Back to Journals » Infection and Drug Resistance » Volume 12
Multi-Drug Resistant Escherichia coli Causing Early-Onset Neonatal Sepsis – a Single Center Experience from China
Authors Zhu M, Jin Y, Duan Y, He M, Lin Z , Lin J
Received 4 September 2019
Accepted for publication 15 November 2019
Published 27 November 2019 Volume 2019:12 Pages 3695—3702
DOI https://doi.org/10.2147/IDR.S229799
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Minli Zhu,1 Yuting Jin,1 Yue Duan,1 Minzhi He,1 Zhenlang Lin,1 Jing Lin1,2
1Department of Neonatology, The Second Affiliated Hospital and Yuying Children’s Hospital, Wenzhou Medical University, Wenzhou 325027, People’s Republic of China; 2Department of Pediatrics, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA
Correspondence: Jing Lin
Department of Pediatrics, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA
Tel +1 212 2416186
Fax +1 212 5345207
Email [email protected]
Zhenlang Lin
Department of Neonatology, The Second Affiliated Hospital and Yuying Children’s Hospital, Wenzhou Medical University, Wenzhou 325027, People’s Republic of China
Tel +86 13806689800
Email [email protected]
Background and objective: Infections caused by extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli (E. coli) have raised public-health concerns and are becoming a global health challenge. This study aimed to investigate changes in antimicrobial resistance of E. coli responsible for early-onset sepsis (EOS) in a perinatal center in eastern China.
Methods: Two periods, 2002 to 2008 and 2012 to 2018, were investigated. EOS was defined as the presence of a single potentially pathogenic bacterium grown from blood or cerebrospinal fluid in cultures drawn in any newborn infant within 72 hrs of birth. The changes in antimicrobial resistance of E. coli were analyzed.
Results: A total of 163 cases of EOS were identified, and E. coli continued to be the leading pathogen in our neonatal intensive care unit (NICU). Overall resistance of E. coli to third-generation cephalosporins increased from 14.3% in 2002–2008 to 46.7% in 2012–2018 (p<0.05). This resistance pattern closely parallels ESBL production. Compared to that from term infants, E. coli isolated from preterm infants had a significantly higher rate of resistance to ampicillin (93.3% vs 48.4%, p<0.01) and gentamicin (60.0% vs 9.4%, p<0.01), as well as a higher rate of ESBL production (66.7% vs 15.6%, p<0.01).
Conclusion: We conclude that ESBL-producing multi-drug resistant E. coli has emerged as the major pathogen responsible for early-onset neonatal sepsis, particularly in preterm infants. Clinicians should consider this trend and attempt to select proper effective antibiotics as the empirical treatment for early-onset neonatal sepsis.
Keywords: Escherichia coli, extended-spectrum beta-lactamase, early-onset sepsis, neonatal intensive care unit, newborn
Introduction
Neonatal sepsis remains a major cause of neonatal morbidity and mortality despite significant advances in perinatal care over the last few decades.1 Early-onset sepsis (EOS) which is defined as infection occurring within 72 hrs after birth is usually acquired through the vertical transmission of organisms from mother to infant. The overall incidence of culture-confirmed EOS in industrialized countries is reported to be 0.54–0.9 per 1000 live births.2–4 The reported incidence of culture-confirmed EOS from low- and middle-income countries appears to be higher.5,6 The presence of maternal risk factors such as prolonged rupture of membranes (PROM), chorioamnionitis and premature delivery is usually associated with a higher incidence of EOS in neonates. Group B Streptococcus (GBS) and Escherichia coli (E. coli) are the two most common pathogenic organisms for EOS.5,7 To prevent EOS due to GBS, either a risk-based approach to identify women who may benefit from intrapartum antimicrobial prophylaxis (IAP) or the administration of IAP to all colonized women based on the result of universal antenatal screening for GBS at 35 to 37 weeks’ gestational age has been recommended and implemented in most countries.2,3
Symptoms of EOS are usually non-specific and most neonatologists start empiric antibiotics when EOS is suspected, prior to the availability of blood culture results. Selection of appropriate initial empiric antibiotics is based mainly on the sensitivity patterns of GBS and E. coli in different hospitals and regions since these are the two most common organisms. Limited data are available from low- and middle-income countries on the epidemiology and antimicrobial resistance patterns of EOS, particularly from China where dramatic socioeconomic changes have occurred in the last two decades due to industrialization. While reports of EOS due to GBS in China are relatively rare,8 all report GBS isolates from Chinese neonates thus far are susceptible to penicillin.9–11 On the other hand, our recent meta-analysis based on a systematic review of the published studies in Chinese literature demonstrates that in newborn infants hospitalized in Chinese NICUs, roughly 50% of all E. coli bloodstream isolates (regardless of early onset or late onset) are multi-drug resistant due to extended-spectrum beta-lactamase (ESBL) production.12 Therefore, the objective of this study was to investigate changes over time of the clinical characteristics and antimicrobial resistance patterns of EOS caused by E. coli in a tertiary neonatal intensive care unit in eastern China. This may contribute to a more informed selection of appropriate antibiotics for empirical therapy in developing countries with similar bacterial profile and sensitivity patterns.
Materials and Methods
Data Collection
All newborn infants admitted into the neonatal intensive care unit (NICU) of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University with a diagnosis of EOS were included in this retrospective cohort study. EOS was defined as the presence of a single potentially pathogenic bacterium grown from blood or cerebrospinal fluid (CSF) in cultures drawn in any newborn infant within 72 hrs of birth. The sensitivity/specificity test results and ESBL status were obtained from our clinical laboratory reports. The clinical laboratory in our center practices routine microbiological tests according to the standard set by the Clinical & Laboratory Standards Institute (USA). Due to the long time span covered in this study, either the conventional biochemical techniques or automated methods with the VITEK system (Vitek 2 compact, BioMerieux, France) was used to identify the specific bacterial species. Initially, the manual Kirby-Bauer disc diffusion method or more recently the Gram-Negative Susceptibility card (BioMerieux, France) was utilized to determine antibiotic susceptibility of bacterial isolates. Two periods, 2002 to 2008 and 2012 to 2018, were covered in the study. All cases were identified from a registry and hospital records of diagnosis with a confirmation from a detailed chart review.
Demographics and relevant clinical data were collected from the medical records. We collected the information about the birth place (inborn or outborn), gestational age, mode of delivery, prolonged rupture of membranes (PROM>18 hrs), intrapartum fever (>38ºC), and the use of antepartum antibiotics. Data related to infants such as gender, birth weight, clinical symptoms such as respiratory distress, septic shock and meningitis were also collected. To calculate the incidence of EOS, data on the number of total live births in the hospital during these two periods were also collected. The Institutional Ethics Committee of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University approved the study protocol. A waver for patient parental consent to review their medical records was granted by the Institutional Ethics Committee. The handling of the patient data confidentiality strictly followed the rules set by the institution and were in compliance with the Declaration of Helsinki.
Statistical Analysis
SPSS 24.0 software was used to perform the statistical analysis. The basic clinical characteristics and the results of blood culture and antimicrobial susceptibilities were analyzed. Continuous variables were tested for normality using the Kolmogorov–Smirnov test. Normally distributed data are described as the mean ± standard deviation (M±SD) and were analyzed using Student’s t-test. Non-normally distributed data are described as median and range and were analyzed using the Wilcoxon signed rank test or the Mann–Whitney U-test. Categorical data were analyzed using the Chi-square test or Fisher’s exact test. The incidences of EOS were calculated by dividing the number of inborn infants with EOS by the number of live births in the hospitals. A p-value of < 0.05 for the predictive variables was considered significant.
Results
The total number of live births was 27,522 in 2002–2008 and 58,619 in 2012–2018, respectively, in the study hospital. A total of 163 cases of culture-confirmed EOS were identified. Of these, 65 were from years 2002–2008 and 98 were from years 2012–2018. Of the 163 cases, 46 infants (5 from 2002–2008 and 41 from 2012–2018) who were inborn developed EOS, and the other 117 cases were either transferred from other hospitals that did not have NICU services or directly admitted from the community after home birth. Therefore, the calculated incidence of culture-confirmed EOS in the inborn infants was 0.18 per 1000 live-births in 2002–2008 and 0.70 per 1000 live-births in 2012–2018.
Table 1 illustrates the distribution of bacterial pathogens for EOS in 2002–2008 and 2012–2018. E. coli continued to be the leading bacterial pathogen for EOS despite GBS emerging as equally important in 2012–2018. The proportion of E. coli as the pathogen in EOS remained relatively stable over the last two decades, while GBS became the most frequently isolated Gram-positive bacteria in 2012–2018.
 |
Table 1 Distribution of Pathogens of EOS in 2002–2008 and 2012–2018 |
Table 2 shows the general characteristics of patients with early-onset E. coli sepsis from 2002 to 2008 and 2012 to 2018. Compared with 2002–2008, children with early-onset E. coli sepsis from 2012 to 2018 were born at an earlier gestational age and with lower birth weight (P<0.001 and P<0.05, respectively), and their mothers were older as well (P<0.05). A significantly higher proportion of preterm infants or low birth infants were diagnosed with early-onset E. coli sepsis from 2012 to 2018 compared to 2002–2008. The number of early-onset E. coli sepsis infants whose mothers were treated with antepartum antibiotics was also significantly increased in 2012–2018 (P<0.01).
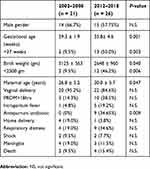 |
Table 2 General Characteristics of Patients with Early-Onset E. coli Sepsis |
The changes in antibiotic susceptibility for all E. coli isolated from infants with EOS in 2002–2008 and 2012–2018 are presented in Figure 1. As the figure shows, overall resistance of E. coli to third-generation cephalosporins increased from 14.3% in 2002–2008 to 46.7% in 2012–2018 (p<0.05). This resistance pattern closely paralleled to ESBL production which increased from 13.3% in 2002–2008 to 46.2% in 2012–2018 (p<0.05). The resistance of E. coli to ciprofloxacin increased from 9.5% in 2002–2008 to 38.5% in 2012–2018 (p<0.05). Although 73.1% of E. coli isolates in 2012–2018 were ampicillin-resistant, while 50.0% were ampicillin-resistant in 2002–2008, this difference was not statistically significant. The incidence of resistance of E. coli to gentamicin remained relatively unchanged (23.8% in 2002–2008 vs 26.9% in 2012–2018, p>0.05).
 |
Figure 1 Antimicrobial susceptibility of all isolated E. coli in 2002–2008 and 2012–2018. *P < 0.05. |
Figure 2 shows the results of antibiotic susceptibility testing on E. coli causing EOS grouped by term vs preterm infants. Compared to that from term infants, E. coli isolated from preterm infants had a significantly higher rate of resistance to both ampicillin (93.3% vs 48.4%, p<0.01) and gentamicin (60.0% vs 9.4%, p<0.01). Two-thirds of E. coli (66.7%) isolated from preterm infants was resistant to third-generation cephalosporins, which is significantly higher than those isolated from term infants (15.6%, p<0.001). This resistance difference is mainly due to ESBL-producing E. coli which represented 66.7% of all E. coli isolated from preterm infants as compared to 15.6% of E. coli in term infants (p<0.001). Overall, almost all isolated E. coli from EOS infants from our NICU were still susceptible to amoxicillin-clavulanic acid, piperacillin-tazobactam, amikacin, cefoxitin, and imipenem.
 |
Figure 2 Antimicrobial susceptibility of all isolated E. coli from term and preterm infants. |
Discussion
EOS is mainly caused by vertical transmission of organisms from mother to infant during labor and delivery.2–5 The most recent guidelines for the management of EOS are based on epidemiologic studies that are from industrialized countries.13,14 Data about EOS from developing countries are relatively rare, and the bacterial profile may be significantly different.6 The current study from a large tertiary perinatal center in eastern China demonstrates that the current incidence of culture-confirmed EOS is around 0.7 per 1000 live-births. E. coli remains the leading bacterial pathogen for EOS despite GBS emerging as equally important in recent years. Although the proportion of E. coli as the pathogen for EOS has remained relatively stable over the last 2 decades, increasing numbers of multi-drug resistant E. coli due to ESBL production are being isolated as the responsible pathogen in at least one NICU in eastern China. This poses a serious challenge concerning the selection of appropriate antibiotics for empirical therapy.
Despite a relative low incidence, EOS accounts for approximately 16% of all neonatal mortality.3 While GBS remains the most common etiologic agent for EOS in industrialized countries, Staphylococcus and gram-negative bacteria such as Klebsiella and E. coli are the most frequent causative organisms responsible for EOS in most low- and middle-income countries.6,15 In the last decade, the annual deliveries in our hospital have increased significantly. This has made our center one of the largest perinatal centers in eastern China where dramatic socioeconomic changes have occurred due to industrialization. The incidence of culture-confirmed EOS from our inborn infants now appears to be comparable to that reported from industrialized countries in the range of 0.54–0.9 per 1000 live births.2–4 Interestingly, along with the industrialization in the region, the pathogen profile for EOS in our center has also changed to that similar to those reported from developed countries. In addition to E. coli, GBS has now emerged to be equally important as the pathogen for EOS.
EOS due to E. coli usually has a higher mortality rate than that caused by gram-positive bacteria.16 The incidence of invasive early-onset GBS disease in developed countries has significantly decreased due to the implementation of IAP guidelines.17 However, the widespread use of IAP for GBS disease has led to concerns about a potential adverse impact on E. coli incidence. Indeed, in an epidemiologic study from the United States, although the overall incidences of EOS due to E. coli over the last decade remained relatively stable, E. coli cases were found to be more common than GBS in some region.7 Early-onset E. coli sepsis is more common in premature and very low birth weight infants and is more likely to be associated with intrapartum fever, preterm premature rupture of membranes, PROM, antibiotic use, and sepsis onset on the first day of life.7,18 In our study, we found that when compared to those of the year 2002–2008, infants with early-onset E. coli sepsis from years 2012–2018 were more likely to be premature, have lower birth weight, and have received IAP. Our results are consistent with the previous findings that prematurity has become one of the most important risk factors for early-onset E. coli infection.8
Roughly 50% of E. coli bloodstream isolates (regardless of early onset or late onset) from Chinese NICUs are multi-drug resistant due to ESBL production.12 In the current study, among all E. coli isolates from infants with EOS in 2012–2018, 73.1% are ampicillin-resistant and 46.7% are resistant to third-generation cephalosporins, which are significantly higher than the percentages of resistance from 2002 to 2008. Our data indicate that increasing numbers of ESBL-producing multi-drug resistant E. coli are being isolated as the pathogens responsible for EOS. The changing patterns of antibiotic susceptibility for all E. coli isolated from infants with EOS in China are very worrisome. Our results indicate that the prevalence of multi-drug resistant E. coli isolated from infants with EOS in our NICU is much higher than that from the United States, where a high rate of ampicillin resistance, no aminoglycoside resistance, and a very low rate of resistance to third-generation cephalosporins are reported.19 On the other hand, the antimicrobial resistance pattern in India appears to be even worse based on a recent report.15 Almost half of the E. coli isolates from newborn infants in Delhi are resistant to commonly used third generation of cephalosporins. Moreover, 15% of their E. coli isolates are even carbapenem resistant, which is not the case for our isolates. Data obtained from the China Antimicrobial Resistance Surveillance Report show that the rate of carbapenem resistance in clinical E. coli strains is around 0.6–3.6% in different provinces of China.20 All isolated E. coli from EOS infants in our NICU thus far are still carbapenem susceptible.
We have previously speculated that ESBL-producing multi-drug resistant gram-negative bacterial infections in Chinese NICUs are likely due to unrestricted use in neonates of broad-spectrum antibiotics, especially third generation of cephalosporins.12 This may be true for late-onset sepsis caused by multi-drug resistant bacteria. Clearly, causes other than NICU practice were responsible for the fact that ESBL-producing E. coli has emerged as the main pathogen for EOS in our NICU. There are several studies that have demonstrated the relationship between the antibiotic chosen for IAP and resistant E. coli infections in neonates.21,22 Colonization of resistant bacteria in pregnant women during hospitalization may be another reason. Indeed, significantly higher numbers of ESBL-producing E. coli were isolated from premature infants as demonstrated in the current study. A higher percentage of resistance to ampicillin, gentamicin, and third-generation cephalosporins was observed among E. coli strains causing EOS from preterm infants in comparison with those from term infants. In the current study, the median hospitalization days prior to delivery in women with preterm delivery were 1 (range 0–11 days) which was significantly longer than those with term delivery (median=0, range 0–0, p<0.001). Therefore, the mothers of preterm infants were at higher risk of being exposed and colonized with resistant E. coli prior to delivery.
Globally, ESBL-producing E. coli infection has been increasingly reported with evidence of spread in the community.23,24 An increasing proportion of ESBL-producing E. coli among patients without any healthcare risk factors was observed in South Korea.24 In a report from Japan, 26.3% patients with ESBL-producing E. coli infection were considered to be community-associated because there were no discernible healthcare-associated risk factors.25 Community-associated infections caused by ESBL-producing E. coli have already raised public-health concerns and are gradually becoming a clinical challenge.26 Infections with community-associated ESBL-producing E. coli in China are not rare.27 In a recent study from China, a surprisingly high number (50.5%, 55/109) of fecal samples from healthy adults showed the presence of ESBL-producing E. coli.28 Even some (2.8%) of E. coli isolates obtained from rivers and lakes in Northwest China were found to be ESBL producers.29 This is because a significant increase in antibiotic consumption has been seen in China and other developing countries both as prescriptions for patients and feed additives in the agriculture industry.30 Such unrestricted use of antibiotics has exerted strong selective pressure in the environment for resistant bacteria, especially for zoonotic pathogens such as E. coli. Mounting scientific evidence has shown that the routine feeding of antibiotics to healthy farm animals, which occurs without a prescription, promotes the development of antibiotic-resistant bacteria that can be transferred to human beings.31 Vaginally delivered neonates are colonized first with maternal fecal and vaginal flora. Therefore, high prevalence of ESBL-producing E. coli colonization in pregnant mothers in China may be another explanation for the fact that increasing numbers of ESBL-producing multi-drug resistant E. coli are being identified as the pathogen for early-onset sepsis in our NICU.
In summary, we have focused on the changing pattern of antimicrobial resistance of E. coli responsible for early-onset neonatal sepsis in a perinatal center in eastern China and found that ESBL-producing multi-drug resistant E. coli has emerged as a main pathogen responsible for early-onset neonatal sepsis in our region. Although the trend of increasing antibiotic resistance of E. coli is threatening the entire global population, it is more so to neonates since neonatal sepsis remains a major cause of neonatal mortality, especially in developing countries. Continuous surveillance for antibiotic susceptibility is needed to ensure proper empirical therapy. It is critical for clinicians to consider this trend and attempt to select proper effective antibiotics as the empirical treatment for early-onset neonatal sepsis.
Acknowledgement
We would like to thank Drs. Robert Green and Alan Groves for advice and critical review of the manuscript.
Disclosure
The authors report having no conflicts of interest relevant to this article to disclose.
References
1. Shane AL, Sanchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390:1770–1780. doi:10.1016/S0140-6736(17)31002-4
2. Fjalstad JW, Stensvold HJ, Bergseng H, et al. Early-onset sepsis and antibiotic exposure in term infants: a nationwide population-based study in Norway. Pediatr Infect Dis J. 2016;35:1–6. doi:10.1097/INF.0000000000000906
3. Stoll BJ, Hansen NI, Sanchez PJ, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127:817–826. doi:10.1542/peds.2010-2217
4. Vergnano S, Menson E, Kennea N, et al. Neonatal infections in England: the NeonIN surveillance network. Arch Dis Child Fetal Neonatal Ed. 2011;96:F9–F14. doi:10.1136/adc.2009.178798
5. Hammoud MS, Al-Taiar A, Al-Abdi SY, et al. Culture-proven early-onset neonatal sepsis in Arab states in the Gulf region: two-year prospective study. Int J Infect Dis. 2017;55:11–15. doi:10.1016/j.ijid.2016.12.006
6. Ganatra HA, Stoll BJ, Zaidi AK. International perspective on early-onset neonatal sepsis. Clin Perinatol. 2010;37:501–523. doi:10.1016/j.clp.2010.02.004
7. Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138:e20162013. doi:10.1542/peds.2016-2013
8. Al-Taiar A, Hammoud MS, Cuiqing L, et al. Neonatal infections in China, Malaysia, Hong Kong and Thailand. Arch Dis Child Fetal Neonatal Ed. 2013;98:F249–F255. doi:10.1136/archdischild-2012-301767
9. Dong Y, Jiang SY, Zhou Q, Cao Y. Group B Streptococcus causes severe sepsis in term neonates: 8 years experience of a major Chinese neonatal unit. World J Pediatr. 2017;13:314–320. doi:10.1007/s12519-017-0034-5
10. Lu B, Chen X, Wang J, et al. Molecular characteristics and antimicrobial resistance in invasive and noninvasive Group B Streptococcus between 2008 and 2015 in China. Diagn Microbiol Infect Dis. 2016;86:351–357. doi:10.1016/j.diagmicrobio.2016.08.023
11. Wang P, Ma Z, Tong J, et al. Serotype distribution, antimicrobial resistance, and molecular characterization of invasive group B Streptococcus isolates recovered from Chinese neonates. Int J Infect Dis. 2015;37:115–118. doi:10.1016/j.ijid.2015.06.019
12. Li JY, Chen SQ, Yan YY, et al. Identification and antimicrobial resistance of pathogens in neonatal septicemia in China − A meta-analysis. Int J Infect Dis. 2018;71:89–93. doi:10.1016/j.ijid.2018.04.794
13. Puopolo KM, Benitz WE, Zaoutis TE, COMMITTEE ON FETUS AND NEWBORN, COMMITTEE ON INFECTIOUS DISEASES. Management of neonates born at ≥35 0/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics. 2018;142:e20182894. doi:10.1542/peds.2018-2894
14. Puopolo KM, Benitz WE, Zaoutis TE, COMMITTEE ON FETUS AND NEWBORN, COMMITTEE ON INFECTIOUS DISEASES. Management of neonates born at ≤34 6/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics. 2018;142:e20182896. doi:10.1542/peds.2018-2896
15. Investigators of the Delhi Neonatal Infection Study (DeNIS) collaboration. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: a cohort study. Lancet Glob Health. 2016;4:e752–e760. doi:10.1016/S2214-109X(16)30148-6
16. Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27:21–47. doi:10.1128/CMR.00031-13
17. Nanduri SA, Petit S, Smelser C, et al. Epidemiology of invasive early-onset and late-onset group B streptococcal disease in the United States, 2006 to 2015: multistate laboratory and population-based surveillance. JAMA Pediatr. 2019;173:224–233. doi:10.1001/jamapediatrics.2018.4826
18. Tsai CH, Chen YY, Wang KG, Chen CY, Chen CP. Characteristics of early-onset neonatal sepsis caused by Escherichia coli. Taiwan J Obstet Gynecol. 2012;51:26–30. doi:10.1016/j.tjog.2012.01.006
19. Weissman SJ, Hansen NI, Zaterka-Baxter K, Higgins RD, Stoll BJ. Emergence of antibiotic resistance-associated clones among Escherichia coli recovered from newborns with early-onset sepsis and meningitis in the United States, 2008-2009. J Pediatric Infect Dis Soc. 2016;5:269–276. doi:10.1093/jpids/piv013
20. Zhang R, Liu L, Zhou H, et al. Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. Ebio Med. 2017;19:98–106. doi:10.1016/j.ebiom.2017.04.032
21. Edwards RK, Clark P, Sistrom CL, Duff P. Intrapartum antibiotic prophylaxis 1: relative effects of recommended antibiotics on gram-negative pathogens. Obstet Gynecol. 2002;100:534–539. doi:10.1016/s0029-7844(02)02096-3
22. Das S, Adler AL, Miles-Jay A, et al. Antibiotic prophylaxis is associated with subsequent resistant infections in children with an initial extended-spectrum-cephalosporin-resistant Enterobacteriaceae infection. Antimicrob Agents Chemother. 2017;61:e02656–16. doi:10.1128/AAC.02656-16
23. Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159–166. doi:10.1016/S1473-3099(08)70041-0
24. Park SH, Choi SM, Lee DG, et al. Emergence of extended-spectrum β-lactamase-producing Escherichia coli as a cause of community-onset bacteremia in South Korea: risk factors and clinical outcomes. Microb Drug Resist. 2011;17:537–544. doi:10.1089/mdr.2011.0072
25. Hayakawa K, Nagamatsu M, Mezaki K, et al. Epidemiology of extended-spectrum beta-lactamase (ESBL) producing Escherichia coli in Japan: characteristics of community-associated versus healthcare-associated ESBL E. coli. J Infect Chemother. 2017;23:117–119. doi:10.1016/j.jiac.2016.08.010
26. Rodríguez-Baño J, Picón E, Gijón P, et al. Community-onset bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: risk factors and prognosis. Clin Infect Dis. 2010;50:40–48. doi:10.1086/649537
27. Quan J, Zhao D, Liu L, et al. High prevalence of ESBL-producing Escherichia coli and Klebsiella pneumoniae in community-onset bloodstream infections in China. J Antimicrob Chemother. 2017;72:273–280. doi:10.1093/jac/dkw372
28. Li B, Sun JY, Liu QZ, Han LZ, Xin-Hong Huang XH, Ni YX. High prevalence of CTX-M β-lactamases in faecal Escherichia coli strains from healthy humans in Fuzhou, China. Scand J Infect Dis. 2011;43:170–174. doi:10.3109/00365548.2010.538856
29. Liu H, Zhou H, Li Q, et al. Molecular characteristics of extended-spectrum β-lactamase-producing Escherichia coli isolated from the rivers and lakes in Northwest China. BMC Microbiol. 2018;18:125. doi:10.1186/s12866-018-1270-0
30. Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115:E3463–E3470. doi:10.1073/pnas.1717295115
31. Goldman E. Antibiotic abuse in animal agriculture: exacerbating drug resistance in human pathogens. Hum Ecol Risk Assess. 2004;10:121–134. doi:10.1080/10807030490281016
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
