomes modified with MAN and WGA could markedly improve the transport of epirubicin and resveratrol across the blood–brain barrier and the survival of brain tumor-bearing animals, showing a multifunctional targeting effect. These findings would promote further developments by noninvasive therapy and provide a promising application for treating brain tumors.
Acknowledgments
This work was supported by grants from the National Science Foundation of China (No. 81760421), the Foundation of Chengdu Medical College (No. CYZ19-17 and No. CYZ17-03), the program of innovation and entrepreneurship training for college students in Sichuan Province (No. S202113705041 and No. S201913705131), Youth Innovation Research Project of Sichuan Medical Association (No.Q20033), and the Collaborative Innovation Center of Sichuan for Elderly Care and Health, Chengdu Medical College (No.19Z05, YLZBZ2006). Dehua Kong and Wenyu Hong made equal contributions and are joint first authors of this article.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392(10145):432–446.
2. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl_5):v1–v88.
3. Ying X, Wen H, Lu W-L, et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 2010;141(2):183–192.
4. Jia Y, Wang X, Hu D, et al. Phototheranostics: active targeting of orthotopic glioma using biomimetic proteolipid nanoparticles. ACS nano. 2019;13(1):386–398.
5. Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20(1):12–25.
6. Furtado D, Björnmalm M, Ayton S, Bush AI, Kempe K, Caruso F. Overcoming the blood-brain barrier: the role of nanomaterials in treating neurological diseases. Advan mater. 2018;30(46):e1801362.
7. Sarkaria JN, Hu LS, Parney IF, et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018;20(2):184–191.
8. Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19(6):771–783.
9. Xu -P-P, Fu D, Li J-Y, et al. Anthracycline dose optimisation in patients with diffuse large B-cell lymphoma: a multicentre, Phase 3, randomised, controlled trial. Lancet Haematol. 2019;6(6):e328–e337.
10. Perveen K, Masood F, Hameed A. Preparation, characterization and evaluation of antibacterial properties of epirubicin loaded PHB and PHBV nanoparticles. Int J Biol Macromol. 2020;144:259–266.
11. Pang -H-H, Chen P-Y, Wei K-C, et al. Convection-enhanced delivery of a virus-like nanotherapeutic agent with dual-modal imaging for besiegement and eradication of brain tumors. Theranostics. 2019;9(6):1752–1763.
12. Feijen EAM, Leisenring WM, Stratton KL, et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncol. 2019;5(6):864–871.
13. Liu -J-J, Tang W, Fu M, et al. Development of R modified epirubicin-dihydroartemisinin liposomes for treatment of non-small-cell lung cancer. Artif Cells Nanomed Biotechnol. 2019;47(1):1947–1960.
14. van den Brand AD, Villevoye J, Nijmeijer SM, van den Berg M, van Duursen MBM. Anti-tumor properties of methoxylated analogues of resveratrol in malignant MCF-7 but not in non-tumorigenic MCF-10A mammary epithelial cell lines. Toxicology. 2019;422:35–43.
15. Yousef M, Vlachogiannis IA, Tsiani E. Effects of resveratrol against lung cancer: in vitro and in vivo studies. Nutrients. 2017;9:11.
16. Jeyaraman MM, Al-Yousif NSH, Singh Mann A, et al. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2020;1:CD011919.
17. Mojarad-Jabali S, Farshbaf M, Walker PR, et al. An update on actively targeted liposomes in advanced drug delivery to glioma. Int J Pharm. 2021;602:120645.
18. Pardridge WM, Boado RJ, Farrell CR. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative Western blotting and in situ hybridization. J Biol Chem. 1990;265(29):18035–18040.
19. McMullen E, Weiler A, Becker HM, Schirmeier S. Plasticity of carbohydrate transport at the blood-brain barrier. Front Behav Neurosci. 2020;14:612430.
20. Libbrecht S, Van den Haute C, Malinouskaya L, Gijsbers R, Baekelandt V. Evaluation of WGA-Cre-dependent topological transgene expression in the rodent brain. Brain Struct Funct. 2017;222(2):717–733.
21. Wang C, Ho PC, Lim LY. Wheat germ agglutinin-conjugated PLGA nanoparticles for enhanced intracellular delivery of paclitaxel to colon cancer cells. Int J Pharm. 2010;400(1–2):201–210.
22. Aires V, Colin DJ, Doreau A, et al. P-Glycoprotein 1 affects chemoactivities of resveratrol against human colorectal cancer cells. Nutrients. 2019;11:9.
23. Yang S, Jin H, Zhao Z. Paracellular tightness and the functional expression of efflux transporters P-gp and BCRP in bEnd3 cells. Neurol Res. 2018;40(8):644–649.
24. Li W, Ren L, Zheng X, et al. 3–Acetyl-11-keto- -boswellic acid ameliorated aberrant metabolic landscape and inhibited autophagy in glioblastoma. Acta pharmaceutica Sinica B. 2020;10(2):301–312.
25. Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15(4):275–292.
26. Tosi G, Duskey JT, Kreuter J. Nanoparticles as carriers for drug delivery of macromolecules across the blood-brain barrier. Expert Opin Drug Deliv. 2020;17(1):23–32.
27. Villaseñor R, Lampe J, Schwaninger M, Collin L. Intracellular transport and regulation of transcytosis across the blood-brain barrier. Cell Mol Life Sci. 2019;76(6):1081–1092.
28. Tang W, Fan W, Lau J, Deng L, Shen Z, Chen X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem Soc Rev. 2019;48(11):2967–3014.
29. Niu X, Chen J, Gao J. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: focus on recent advances. Asian J Pharm Sci. 2019;14(5):480–496.
30. Francis GE, Delgado C, Fisher D, Malik F, Agrawal AK. Polyethylene glycol modification: relevance of improved methodology to tumour targeting. J Drug Target. 1996;3(5):321–340.
31. Shen Z, Ye H, Kröger M, Li Y. Aggregation of polyethylene glycol polymers suppresses receptor-mediated endocytosis of PEGylated liposomes. Nanoscale. 2018;10(9):4545–4560.
32. Mastrotto F, Brazzale C, Bellato F, et al. In vitro and in vivo behavior of liposomes decorated with PEGs with different chemical features. Mol Pharm. 2020;17(4):1444.
33. Steffes VM, Zhang Z, MacDonald S, et al. PEGylation of paclitaxel-loaded cationic liposomes drives steric stabilization of bicelles and vesicles thereby enhancing delivery and cytotoxicity to human cancer cells. ACS Appl Mater Interfaces. 2020;12(1):151–162.
34. Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51.
35. Zhong L, Xu L, Liu Y, et al. Transformative hyaluronic acid-based active targeting supramolecular nanoplatform improves long circulation and enhances cellular uptake in cancer therapy. Acta pharmaceutica Sinica B. 2019;9(2):397–409.
36. Teleanu DM, Chircov C, Grumezescu AM, Volceanov A, Teleanu RI. Blood-brain delivery methods using nanotechnology. Pharmaceutics. 2018;10:4.
37. Zhu Q, Ling X, Yang Y, et al. Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Advan mater. 2019;6(6):1801899.
38. Dallavalle S, Dobričić V, Lazzarato L, et al. Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors. Drug Resist Updat. 2020;50:100682.
39. Maleklou N, Allameh A, Kazemi B. Targeted delivery of vitamin D3-loaded nanoparticles to C6 glioma cell line increased resistance to doxorubicin, epirubicin, and docetaxel in vitro. Vitro Cell Dev Biol Anim. 2016;52:10.
40. Han W, Shi J, Cao J, Dong B, Guan W. Emerging roles and therapeutic interventions of aerobic glycolysis in glioma. Onco Targets Ther. 2020;13:6937–6955.
41. Apfelthaler C, Skoll K, Ciola R, Gabor F, Wirth M. A doxorubicin loaded colloidal delivery system for the intravesical therapy of non-muscle invasive bladder cancer using wheat germ agglutinin as targeter. Eur J Pharm Biopharm. 2018;130:177–184.
42. Xiao Y, Cheng L, Xie H-J, et al. Vinorelbine cationic liposomes modified with wheat germ agglutinin for inhibiting tumor metastasis in treatment of brain glioma. Artif Cells, Nanomed Biotechnol. 2018;46(sup3):):S524–S537.
43. Du D, Chang N, Sun S, et al. The role of glucose transporters in the distribution of p-aminophenyl-α-d-mannopyranoside modified liposomes within mice brain. J Control Release. 2014;182:99.
44. Liu L, Mu L-M, Yan Y, et al. The use of functional epirubicin liposomes to induce programmed death in refractory breast cancer. Int J Nanomedicine. 2017;12:4163–4176.
45. Du J, Lu W-L, Ying X, et al. Dual-targeting topotecan liposomes modified with tamoxifen and wheat germ agglutinin significantly improve drug transport across the blood-brain barrier and survival of brain tumor-bearing animals. Mol Pharm. 2009;6(3):905–917.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.