Back to Journals » International Medical Case Reports Journal » Volume 13
Multi-Drug Resistant Post Corneal Repair Klebsiella oxytoca’s Keratitis
Authors Dago TR , Zewudie A , Mamo Y, Feyissa D , Geleta S
Received 28 August 2020
Accepted for publication 23 September 2020
Published 20 October 2020 Volume 2020:13 Pages 537—541
DOI https://doi.org/10.2147/IMCRJ.S278625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Tolcha Regasa Dago,1 Ameha Zewudie,1 Yitagesu Mamo,1 Desalegn Feyissa,1 Sinbona Geleta2
1Department of Pharmacy, College of Medicine and Health Science, Mizan-Tepi University, Mizan-Aman, Ethiopia; 2Department of Ophthalmology, Institute of Health Science, Jimma University, Jimma, Ethiopia
Correspondence: Tolcha Regasa Dago
Department of Pharmacy, College of Medicine and Health Science, Mizan Tepi University, Mizan-Aman, Ethiopia
Tel +251917233151
Email [email protected]
Background: Bacterial keratitis can threaten vision through permanent corneal scarring and even perforation, resulting in loss of the eye. Klebsiella oxytoca is resistant to several antibiotics because it produces extended-spectrum β-lactamase encapsulated with polysaccharide. Thus, this article is aimed at reporting a rare case of Klebsiella oxytoca-induced keratitis in Jimma University Medical Centre, Jimma, Ethiopia.
Case Presentation: TA 25-year-old female patient presented with photophobia, redness, and purulent discharge from the right eye. She had matted cilia of the eyelid, conjunctiva injection, corneal ulcer, and deep fibrinous anterior chamber reaction. She had light perception (LP) visual acuity for the same eye and it was firm when examined digitally. The cornea-scleral repair was performed one month earlier, due to open globe injury. The patient had taken empirical fortified antibiotics before the identification of the specific pathogen. Culture and drug sensitivity test was performed in order to identify the aetiology. The result of the test revealed that the identified pathogen was multi-drug-resistant Klebsiella oxytoca. Based on this result and drug availability, high dose topical fluoroquinolones eye drops (Ciprofloxacin eye drop 0.3% and Ofloxacin 0.3%) were given. Besides, dexamethasone 0.1% eye drop was added to the aforementioned antibiotics. After four months of treatment, the visual outcome was changed from LP to hand motion.
Conclusion: A rare case of multi-drug resistant Klebsiella oxytoca induced keratitis which was isolated in a biochemical test was successfully treated with a high dose of fluoroquinolones.
Keywords: Klebsiella oxytoca, keratitis, anti-microbial resistant, Ethiopia
Introduction
Bacterial keratitis is a serious ocular infectious disease that can lead to severe visual disability. The severity of the corneal infection usually depends on the underlying conditions of the cornea and the pathogenicity of the infecting bacteria. The eye is hard and protected by a continuous flow of tears which contain antibacterial compounds. However, underlying conditions such as inflammation, previous surgical procedures, scarring, and immunity-compromising conditions may predispose the cornea for infection.1–3
Klebsiella oxytoca is rare but it can cause severe bacterial keratitis. It is a gram-negative bacterial pathogen which is encapsulated with cylindrical polysaccharide. It is also a rod-shape, non-motile in nature, belonging to the Enterobacteriaceae family and the genus of Klebsiella.4,5
Bacterial keratitis can threaten vision through permanent corneal scarring and even perforation, resulting in the loss of the eye. If definitive identification and treatment are given, significant morbidity associated with severe microbial keratitis may be avoided. However, in this case complications occurred due to the misidentification of the responsible microbial organism and subsequent inadequate and/or inappropriate treatment.6,7 Isolation of the indole-positive Klebsiella species- Klebsiella oxytoca is difficult. This characteristic increases the incidence of extended-spectrum β-lactamase producing Klebsiella oxytoca. Klebsiella oxytoca is a serious problem worldwide, because of their incrimination in antibiotic resistance.8,9 Polysaccharide capsule and different mutation of Klebsiella oxytoca contributes to great resistance against the host defence mechanism. Therefore, this case reports the isolation of Klebsiella oxytoca’ without polymerase chain reaction and successful treatment of multi-drug resistant Klebsiella oxytoca- induced keratitis with fluoroquinolone eye drops (Ciprofloxacin eye drop 0.3% and Ofloxacin 0.3%).
Case Presentation
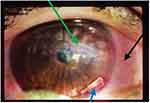
A 25-year-old female patient admitted to the Jimma University Medical Centre, Jimma, Ethiopia, Ophthalmology clinic on May 9, 2019 with the complaint of photophobia, redness, and purulent discharge from the right eye. The slit-lamp examination also showed matted cilia of the eyelid, conjunctiva injection which had eroded around the stitch, corneal ulcer of 3.5 x 3 mm, negative sidle test, hypopyon, deep fibrinous anterior chamber reaction, pupil not visible well due to stitch-site abscess, the lens was transparent and located around thestitch. She had also a light perception (LP) visual acuity for the same eye. The eye was firm when examined digitally (Figure 1).
She had a traumatic globe injury of the same eye one month earlier. At that time, she had bleeding from the eye. There was also laceration of the cornea and iris. For this reason, the cornea-scleral repair was performed under aseptic technique and corneal wound repair was performed with nylon 10/0 while sclera’s wound was repaired with vicryl 5/0. Tetracycline and sub-conjunctiva vancomycin and ceftazidime were given at that time.
An abscess was debrided and 3–5 stitches were removed during admission one month after cornea-scleral repair. The corneal scraped sample was then sent to the microbiology department for culture and drug susceptibility test and an empirical fortified antibiotic was prescribed. Fortified vancomycin eye drop 50mg/mL (5%) and fortified gentamicin eye drops 14mg/mL (1.4%, tropicamide 0.3% eye drop, and acetazolamide 250mg orally were given for patient). The patient was taking the above medication until the result of the microbiology report (7days) but no improvement was seen. Ophthalmologist collected corneal scrapings with a 20 gauge sterile needle during admission. A topical anaesthetic (1% tetracaine) was used during taking of the corneal specimens. Then the scraping was inserted into a 2mL brain heart infusion broth (Oxoid, Hampshire, UK) tube. After that, it was immediately inoculated into chocolate and blood agar. A chocolate and a blood agar plate were placed into a candle jar for fastidious bacterial pathogens, which require CO2, while one blood agar plate was placed into a cold chain to maintain the viability of the bacterial pathogens during transport. This three culture plates and one tube were incubated appropriately. After incubation, subcultures were made onto sheep blood agar (5%), chocolate agar, mannitol salt agar and MacConkey agar (Oxoid, Hampshire, UK) using the standard methods. The inoculated media plates were incubated at their respective optimal temperatures. The plates were then checked for growth after 48 hours. The bacterial isolates from the corneal scrapings was identified up to species-level based on different criteria which included morpho-cultural and biochemical tests. Currently using a molecular method such as polymerase chain reaction is recommended to identify different species of Klebsiella but we used a biochemical test method (old method). Biochemical test on isolated organism showed negative methyl red, positive Voges-Proskauer reaction, positive citrate, urea and growth on sulfide-indole motility medium (indole-positive). It was also ornithine decarboxylase negative and lysine decarboxylase positive. Finally, these were all test-proven characteristics of Klebsiella oxytoca which as reported by Jimma University Medical Center Microbiology Department. In vitro antibiotic susceptibility testing of the bacterial isolates was performed by Kirby-Bauer disc diffusion method.10 The interpretation of the results was according to the Clinical and Laboratory Standards Institute methodology as susceptible, intermediate and resistant (Table 1).
 |
Table 1 Culture Result and Drug Susceptibility of Isolated Organism from Sample of Case Report |
Due to this, drug susceptibility test and local availability of eye drop fortified antibiotics were discontinued and a high dose of ciprofloxacin 0.3% eye drop was prescribed every 2 hours with dexamethasone eye drops every 6 hours. Ibuprofen 400mg as required was also given for pain relief. She had also taken cloxacillin 500mg orally every 6 hours for 7days. Pain, photophobia, and discharges were much decreased. Ulcer size also decreased to less than 1.5 x 1mm She was discharged after one month on June 8, 2019 (Figure-2). After discharge, she was kept on weekly follow-up and stayed on follow-up until September 21, 2019. At follow-up, she was taking tetracycline eye ointment, ciprofloxacin eye drops, and tropicamide eye drops. Sometimes, she took ofloxacillin eye drops in place of ciprofloxacin eye drops. At the end of follow-up, a scar was formed around the stitch and improvement of vision was seen from LP to hand motion at the end of 4 months (Figure-3).
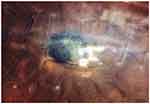 |
Figure 3 Photograph of Klebsiella oxytoca-induced infectious keratitis showing formed scar from ulcer and scar around removed stitch at final follow-up. |
Discussion
This case reported the isolation of Klebsiella oxytoca without polymerase chain reaction and successful treatment of multi-drug resistant Klebsiella oxytoca- induced keratitis with fluoroquinolones eye drops (ciprofloxacin eye drop 0.3% and ofloxacin 0.3%).
Klebsiella oxytoca is a rare cause of infectious keratitis. For instance, a study conducted in British Columbia University, Canada showed that the incidences of bloodstream infection due to Klebsiella Pneumoniae and Klebsiella oxytoca were 9.1 and 2.9 per 100,000 per year, respectively. About 75% of those pathogens were healthcare-associated. The incidence case of Klebsiella oxytoca-induced keratitis is far less than those of blood steam infections.11
As far as our knowledge, there is only one case that was reported worldwide before this case.4
In this case report, the isolated Klebsiella oxytoca strain was resistant to ampicillin, cefepime, chloramphenicol, and gentamicin. However, it was susceptible to ampicillin-sulbactam and intermediate resistant for ciprofloxacin and meropenem. Since the susceptible drugs were not available in the study setting, fluoroquinolones (ciprofloxacin eye drop and ofloxacin eye drop) were prescribed for the patients. Finally, the patient’s visual acuity was improved from LP to hand motion after four month of treatment with fluoroquinolones. This is in line with the study conducted in India which revealed that the isolated Klebsiella oxytoca strains were resistant to cefepime and gentamicin. However, the finding of the above report showed out of 175 Klebsiella oxytoca isolated, 117 were fluoroquinolone-resistant.12 Similar to our report, a study conducted at Hinduja Hospital, Mumbai identified, Klebsiella oxytoca as resistant to ampicillin, cefepime, and gentamicin.12 In contrast to our report, a study conducted at Hinduja Hospital, Mumbai identified Klebsiella oxytoca resistant to ampicillin/sulbactam.13
Fluoroquinolones have good clinical effect against Enterobacteriaceae including Klebsiella. However, the recurrence of ciprofloxacin-resistant Klebsiella species has expanded worldwide lately.14 Klebsiella pneumonia and Klebsiella oxytoca are the two most common pathogens causing nosocomial infections in humans, among different genus of Klebsiella. Because of this reason, they are of great concern for developing multi-drug resistance.15 The emergence of strains of Klebsiella oxytoca that are resistant to extended-spectrum β-lactam antibiotics enforced the physicians to choice of an appropriate antimicrobial agent for treating these infections.16
The risk factors for Klebsiella oxytoca-induced keratitis in our case report may include previous cornea-scleral surgery and use of dexamethasone eye drops. This is similar to studies conducted by Karp et al and Chang et al which showed that the history of corneal surgery and use of topical corticosteroids were the risk factors for Klebsiella oxytoca induced keratitis. The incidence of post-laser surgery infectious keratitis is estimated to be between 1/1000 and 1/5000.17,18 Physicians routinely order antibiotics to treat Klebsiella oxytoca-induced keratitis infection. However, some strains are resistant to most commonly used antibiotics. In such cases, specialized laboratory tests may be used in order to prescribe alternative antibiotics to treat the infections.6
In this case report, we used an old method (biochemical test) to differentiate Klebsiella oxytoca from other Klebsiella species. Molecular methods such as polymerase chain reaction have been evaluated for the rapid and accurate identification of Klebsiella in human clinical specimens.19
In conclusion, a rare case of multi-drug resistant Klebsiella oxytoca-induced keratitis was successfully treated with high dose topical fluoroquinolone eye drops (ciprofloxacin eye drop 0.3% and ofloxacin 0.3%).
Ethics
Ethical approval was obtained from the institutional review board of Jimma University Medical Centre. The patient has provided written informed consent to have the case details published.
Acknowledgment
We would like to acknowledge our colleague from Jimma University Medical Centre, the Ophthalmology hospital where the patient was initially diagnosed and managed. We would also like to acknowledge the patient for her willingness in providing all the necessary information.
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. Vajpayee RB, Dada T, Saxena R, et al. Study of the first contact management profile of cases of infectious keratitis: a hospital-based study. Cornea. 2000;19(1):52–56. doi:10.1097/00003226-200001000-00011
2. Miedziak AI, Miller MR, Rapuano CJ, Laibson PR, Cohen EJ. Risk factors in microbial keratitis leading to penetrating keratoplasty. Ophthalmology. 1999;106(6):1166–1171.
3. Teweldemedhin M, Gebreyesus H, Atsbaha AH, Asgedom SW, Saravanan M. Bacterial profile of ocular infections: a systematic review. BMC Ophthalmol. 2017;17(1):212. doi:10.1186/s12886-017-0612-2
4. Chou TY, Adyanthaya R. Infectious crystalline keratopathy associated with Klebsiella oxytoca. J Ophthalmic Inflamm Infect. 2012;2(4):211. doi:10.1007/s12348-012-0071-0
5. Gorkiewicz G. Nosocomial and antibiotic-associated diarrhoea caused by organisms other than Clostridium difficile. Int J Antimicrob Agents. 2009;33:S37–S41. doi:10.1016/S0924-8579(09)70015-9
6. Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87(7):834–838. doi:10.1136/bjo.87.7.834
7. Karsten E, Watson SL, Foster LJ. Diversity of microbial species implicated in keratitis: a review. Open Ophthalmol J. 2012;6:110. doi:10.2174/1874364101206010110
8. Mori M, Ohta M, Agata N, et al. Identification of species and capsular types of Klebsiella clinical isolates, with special reference to Klebsiella planticola. Microbiol Immunol. 1989;33(11):887–895. doi:10.1111/j.1348-0421.1989.tb00976.x
9. Hori K, Yasoshima H, Yamada A, et al. Adrenal hemorrhage associated with Klebsiella oxytoca bacteremia. Intern Med. 1998;37(11):990–994. doi:10.2169/internalmedicine.37.990
10. Cheesbrough M. District Laboratory Practice in Tropical Countries.
11. Reid CB, Steele L, Pasquill K, Parfitt EC, Laupland KB. Occurrence and determinants of Klebsiella species bloodstream infection in the western interior of British Columbia, Canada. BMC Infect Dis. 2019;19(1):1–7. doi:10.1186/s12879-019-4706-8
12. Rath S, Padhy RN. Prevalence of two multidrug-resistant Klebsiella species in an Indian teaching hospital and adjoining community. J Infect Public Health. 2014;7(6):496–507.
13. Trivedi MK, Branton A, Trivedi D, et al. Characterization of antimicrobial susceptibility profile of biofield treated multidrug-resistant klebsiella oxytoca. Appl Microbiol. 2015;1(1). Open Access, Longdom Publishing SL.
14. Thomson CJ. The global epidemiology of resistance to ciprofloxacin and the changing nature of antibiotic resistance: a 10 year perspective. J Antimicrob Chemother. 1999;43(suppl_1):31–40. doi:10.1093/jac/43.suppl_1.31
15. Chakraborty S, Mohsina K, Sarker PK, Alam MZ, Karim MI, Sayem SA. Prevalence, antibiotic susceptibility profiles and ESBL production in Klebsiella pneumoniae and Klebsiella oxytoca among hospitalized patients. Period Biol. 2016;118(1):53–58. doi:10.18054/pb.2016.118.1.3160
16. Arakawa Y, Ohta M, Kido N, et al. Chromosomal b-lactamase of Klebsiella oxytoca, a new class A enzyme that-hydrolyzes broad-spectrum b-lactam antibiotics. Antimicrob Agents Chemother. 1989;33:63–70. doi:10.1128/AAC.33.1.63
17. Karp CL, Tuli SS, Yoo SH, et al. Infectious keratitis after LASIK. Ophthalmology. 2003;110(3):503–510. doi:10.1016/S0161-6420(02)01760-8
18. Chang MA, Jain S, Azar DT. Infections following laser in situ keratomileusis: an integration of the published literature. Surv Ophthalmol. 2004;49(3):269–280. doi:10.1016/j.survophthal.2004.02.007
19. Chander Y, Ramakrishnan MA, Jindal N, Hanson K, Goyal SM. Differentiation of Klebsiella pneumoniae and K. oxytoca by multiplex polymerase chain reaction. Int J Appl Res Vet Med. 2011;9(2):138.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.