Back to Journals » Clinical Ophthalmology » Volume 15
Multicenter Evaluation of Time, Operational, and Economic Efficiencies of a New Preloaded Intraocular Lens Delivery System versus Manual Intraocular Lens Delivery
Authors Mendicute J, Bascarán L, Pablo L, Schweitzer C , Velasque L , Bouchet C, Martinez AA
Received 28 May 2020
Accepted for publication 6 January 2021
Published 16 February 2021 Volume 2021:15 Pages 591—599
DOI https://doi.org/10.2147/OPTH.S263658
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Javier Mendicute,1 Lucia Bascarán,1 Luis Pablo,2 Cédric Schweitzer,3 Laurent Velasque,4 Christine Bouchet,5 Aldo A Martinez5
1Department of Ophthalmology, Hospital Universitario Donostia, San Sebastián, Spain; 2Department of Ophthalmology, Hospital Universitario Miguel Servet, Zaragoza, Spain; 3Department of Ophthalmology, Bordeaux University Hospital, Bordeaux, France; 4Centre Retine Gallien, Bordeaux, France; 5Alcon Research LLC, Fort Worth, TX, USA
Correspondence: Javier Mendicute
Department of Ophthalmology, Hospital Universitario Donostia, Paseo Dr. Beguiristáin, 115, San Sebastián, 20014, Spain
Tel +34-943322233
Email [email protected]
Purpose: To evaluate intraoperative intraocular lens (IOL) delivery time and total surgical case time using the UltraSert preloaded delivery system (System U) during routine cataract surgeries and to compare with the manually loaded Monarch delivery system (System M). Physician satisfaction with System U was also assessed.
Patients and Methods: In this prospective observational study, subjects ≥ 18 years old underwent cataract surgery in 1 eye and received the AcrySof IQ IOL via the manually loaded System M (n=103) or the AcrySof IQ IOL model AU00T0 via the preloaded System U (n=93). Procedures were digitally recorded by an external camera or by a camera within the operating microscope. Device preparation, IOL delivery, and IOL positioning times were evaluated by 2 independent graders. Pearson χ2 test or Fisher exact test was used for categorical variables and Student’s t-test or Wilcoxon rank-sum test for continuous variables (all tests were 2-sided and performed at a 5% α-level). Physician satisfaction levels were assessed using questionnaires.
Results: Lens delivery time was similar for System U and System M (12.9± 5.1 and 12.2± 6.3 s; P=0.412). Mean device preparation time for System U was significantly shorter compared with System M (30.3± 6.6 versus 59.8± 31.0 s; P< 0.05). This resulted in a significantly shorter total intraoperative time (device preparation + lens delivery) with System U versus System M (43.0± 8.6 versus 72.0± 32.5 s; P< 0.05). Total surgical case time (device preparation + lens delivery + lens positioning and unfolding) was shorter for System U versus System M (56.6± 12.6 versus 89.6± 34.6 s; P< 0.05). Physicians reported greater satisfaction levels with System U compared with other devices.
Conclusion: Use of the preloaded delivery system (System U) resulted in faster device preparation and reduced total surgical time compared with the manually loaded system (System M). System U was intuitive to use, and physicians preferred it to other devices.
Keywords: cataract surgery, intraocular lens delivery time, Monarch, UltraSert
Introduction
Cataract has been reported to cause 33% of visual impairment cases and 51% of blindness cases worldwide.1 In 2015, cataract was the most common cause of blindness in Central, Eastern, and Western Europe (25%, 21%, and 21%, respectively), and these rates were projected to remain similarly high in 2020.2 Cataract surgery to remove the opaque lens (ie, phacoemulsification) followed by the implantation of an intraocular lens (IOL) is an effective treatment to restore and maintain vision.3 However, the cost of cataract surgery varies globally,4 and the specific factors that contribute to the economic burden of cataract surgery vary regionally. Economic studies in Europe have shown that direct labor contributed from 17% (Denmark) to 58% (Netherlands) of the total cost, whereas overhead costs contributed from 6% (Hungary) to 47% (England).5 Increased labor time was associated with an increase in total costs, with each additional hour of labor time leading to an estimated 10% increase in cost.5
Development of new IOL delivery systems may improve surgical time efficiencies.6,7 IOL delivery systems have been designed to facilitate implantation of the IOL through microincisions. Minimal incision enlargement reduces the risk of surgically induced astigmatism or corneal wound damage.8,9 Preloaded delivery systems also limit the risk of infection caused by manual handling of the IOL and lead to fewer surgical errors.10,11 Most studies of preloaded versus manual IOL delivery systems have focused on performance, safety, and postoperative results.12–16 However, the use of preloaded systems may also decrease surgical time.6,7,17 Recently, comparison studies of preloaded and manually loaded IOL delivery systems showed that preloaded systems resulted in fewer surgical delays and reduced total case time.6,18
The UltraSertTM Preloaded Delivery System (System U; Alcon Laboratories, Inc., Fort Worth, TX, USA) has been developed for use with the AcrySof® IQ IOL (Alcon Laboratories) to provide control and reliability, to reduce complications, and improve time efficiency during cataract surgery (Figure 1). This single-use system, consisting of a nozzle, main body, and plunger, has been designed so that the preloaded IOL is delivered by manually advancing the plunger using a one-handed push mechanism. Unlike other delivery systems, System U has a depth guard and a plunger with a TensionGlideTM system. The depth guard was designed to help surgeons control insertion depth; it is intended to provide a counterforce during insertion, minimize the stretch of the incision, and preserve incision architecture. The TensionGlide spring was designed to provide a smooth, consistent, and controlled delivery of the IOL in the capsular bag. Compared with manually loaded devices, such as the Monarch® D Cartridge (Alcon Laboratories) system (System M), use of System U led to a smaller final corneal incision size after IOL implantation.13 Overall, System U caused less widening of the corneal incision compared with System M and with other preloaded systems, leading to less surgically induced astigmatism.13
 |
Figure 1 UltraSert preloaded IOL delivery system. |
The purpose of this study was to assess intraoperative lens delivery time and total surgical case time for AcrySof IQ IOL model AU00T0 with System U preloaded delivery versus System M manually loaded delivery during routine cataract surgeries. The primary objective was to investigate if there was a decrease in lens delivery time using System U compared with System M. The secondary objective was to investigate if lens delivery with System U led to a decrease in total intraoperative surgical case time (device preparation time plus lens delivery time) compared with System M. The individual components of total surgical case time, including device preparation time and lens unfolding time, were characterized to assess economic efficiencies that may be achieved with System U. Physician satisfaction with System U compared with other injector systems was also evaluated.
Methods
Study Population
Included in the study were subjects ≥18 years of age who received cataract extraction by phacoemulsification in 1 eye in accordance with the directions for use of the study device in routine clinical practice. Subjects received the AcrySof IQ IOL via the manually loaded System M or the AcrySof IQ IOL model AU00T0 via the preloaded System U under standard care conditions (2.2 or 2.4 mm width incisions were used for System U or System M); surgeons participating in this observational study performed cataract surgeries according to their regular schedules. Excluded from the study were patients who required cataract extraction in 2 eyes during the same surgery or patients receiving both manual and System U preloaded IOL delivery.
Study Design
This was a prospective observational study conducted in France (3 sites) and Spain (2 sites) under a protocol approved by a Medicinal Research Ethics Committee (Comité Ético de Investigación Clínica) and in compliance with local regulatory bodies (Commission Nationale de l’Informatique et des Libertés). Additionally, the protocol was reviewed and approved by the Ethics Committees of the respective centers, and the study followed the recommendations of Good Clinical Practice and the principles of the Declaration of Helsinki. All patients were informed about the characteristics of the study and signed the informed consent. All stages of the procedure, from initiation of device preparation through lens unfolding, were recorded (Figure 2). Delivery device preparation was recorded by an external digital video camera and was defined starting with the time of device preparation by the nurse/surgical assistant (T0). Device preparation time comprised the time at which the plastic device tray was opened (T01), the time at which the leading haptic of the lens hit the nozzle line (T02), the beginning of the lens inspection (T03), and the end of the lens inspection (T04). The intraoperative procedure was recorded by the camera within the operating microscope and comprised the time the device touched the eye for purpose of insertion (T1), the time at which the trailing haptic left the plunger (T2), and the time at which lens unfolding was complete (T3). Two independent graders assessed the recordings to determine device preparation time, lens delivery time, and lens positioning time.
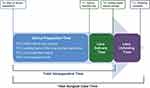 |
Figure 2 Lens delivery process. |
Outcome Measures
The primary outcome measure was the lens delivery time, defined as the time from when the device first touched the eye to when the leading haptic left the plunger (T1–T2; Figure 2). The secondary outcome was the total intraoperative time, T0–T2. Three exploratory outcomes were also assessed, including device preparation time (T01–T04), lens unfolding time (T2–T3), and total surgical case time (T0–T3).
Physician Experience and Satisfaction
Questionnaires were collected at all sites to assess satisfaction levels with System U on a scale of 1 to 7 (1=not satisfied at all; 7=extremely satisfied; Supplementary Table 1). Satisfaction with System U compared with “most often used manually loaded devices” and with “most often used preloaded devices” were rated on a scale of 1 to 9 (1=manual/other preloaded device better; 5=neutral; 9=System U better; Supplementary Table 2 and Supplementary Table 3).
Statistical Analysis
The sample size required to detect a difference between System U and System M was calculated using a porcine eye model; it was determined that 80 observations per group were required to detect a difference of ≥2.6 seconds with a power of ≥80% at a 5% significance level. Descriptive results were presented as mean ± SD for continuous variables. There was no imputation method used for missing data. For categorical variables, frequencies were calculated relative to nonmissing data. Statistical analysis was performed using SAS® 9.2 (SAS Institute, Cary, NC, USA). Statistical comparisons were performed using Pearson χ2 test or Fisher exact test for categorical variables and Student’s t-test or Wilcoxon rank-sum test for continuous variables. All tests were 2-sided and performed at a 5% α-level. Questionnaire data were summarized descriptively, and no statistical analysis was performed.
Results
Subjects
In this study, 93 subjects received an IOL implanted with System U and 103 received an IOL implanted with System M.
Outcomes
The lens delivery time was similar for System U and System M (P=0.412). With the preloaded System U, the mean ± SD lens delivery time from all study sites was 12.9±5.1 seconds and with the manually loaded System M, the mean lens delivery time was 12.2±6.3 seconds (Figure 3).
 |
Figure 3 Mean lens delivery times with System U and System M. |
Lens implantation with System U led to a significantly shorter mean total intraoperative time (43.0±8.6 s) compared with System M (72.0±32.5 s; P<0.05; Figure 4), corresponding to a 40% reduction overall and a mean difference of 28.9 seconds per case. Because there was no significant difference for lens delivery time, the reduction in total intraoperative time (device preparation time plus lens delivery time) resulted from the significantly shorter mean device preparation time for System U (30.3±6.6 s) compared with System M (59.8±31.0 s; P<0.05; Figure 5A). Additionally, the variability in the preparation times was less with System U (range, 20–60 s) than with System M (range, 31–202 s; Table 1). Mean lens positioning and unfolding time was numerically shorter for System U (13.8±7.0 s) compared with System M (17.6±11.0 s; Table 1).
 |
Figure 4 Mean total intraoperative surgical times with System U and System M (device preparation + lens delivery). |
 |
Table 1 Device Preparation Time with System U and System M |
Total surgical case time, which included device preparation time, lens delivery time, and lens positioning and unfolding, was 33 seconds shorter for System U (56.6±12.6 s) compared with System M (89.6±34.6 s; P<0.05; Figure 5B).
Physician Experience and Satisfaction
Physicians were satisfied with the preloaded System U and preferred it to their “most-often used” manually loaded IOL systems and other preloaded IOL delivery systems (Figure 6). Physicians found System U intuitive to use (score of 6.4 out of 7) and reported greater satisfaction levels with System U compared with other devices when evaluating the ease of preparation, the number of steps required for preparation, overall time of delivery, overall control of the procedure, and overall confidence in using the device (scores were ≥7.7 out of 9 when compared with manually loaded devices; scores were ≥7.2 out of 9 when compared with preloaded devices).
 |
Figure 6 Physician satisfaction levels: *1=not satisfied at all; 7=extremely satisfied; †1=manual better, 5=neutral, 9=System U better; ‡1=other preloaded device better, 5=neutral, 9=System U better. |
Discussion
As the age of the global population increases, the number of people with visual impairment caused by cataract is predicted to reach >50 million by 2020.19,20 The incidence of cataract surgery has increased over time, particularly in aging populations, and continued increases in the number of people with pseudophakia are expected.21–25 Other factors, such as improved postoperative visual outcomes achieved with the development of new IOL technology, also drive the demand for cataract replacement surgery.26–28 Thus, to accommodate the increased number of cataract surgeries, there is a need to optimize surgical time, facilitate better operating room performance, and reduce cost.
Cataract surgery in which IOLs are implanted through microincisions requires less surgical time compared with coaxial phacoemulsification and provides patients with better postoperative outcomes, including faster recovery time.29–31 During IOL delivery, preservation of the incision architecture is essential to prevent postoperative endophthalmitis, inflammation, and surgically induced astigmatism.29,32 Delivery systems that inject the IOL into the eye have been developed to limit manual manipulation of the incision and the lens.6,33,34 However, several available delivery devices, like System M, require manual loading of the lens, potentially risking contamination and surface deterioration due to handling of the IOL, and the time required to load the device may affect overall surgical time.6,12,35
Preloaded delivery systems, such as System U, create smaller corneal incisions, resulting in less surgically induced astigmatism than manual delivery systems.13,17,36 In comparison studies of preloaded delivery systems, incision size enlargement after IOL implantation with System U led to better conservation of the incision architecture and less surgically induced astigmatism compared with Tecnis® iTec and Hoya iSert® (Hoya Surgical Optics, Inc., Singapore) systems.13,17 In pig eyes, preloaded delivery systems provided more regular incision architecture compared with other devices.37 Another comparison study reported that mean incision enlargements were significantly smaller for System U compared with iSert.38 Additionally, System U was less prone to mechanical problems, including nozzle tip splitting or IOL adherence to the plunger tip, which was observed with the other preloaded systems.13,17 Recent studies have also evaluated IOL delivery times with use of preloaded devices.6,17,18 Compared with iTec and iSert, device preparation time was shorter with System U.7,17 Surgical time, the time to implant the IOL, was also shorter with System U than with iTec or iSert.7
Although a previous in vitro study reported that device implantation time was shorter with System U compared with System M,17 the outcomes of this study show similar lens delivery times for both System U and System M. However, the use of System U in this study allowed a reduction in device preparation time and total intraoperative time, improving surgical efficiency (37% reduction in mean total surgical case time) compared with the System M device.17 The present study agrees with an in vivo, multicountry study that demonstrated reduced surgery time when a preloaded device was used versus a manual device.6 In the previous study, use of a preloaded delivery system led to a decrease in total case time by 6% to 12%, but total case time included operating room setup and teardown, whereas the surgical case time in this study was limited to time spent manipulating the IOL. With System M, the additional steps of opening and inserting the IOL into the cartridge contributed to increased surgical time. Device preparation time was, on average, 30 seconds shorter with System U (range, 20–60 s) than with System M (range, 31–202 s), which can be attributed to the reduced lens handling time. Additionally, low variability was reported between surgical case times, reflecting the consistency of the preloaded System U device (ie, consistent folding) that resulted in improved reproducibility compared with System M.
The surgical time saved in this study with use of System U potentially increased the patient throughput; using a previously reported mean procedure duration of 13.9 minutes (834 s)39 and taking into consideration the 33 seconds gained per procedure using the System U device, it was estimated that 1 additional procedure could be performed per 25 cataract surgeries. In a study evaluating annual throughput at different geographical sites (Canada, France, and the US), 1 additional surgery could be performed per day with the use of a preloaded delivery device versus a manual device. The average number of manual surgeries performed per day ranged from 10 to 25 depending on the surgical site. There was an estimated additional annual throughput of 4% to 10% with a preloaded device. In a prospective observational study of 200 cases conducted in Northwestern China, the use of preloaded devices significantly reduced total surgery time, and the switch from manual to preloaded delivery device was estimated to increase the annual cataract surgery throughput by 5.2%–7.7%.18 Although the time saved with System U in the present study was used to estimate throughput, more studies are required to assess the direct impact of surgical case time savings, including impact on economic outcomes.
Physicians were satisfied with System U, rating satisfaction as 6.4 out of 7 for “device is intuitive use” and “workflow between ophthalmologist and nurse/technician.” The lowest average satisfaction rating for System U was for “ability to control deployment of the IOL,” (5.7 out of 7, where 1 was “not satisfied at all” and 7 was “extremely satisfied”); however, when asked to rate System U compared with other most often used manual and preloaded systems, physicians indicated that System U provided better control than other devices.
Limitations of this study included the observational, non-interventional design, which allowed for use of routine standard practices that could vary across study sites. This was considered a time and motion study to obtain real-world evidence. Therefore, surgeons were not restricted in the use of available lens power at the time of the surgery, which was 18 to 27 D. Additionally, this study was not randomized, and the graders were not masked to the surgical procedure or the implanted IOLs, leading to potential bias in the reported surgical times. Voluntary participation of physicians and social desirability bias promoted by surveys could also affect the validity of the research findings.
Conclusions
Preloaded System U allowed a significant time reduction in both device preparation and total surgical time compared with System M. No significant difference for lens delivery time was observed when comparing System U and System M. Overall, physicians were satisfied with System U compared with the other delivery devices used in clinical practice.
Abbreviations
IOL, intraocular lens.
Data Sharing Statement
Study data not reported in this publication are confidential.
Acknowledgments
Medical writing assistance was provided by Natalia Zhukovskaya, PhD, and Catherine DeBrosse, PhD, of ICON (North Wales, PA), and was funded by Alcon.
Funding
This study was sponsored by Alcon Research LLC (Fort Worth, TX). The sponsor participated in study design; data management, analysis, and interpretation; and manuscript preparation, review, and approval. IMS Health Solutions France contributed to the management and conduct of the study on behalf of Alcon.
Disclosure
Javier Mendicute, Luis Pablo, Cédric Schweitzer, and Laurent Velasque received honoraria for participating in the study; the study was funded by Alcon Research LLC as a clinical trial, and fees paid were credited to Biodonostia Health Research Institute, a nonprofit public research center. Aldo A Martinez is an employee of Alcon Research LLC. Christine Bouchet was an employee of Alcon Research LLC at the time of the research. The authors report no other conflicts of interest in this work.
References
1. Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614–618. doi:10.1136/bjophthalmol-2011-300539
2. Bourne RRA, Jonas JB, Bron AM, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: magnitude, temporal trends and projections. Br J Ophthalmol. 2018;102(5):575–585. doi:10.1136/bjophthalmol-2017-311258
3. Lundstrom M, Barry P, Henry Y, Rosen P, Stenevi U. Evidence-based guidelines for cataract surgery: guidelines based on data in the European registry of quality outcomes for cataract and refractive surgery database. J Cataract Refract Surg. 2012;38(6):1086–1093. doi:10.1016/j.jcrs.2012.03.006
4. Lansingh VC, Carter MJ. Use of global visual acuity data in a time trade-off approach to calculate the cost utility of cataract surgery. Arch Ophthalmol. 2009;127(9):1183–1193. doi:10.1001/archophthalmol.2009.113
5. Fattore G, Torbica A. Cost and reimbursement of cataract surgery in Europe: a cross-country comparison. Health Econ. 2008;17(S1):S71–82. doi:10.1002/hec.1324
6. Jones JJ, Chu J, Graham J, Zaluski S, Rocha G. The impact of a preloaded intraocular lens delivery system on operating room efficiency in routine cataract surgery. Clin Ophthalmol. 2016;10:1123–1129. doi:10.2147/OPTH.S107726
7. Nanavaty MA, Kubrak-Kisza M. Evaluation of preloaded intraocular lens injection systems: ex vivo study. J Cataract Refract Surg. 2017;43(4):558–563. doi:10.1016/j.jcrs.2017.02.019
8. Masket S, Wang L, Belani S. Induced astigmatism with 2.2- and 3.0-mm coaxial phacoemulsification incisions. J Refract Surg. 2009;25(1):21–24. doi:10.3928/1081597X-20090101-04
9. Berdahl JP, DeStafeno JJ, Kim T. Corneal wound architecture and integrity after phacoemulsification evaluation of coaxial, microincision coaxial, and microincision bimanual techniques. J Cataract Refract Surg. 2007;33(3):510–515. doi:10.1016/j.jcrs.2006.11.012
10. Mayer E, Cadman D, Ewings P, et al. A 10 year retrospective survey of cataract surgery and endophthalmitis in a single eye unit: injectable lenses lower the incidence of endophthalmitis. Br J Ophthalmol. 2003;87(7):867–869. doi:10.1136/bjo.87.7.867
11. Simon JW, Ngo Y, Khan S, Strogatz D. Surgical confusions in ophthalmology. Arch Ophthalmol. 2007;125(11):1515–1522. doi:10.1001/archopht.125.11.1515
12. Weston K, Nicholson R, Bunce C, Yang YF. An 8-year retrospective study of cataract surgery and postoperative endophthalmitis: injectable intraocular lenses may reduce the incidence of postoperative endophthalmitis. Br J Ophthalmol. 2015;99(10):1377–1380. doi:10.1136/bjophthalmol-2014-306372
13. Mendicute J, Amzallag T, Wang L, Martinez AA. Comparison of incision size and intraocular lens performance after implantation with three preloaded systems and one manual delivery system. Clin Ophthalmol. 2018;12:1495–1503. doi:10.2147/OPTH.S166776
14. Khokhar S, Sharma R, Patil B, Aron N, Gupta S. Comparison of new motorized injector vs manual injector for implantation of foldable intraocular lenses on wound integrity: an ASOCT study. Eye (Lond). 2014;28(10):1174–1178. doi:10.1038/eye.2014.162
15. Acar B, Torun IM, Acar S. Evaluation of preloaded IOL delivery system and hydrophobic acrylic intraocular lens in cataract surgery. Open Ophthalmol J. 2018;12(1):94–103. doi:10.2174/1874364101812010094
16. Haldipurkar SS, Shetty V, Haldipurkar T, et al. Incision size changes after cataract surgery with intraocular lens implantation: comparison of 2 preloaded IOL implantation injectors. J Cataract Refract Surg. 2020;46(2):222–227. doi:10.1097/j.jcrs.0000000000000014
17. Wang L, Wolfe P, Chernosky A, et al. In vitro delivery performance assessment of a new preloaded intraocular lens delivery system. J Cataract Refract Surg. 2016;42(12):1814–1820. doi:10.1016/j.jcrs.2016.10.014
18. Wu Y, Yan H, Weijia Y. Preloaded vs manually-loaded IOL delivery systems in cataract surgery in a biggest ambulatory surgery center of Northwestern China: an efficiency analysis. BMC Ophthalmol. 2020;20(1). doi:10.1186/s12886-020-01721-5
19. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. doi:10.1016/S2214-109X(17)30393-5
20. Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451(7179):716–719. doi:10.1038/nature06516
21. Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487–494.
22. Klein BEK, Howard KP, Lee KE, Klein R. Changing incidence of lens extraction over 20 years: the Beaver Dam eye study. Ophthalmology. 2014;121(1):5–9. doi:10.1016/j.ophtha.2013.06.006
23. Daien V, Le Pape A, Heve D, Carriere I, Villain M. Incidence and characteristics of cataract surgery in France from 2009 to 2012: a national population study. Ophthalmology. 2015;122(8):1633–1638. doi:10.1016/j.ophtha.2015.04.017
24. Kanthan GL, Wang JJ, Rochtchina E, et al. Ten-year incidence of age-related cataract and cataract surgery in an older Australian population. The Blue Mountains Eye Study. Ophthalmology. 2008;115(5):808–814 e801. doi:10.1016/j.ophtha.2007.07.008
25. Gollogly HE, Hodge DO, St Sauver JL, Erie JC. Increasing incidence of cataract surgery: population-based study. J Cataract Refract Surg. 2013;39(9):1383–1389. doi:10.1016/j.jcrs.2013.03.027
26. Mendicute J, Kapp A, Levy P, et al. Evaluation of visual outcomes and patient satisfaction after implantation of a diffractive trifocal intraocular lens. J Cataract Refract Surg. 2016;42(2):203–210. doi:10.1016/j.jcrs.2015.11.037
27. Kohnen T, Herzog M, Hemkeppler E, et al. Visual performance of a quadrifocal (trifocal) intraocular lens following removal of the crystalline lens. Am J Ophthalmol. 2017;184:52–62. doi:10.1016/j.ajo.2017.09.016
28. Kretz FT, Breyer D, Klabe K, et al. Clinical outcomes after implantation of a trifocal toric intraocular lens. J Refract Surg. 2015;31(8):504–510. doi:10.3928/1081597X-20150622-01
29. Alio J, Rodriguez-Prats JL, Galal A, Ramzy M. Outcomes of microincision cataract surgery versus coaxial phacoemulsification. Ophthalmology. 2005;112(11):1997–2003. doi:10.1016/j.ophtha.2005.06.024
30. Hayashi K, Hayashi H, Nakao F, Hayashi F. The correlation between incision size and corneal shape changes in sutureless cataract surgery. Ophthalmology. 1995;102(4):550–556. doi:10.1016/S0161-6420(95)30983-9
31. Dick HB. Controlled clinical trial comparing biaxial microincision with coaxial small incision for cataract surgery. Eur J Ophthalmol. 2012;22(5):739–750. doi:10.5301/ejo.5000100
32. Elkady B, Pinero D, Alio JL. Corneal incision quality: microincision cataract surgery versus microcoaxial phacoemulsification. J Cataract Refract Surg. 2009;35(3):466–474. doi:10.1016/j.jcrs.2008.11.047
33. Kohnen T, Koch DD. Experimental and clinical evaluation of incision size and shape following forceps and injector implantation of a three-piece high-refractive-index silicone intraocular lens. Graefes Arch Clin Exp Ophthalmol. 1998;236(12):922–928. doi:10.1007/s004170050181
34. Kohnen T, Klaproth OK. Incision sizes before and after implantation of SN60WF intraocular lenses using the Monarch injector system with C and D cartridges. J Cataract Refract Surg. 2008;34(10):1748–1753. doi:10.1016/j.jcrs.2008.06.031
35. Bainbridge JW, Teimory M, Tabandeh H, et al. Intraocular lens implants and risk of endophthalmitis. Br J Ophthalmol. 1998;82(11):1312–1315. doi:10.1136/bjo.82.11.1312
36. Wang L, Wolfe P, Paliwal S, Chernosky A, Kohnen T. Comparative evaluation of corneal incision enlargement after intraocular lens delivery of new preloaded and manual implantation systems. Eur J Ophthalmol. 2019;1120672119882334. doi:10.1177/1120672119882334
37. Mencucci R, Favuzza E, Salvatici MC, Spadea L, Allen D. Corneal incision architecture after IOL implantation with three different injectors: an environmental scanning electron microscopy study. Int Ophthalmol. 2019;39(2):397–403. doi:10.1007/s10792-018-0825-2
38. Liu J, Wolfe P, Hernandez V, Kohnen T. Comparative assessment of the corneal incision enlargement of four preloaded IOL delivery systems. J Cataract Refract Surg. 2020;Publish Ahead of Print. doi:10.1097/j.jcrs.0000000000000214
39. Rothschild PR, Grabar S, Le Du B, et al. Patients’ subjective assessment of the duration of cataract surgery: a case series. BMJ Open. 2013;3(5):
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

