Back to Journals » Hepatic Medicine: Evidence and Research » Volume 15
Mucinous Cystic Neoplasms of the Liver: Epidemiology, Diagnosis, and Management
Authors Hutchens JA , Lopez KJ, Ceppa EP
Received 24 December 2022
Accepted for publication 1 March 2023
Published 29 March 2023 Volume 2023:15 Pages 33—41
DOI https://doi.org/10.2147/HMER.S284842
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Jeffrey A Hutchens, Kevin J Lopez, Eugene P Ceppa
Department of Surgery, Division of Surgical Oncology, Indiana University School of Medicine, Indianapolis, IN, USA
Correspondence: Eugene P Ceppa, Associate Professor of Surgery, Section Chief of HPB Surgery, Division of Surgical Oncology, Indiana University School of Medicine, 545 Barnhill Dr, EH 541, Indianapolis, IN, 46202, USA, Tel +1-317-944-5013, Fax +1-317-968-1031, Email [email protected]
Abstract: Mucinous cystic neoplasms (MCNs) are rare tumors of the liver, occasionally seen in the biliary tree. Epidemiologic data are limited by their indolence and recent changes to diagnostic criteria. They are considered premalignant lesions capable of invasive behavior. While their etiology remains unknown, their female predominance, age of onset, and hormonally responsive ovarian-type stroma suggest ectopic organogenesis during embryologic development. MCNs can typically be recognized on imaging; yet, invasiveness is often indeterminate, and percutaneous tissue biopsy has shown limited value. Therefore, complete excision is recommended for all lesions as focal malignant transformation and metastatic disease has been reported.
Keywords: mucinous cystic neoplasm, hepatobiliary cystadenoma, hepatobiliary cystadenocarcinoma
Introduction
Nearly one in five adults have a hepatic cyst detectable by modern imaging modalities, with the vast majority appearing after the fourth decade of life.1,2 Most are benign unilocular and thin-walled simple cysts.3 Few are multilocular with septations and thickened walls.4,5 For decades most multilocular cysts were referred to as cystadenomas, and those with collagen and spindle-shaped cells between the inner epithelium and outer capsule were referred to as cystadenomas with mesenchymal stroma (CMS).6,7
As immunohistochemistry techniques evolved to identify the presence of estrogen and progesterone receptor (ER and PR) positivity within the supportive tissue of CMS neoplasms, the consensus descriptor changed to cystadenomas with ovarian-like stroma.8,9 In 2010 the WHO renamed these tumors to align with their pancreatic counterparts, making the presence of ovarian-type stroma a prerequisite for diagnosis. Mucinous cystic neoplasms, distinguishable by their subepithelial ovarian-type stroma, are now a unique entity.10,11
Epidemiology
Mucinous cystic neoplasms (MCNs) of the hepatobiliary tract account for less than 5% of all hepatic cysts.5,12 More precise prevalence estimates are restricted by their relative indolence and recent addition to the WHO disease lexicon. Much of the available data stems from when MCNs were called cystadenomas. For decades, this designation was shared with what is now differentiated as intraductal neoplasms and non-neoplastic hamartomas, congenital and choledochal cysts, or polycystic liver disease.8,13
Before 2005 there were fewer than 200 documented cases of cystadenomas.4 That number had only grown to 250 ten years later.14 The small size of many of these publications limited population-based conclusions about incidence and risk factors. Consequently, a Mayo Clinic study published in 1974 which found cystadenomas responsible for 5 of 227 (2.2%) grossly detectable hepatic cysts has remained the most robust and frequently cited epidemiologic series.15 Unfortunately, the Mayo Clinic report, like many others at the time, did not report the frequency of mesenchymal (“ovarian-like”) stroma.15–17 Little attention was paid to this unique supportive layer until it was documented in malignant cystic neoplasms of the hepatobiliary tract.6,18 Subsequently, several small publications confirmed cystadenocarcinomas with mesenchymal stroma, like their benign counterparts, occurred almost exclusively in females (96–100%) of middle age, though those with malignant change were noticeably older (57–64 vs 41–44 years).6–8 A theory for malignant transformation arose when focal epithelial dysplastic changes were correlated with nodular cystic wall enhancement on CT imaging over time.8,19
As evidence for the malignant potential of these previously assumed benign tumors accumulated, documentation of the ovarian-like histologic subtype increased. In 2005 Cleveland Clinic released the largest single-institution cystadenoma case series at the time.4 They reported ovarian-type stroma in 10 of 18 (56%) patients with benign disease and 2 of 4 with malignant change.4 All patients with ovarian-like stroma were female, with a mean age of 48 in the benign group and 60 in those with malignant disease.4 Shortly thereafter, a series of immunohistochemistry studies on this cystadenoma subtype were released.9,20 These confirmed previous reports of biliary epithelium, defusing speculation that these neoplasms originated from ectopic reproductive tissue.9,20 It also highlighted the hormonally responsive ovarian-type stroma as likely driving the tumor’s growth.8,9,20 To account for this unique biology and provide reference to the often present mucin-containing cystic fluid, in 2010, the WHO officially reclassified all hepatobiliary cysts with ovarian-type stroma as Mucinous Cystic Neoplasms (MCN) with either low-, intermediate-, or high-grade dysplasia, or an associated invasive carcinoma.11
Since the revised WHO diagnostic criteria were released, numerous studies have reported near-exclusive female presentation of MCN. Two studies of nearly 500 subjects found MCN in 10.5–11% of primary resected hepatobiliary cysts, while a multinational study of over 5000 subjects found rates of 0.6–2.2% depending on the institution.21–24 The reason for the discrepancy is unclear but may be related to the radiographic criteria or the use of serum biomarkers to determine the need for resection.
Apart from the beforementioned multinational study, few have commented on the racial demographics of MCN populations. Early reports suggested an overwhelming majority (80–90%) of cystadenomas were seen in the white/Caucasian population.8,25 However, it is unclear whether there is an actual racial predilection or whether racial disparities are secondary to recruitment and observation biases.
However, there is evidence for the disparity in the relative rate of MCN to intraductal papillary neoplasms of the bile duct (IPNB) across populations. IPNB were formerly grouped with MCNs as cystadenomas but are now known to have a nearly 10-fold higher malignancy risk (Table 1). Zen et al found the relative prevalence of IPNB to MCN was 3.5x higher in South Korea than in the UK or the US.24 They suggested this could be secondary to rates of hepatolithiasis or Clonorchis infections, known risk factors for IPNB, or diagnosis bias in favor of MCN in the West akin to what had been seen with pancreatic MCN decades prior.26 Currently, there are no known modifiable risk factors for MCN. Risk factors that have been explored include tobacco, alcohol, cirrhosis, HBV, HCV, and hepatobiliary stone. These have all failed to yield associations of greater than 15%, with the majority less than 5%.22,24
 |
Table 1 Comparison of Mucinous Cystic Neoplasm of the Liver with Intraductal Papillary Neoplasms of the Bile Duct |
Etiology
The precise etiology of MCNs remains unknown.5 It was originally postulated that the epithelium arose from ectopic rests of embryonic bile ducts while the stroma was a layer of primitive mesenchyme.6 When immunohistochemistry revealed the presence of α-inhibin, estrogen, and progesterone receptors within the stromal layer, a new theory began to circulate that perhaps it was secondary to ectopic tissue incorporated as part of a disease process like endometriosis.8 The theory hinged on the hormonally responsive stroma becoming activated in the setting of hormonal imbalance and promoting the proliferation of the nearby epithelium.9 It was supported by the near female exclusivity and perimenopausal age of onset discussed previously.5,22 However, the absence of uniform CD10 expression, as seen in endometrial tissue, rendered the endometrial implant explanation less plausible.20 More recently, transcriptome sequencing identified sex cord-stromal markers consistent with not just hormone responsiveness but also hormone production. This provides credence to an existing pancreatic MCN theory suggesting an etiology secondary to ectopic primordial germ cells of the gonads implanted during embryogenesis.27
Localization
Nearly all MCNs are solitary and located within the liver parenchyma.4,5,8 Three in four localize to the smaller left lobe for unclear reasons, with segment 4B being most affected.20,23 The remainder distributes in an approximate 2:1 ratio to the right lobe or bilaterally with only the rare case in the extrahepatic biliary system (including the gallbladder).12,20–24
Malignancy Risk
Invasive MCNs, previously known as cystadenocarcinomas, are very rare. There were fewer than 100 documented cases of cystadenocarcinomas through 2005. Many were unlikely to have contained the requisite ovarian-type stroma which has since been associated with indolent behavior and female predominance.4 Rates have been reported as less than 5% to as high as 9% using the new WHO diagnostic criteria.21,23,24 The rates are well below the carcinoma rate for their pancreatic counterparts, which have been reported in the range of 11–36%.21,28
Tumorigenic progression occurs in a stepwise fashion over a period of years and possibly decades.6–8 The early nonmucinous biliary epithelium is focally replaced by mucinous epithelium, followed by malignant transformation to a tubular or tubulopapillary adenocarcinoma.6 More than 80% of MCNs have a mixture of mucinous and nonmucinous epithelium.28 However, those with high-grade dysplasia or an associated invasive carcinoma have little to no retained nonmucinous epithelium.28 Due to the prognostic significance of the epithelium, some have recently called into question the new classification system that focuses on stromal type suggesting a better division would have been mucinous and nonmucinous cystadenomas.29
Diagnosis
Mucinous cystic neoplasm (MCN) of the hepatobiliary system, as defined by the WHO, is a
Cyst-forming epithelial neoplasm, typically showing no communication with the bile ducts, composed of cuboidal to columnar, variably mucin-producing epithelium, associated with ovarian-type subepithelial stroma.5,11
Ovarian-type stroma is an entity-defining prerequisite for diagnosis. It is recognized histologically by densely packed spindle cells with elongated nuclei and scant cytoplasm with variable amounts of collagen between the inner epithelium and outer capsule.21,23
The differential diagnosis of hepatobiliary cystic lesions is broad, making accurate diagnosis challenging. In general, hepatic cystic lesions can be classified into three categories: those due to secondary cystic change, congenital cystic dilations, and infectious etiologies.8 Specific examples within these categories include simple cysts, parasitic cysts, mucinous cystic neoplasms, congenital cystic dilation, degenerative metastatic tumors, mucin-producing metastatic tumors, cystic hemangioma, lymphangioma, hepatic foregut cysts, and mesenchymal hamartoma, and teratoma.22 The etiologies which most closely resemble MCN on imaging and which must be distinguished histologically based on the epithelium, stroma, presence of a papillary lesion, or communication with duct lumen include endometrial cyst, intraductal papillary neoplasm, bile duct cyst, peribiliary cyst, hepatic microcystic serous cystadenoma, and hepatic foregut cyst.5 Microscopic evaluation of the cyst wall is currently the only definitive means to diagnose MCN.
Clinical Features
Most patients with a mucinous cystic neoplasm will present with vague, non-descript symptoms.5 In a cohort of 248 patients with cystadenomas, approximately 10% of which were invasive cystadenocarcinomas, the most common presenting symptoms were abdominal pain (57.3%), abdominal fullness (20%), early satiety (12.5%), jaundice (11.3%), or weight loss (3.6%).25 Fewer than 15% were completely asymptomatic.25 Similar results were reported in smaller MCN-only cohorts. The duration of symptoms in these small cohorts ranged from 10 days to 2 years.21,22
Laboratory Findings
Standard laboratory tests have a limited diagnostic role in the evaluation of suspected MCN. The median bilirubin and alanine aminotransferase (ALT) in a sample of 248 patients with cystadenomas (average size 10cm) was 0.6mg/dL and 27U/L, respectively.25 There was no difference between benign and malignant lesions.25 Conversely, in a cohort of 21 MCN patients, two of which were malignant (average size of 8.5cm), all but one had normal total bilirubin, while 84% had elevated ALT.22
Biomarkers
Preliminary analysis on cystic fluid indicated carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19-9) were both elevated in cystadenomas though normal in simple cysts, but unfortunately, only 1 of the 22 samples in this study contained mesenchymal (ovarian-type) stroma.30 A subsequent study of 17 cystadenomas found that CEA and CA 19–9 levels were ineffective in differentiating between biliary cystadenomas and hepatic simple cysts.31 This study did not include the percentage containing ovarian stroma.31 Most recently, a cohort of MCN-only patients found that CEA and CA19-9 levels in both serum and cystic fluid were elevated in 19 benign and two malignant patients.22
Imaging
CT and US typically reveal a non-specific multilocular fluid-filled mass, often with smaller cysts within the cyst wall (Figure 1). Septations are common and can help distinguish complex from simple cysts or hamartomas. However, septations may be seen in other hepatobiliary cystic lesions such as echinococcal cysts, mesenchymal hamartomas, abscesses, and undifferentiated (embryonal) sarcomas.19 Magnetic resonance imaging (MRI) with contrast enhancement remains the best modality for characterization and surgical planning. MCNs typically appear with loculations (84.2%) and septations (63.2%).22 Most benign MCNs are thin walled. Thickening or contrast enhancement of the rim suggests possible malignant transformation.20 Malignant MCNs typically display irregular wall thickness and papillary projections.5 They frequently have signs of multiloculation (56.9%), mural nodularity (16.5%), or biliary ductal dilation (17.7%). Unfortunately, these factors were only shown to predict malignant potential with a sensitivity of 81% and specificity of 21%.25
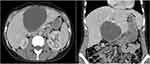 |
Figure 1 Computed Tomography of a Mucinous Cystic Neoplasm of the Liver. Axial (Left) and coronal (Right) CT abdomen images demonstrating a 10×7 cm cystic liver mass. |
In 2017 a small retrospective study revealed that assessing the relationship of septations to the internal cyst wall could improve the differentiation of the traditionally categorized biliary cystadenomas from benign hepatic cysts.32 When blinded, radiologists identified septations arising from a cyst wall without external indentation, as opposed to septations arising from external macrolobulations, as predictors of biliary cystadenomas.32 Boyum et al confirmed the diagnostic value of this finding in a larger series using the updated 2010 WHO criteria for MCNs, although their specificity was lower at 56.3%.33
A recent multicenter follow-up study used machine learning to develop a classification system to differentiate MCNs from benign hepatic cysts.34 The three imaging features that accurately differentiated MCNs from benign hepatic cysts were 1.) the presence of a solid enhancing nodule (100% specific), 2) lack of septations arising from external macrolobulations, and 3.) the presence of a solitary lesion. In accordance with the previously mentioned study, septations from external macrolobulations were consistent with benign hepatic cysts 97% of the time. Lack of septations arising from external macrolobulations and the presence of solitary lesions represented MCNs 88% of the time. Lack of both septations from external macrolobulations and not a solitary lesion was associated with benign hepatic cysts 89% of the time. The total accuracy of the proposed 4 type classification system was 93.5%, making it useful in risk-stratifying lesions for the likelihood of MCN diagnosis.34
An important distinction that can often be made in imaging is between MCNs and IPNBs. While the vast majority of MCNs are multilocular with septations or cyst-in-cyst appearance and rarely contain mural nodules except when malignant, IPNBs are typically multicystic with a grape-like appearance, papillary nodules, and peripheral bile duct dilation26 (Table 1).
Gross Appearance
MCNs appear as multilocular lesions with well-defined edges upon gross resection (Figure 2). The demarcation between the cyst wall and liver parenchyma is maintained in benign lesions by a circumferential fibrous outer capsule.8 Loss of capsular integrity is associated with malignant invasion. With rare exceptions, MCNs do not infiltrate and are easily separated from the biliary tree.5 As their name suggests, most mucinous cystic neoplasms contain jelly-like mucinous fluid beneath the epithelial layer accounting for most of their size. Less often, this cystic fluid may also be clear or hemorrhagic. Purulence suggests a secondary infection. The inner surface is typically smooth or trabeculated. Papillary projections are rarely seen and are more often associated with malignant transformation or an alternative etiology.21,29 MCNs may grow as large as 28–30cm.8 Most patients become symptomatic with abdominal pain, distension, or fullness by the time they reach 10cm, interquartile range of 7–13cm.25 MCNs associated with invasive carcinomas are larger on average than their benign counterparts at the time of diagnosis, 17.5cm vs 11.2cm.21 Malignant lesions may have a solid area of greyish-white tumor secondary to epithelial anaplasia.5
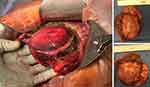 |
Figure 2 Gross appearance of a Mucinous Cystic Neoplasm of the Liver. (Left) Operative resection of a mucinous cystic neoplasm of the liver. (Right) Tumor dimensions post-resection. |
Percutaneous Tissue Sampling
Percutaneous core needle biopsy and fine needle aspiration of suspected mucinous cystic neoplasms are of uncertain utility. The diagnostic ovarian-type stroma is often not seen, making MCNs difficult to distinguish from IPNBs with cystic appearance.5,35 Biopsy results also often require upgrading upon final pathology due to inadequate sampling of the thin walls for focal dysplasia and falsely low rates of atypia. This has led to recommendations against using percutaneous tissue sampling unless the cystic neoplasm has evidence of wall thickening or mural nodules on imaging.22
When adequate, a typical needle aspiration will reveal aggregates of cuboidal to columnar epithelium. The occasional papillary arrangement may also be seen. The background is typically an abundant, thick gelatinous PAS (+) mucin filled with macrophages; it may be watery or hemorrhagic.5,35
Despite their name, nonmucinous biliary-type epithelium, identical to that seen in ductal malformation cysts, is common in MCNs. It was seen focally in all 27 cases of a recent cohort and was the predominant pattern in nearly half. This has led some to question the utility of aspiration cytology and the revised nomenclature.23
Histology
The wall of mucinous cystic neoplasms is comprised of three layers. The inner epithelial layer is typically columnar or cuboidal but rarely may be squamous-like (Figure 3). Cells are pale with eosinophilic cytoplasm and basally oriented nuclei. The middle layer is the entity-defining subepithelial ovarian-type stroma (Figure 3). This layer is diffuse in only about half of cases, necessitating careful analysis for accurate diagnosis.21 It consists of densely packed spindle-shaped cells with elongated nuclei and scant cytoplasm surrounded by collagen.8 Some of these cells may be focally luteinized, and neuroendocrine cells are present in about 50%. The third outer layer is a fibrous capsule.5
In cases of malignant transformation, the histologic appearance of all three layers changes. The epithelium develops large pseudostratified hyperchromatic nuclei. Cells lose polarity, and there are numerous mitotic figures in high-grade dysplasia. There are often polypoid or papillary projections.26 This has been referred to as gastric or intestinal differentiation with the appearance of abundant PAS+ mucin-producing goblet cells. Invasive carcinomas are adenocarcinomas with tubulopapillary or tubular growth. As the dysplasia progresses, the small or cystically dilated glands appear to undergo a desmoplastic reaction with eosinophilic necrosis analogous to that seen in adenocarcinomas of the colon.29 The middle stromal layer degenerates with signs of hemorrhage, calcification, and necrosis.5,29 While the external fibrous capsule shows focal erosion representative of invasive behavior.29
Immunohistochemistry
Immunohistochemistry continues to play a significant role in diagnosing and understanding hepatobiliary MCNs. The stroma was first described as mesenchymal based on the presence of desmin and muscle-specific actin. The term ovarian-like supplanted this with the detection of estrogen and progesterone receptors and the sex cord-stromal marker α-inhibin. Meanwhile, speculation that these tumors may be ectopic endometrial implants was defused by the detection of biliary epithelial cytokeratins and variable expression of CD10.8,9,20
Zen et al showed epithelial antigen markers might have utility in characterizing the invasiveness of these tumors and differentiating them from similar appearing lesions like IPNB. Their sample of 26 benign MCNs all shared the immunophenotype CK7+/CK20-/MUC2-/MUC5AC-/MUC6- while all IPNBs and borderline to invasive MCNs variably expressed gastrointestinal markers CK20, MUC2, and MUC6 (Table 1). MUC5AC was the only antigen detected in all cases of IPNB and borderline to invasive MCNs.26
Albores-Saavedra et al expanded on these findings by showing dysplastic epithelial tissue of MCN expressed CDX2, goblet-cell specific MUC2, MUC5AC, and CK20, while benign lesions did not.29 Increased expression of three MUC core proteins, MUC1, MUC2, and MUC5AC was later correlated with KRAS mutations.36 Mutations in KRAS were recently linked with late malignant change.27
Molecular Pathology
The first genetic study on MCNs of the liver was published in 2017 from a sample of eight neoplasms. Four oncogenic mutations (KRAS, GNAS, RNF43, and PIK3CA) commonly seen in hepatobiliary and pancreatic malignancies were evaluated. Seven of the eight tumors genotyped were low- or intermediate-grade and had no mutations. The only identified mutation was KRAS in the sole high-grade tumor.36
In 2020, Van Treeck et al published an expanded molecular analysis. They did not identify any somatic mutations for KRAS, GNAS, or RNF43 within the 22 samples, none of which displayed high-grade dysplasia. However, RNA sequencing revealed increased expression of hormone receptors (ER and PR) and steroidogenic enzymes (StAR, CYP17, CYP 19, and 17β-hydroxysteroid dehydrogenase 1). Transcriptome sequencing also revealed activation of the hedgehog pathway and downregulation of IFN-γ, TH-1, and TH-2. Together these results suggest KRAS is not an early driver in MCN development but may contribute to late malignant transformation, while aberrations in the hedgehog pathway and hormone responsiveness and production likely contribute to tumor growth in an immunosuppressed microenvironment.27
Management
Expert consensus on the management of hepatobiliary MCNs to prevent recurrence and potential malignant transformation is for complete surgical removal by liver resection preferably or enucleation.12,19 The recurrence rate in a sample of 19 MCNs with a median follow-up of 51.5mths was 0/11 for radical resection and 5/8 (62.5%) for a liver-preserving approach (eg, percutaneous aspiration, surgical fenestration, or marsupialization). Disease-free survival in the liver-preserving approach was 3.7 months.22 In 248 cystadenomas, 86% of which underwent extensive resection, median recurrence-free survival was 12.1 years (95% CI 6.5–18.1). The incidence of recurrence was 48% after unroofing/fenestration vs 15.7% and 10% for partial and major hepatectomy, respectively (p<0.001). Overall survival at 1-year, 3-years, and 5-years was 95%, 87%, and 84%. The median overall survival for those with cystadenocarcinoma was 8.4 years, and the subgroup with ovarian-type stroma was 8.3 years.25
The timing of when to resect MCNs remains challenging. Given their low rate of malignant transformation and generally indolent behavior, it is reasonable to delay intervention to medically optimize a surgical candidate. Unfortunately, as discussed previously, we remain limited in our ability to preoperatively identify the potential invasiveness of these tumors, so all good surgical candidates should be intervened upon promptly.
Staging
TNM staging for mucinous cystic neoplasms of the hepatobiliary tract associated with invasive carcinoma (ie, adenocarcinoma) is in accordance with intrahepatic cholangiocarcinoma and carcinoma of extrahepatic bile ducts.5
Conclusion
Mucinous cystic neoplasms (MCNs) of the hepatobiliary tract are rare, mostly indolent tumors occurring almost exclusively in perimenopausal females. Their true prevalence is lower than the estimates provided when grouped as cystadenomas. There are no additional known risk factors aside from sex and age, and patients are often asymptomatic until the tumor reaches a size of at least 10 cm, at which time generalized abdominal discomfort and fullness may develop.
Preoperative diagnosis of hepatobiliary MCNs remains challenging. A recent multi-center study has developed a novel classification system using the presence of a solid enhancing nodule, lack of septations arising from external macrolobulations, and the presence of a solitary lesion to differentiate MCNs from benign hepatic cyst. With a total accuracy of 93.5%, it has proven useful in risk-stratifying lesions for the likelihood of MCN diagnosis.
Serum and cystic fluid analysis of CEA and CA19-9 have been proposed as a diagnostic tool and incorporated into decision trees.30 Unfortunately, several studies have drawn questions about the utility of these biomarkers.12,22,31 Similarly, percutaneous tissue biopsy has proven unreliable due to the focal nature of the epithelial changes leaving some only to recommend percutaneous tissue biopsy if there is wall thickening or mural nodules.22 For this reason, the use of biomarkers and biopsy is usually reserved in diagnostic uncertainty, where surgical intervention is likely to be particularly challenging.
Histological evaluation remains the only definitive means of diagnosis. Immunohistochemistry should be used when the stromal layer is difficult to visualize, there is limited mucin production, or the diagnosis is otherwise unclear. In the future, there may be identifiable mutations for circulating tumor DNA PCR amplification, but currently, the likes of KRAS and others have failed to provide an early marker of dedifferentiation.27
The cornerstone of management for hepatobiliary MCNs remains surgical resection. Alternative liver-sparing approaches have demonstrated unacceptably high rates of reoccurrence.12,22 Median survival in those with malignant disease is eight years.25 Looking forward, there is a reason for optimism as our understanding of the etiology of hepatobiliary MCNs improves.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gaines PA, Sampson MA. The prevalence and characterization of simple hepatic cysts by ultrasound examination. Br J Radiol. 1989;62(736):335–337. doi:10.1259/0007-1285-62-736-335
2. Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol. 2003;58(8):626–629. doi:10.1016/S0009-9260(03)00165-X
3. Chenin M, Paisant A, Lebigot J, et al. Cystic liver lesions: a pictorial review. Insights Imaging. 2022;13(1):116. doi:10.1186/s13244-022-01242-3
4. Vogt DP, Henderson JM, Chmielewski E. Cystadenoma and cystadenocarcinoma of the liver: a single center experience. J Am Coll Surg. 2005;200(5):727–733. doi:10.1016/j.jamcollsurg.2005.01.005
5. Basturk ONY, Aishima S, Esposito I, Klimstra DS, Komuta M, Zen Y. Mucinous Cystic Neoplasm of the Liver and Biliary System. IARC; 2019.
6. Wheeler DA, Edmondson HA. Cystadenoma with mesenchymal stroma (CMS) in the liver and bile ducts. A clinicopathologic study of 17 cases, 4 with malignant change. Cancer. 1985;56(6):1434–1445. doi:10.1002/1097-0142(19850915)56:6<1434::AID-CNCR2820560635>3.0.CO;2-F
7. Akwari OE, Tucker A, Seigler HF, Itani KM. Hepatobiliary cystadenoma with mesenchymal stroma. Ann Surg. 1990;211(1):18–27. doi:10.1097/00000658-199001000-00004
8. Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma. A light microscopic and immunohistochemical study of 70 patients. Am J Surg Pathol. 1994;18(11):1078–1091. doi:10.1097/00000478-199411000-00002
9. Abdul-Al HM, Makhlouf HR, Goodman ZD. Expression of estrogen and progesterone receptors and inhibin-alpha in hepatobiliary cystadenoma: an immunohistochemical study. Virchows Arch. 2007;450(6):691–697. doi:10.1007/s00428-007-0411-x
10. Zamboni GKG, Hruban RH, et al. Mucinous Cystic Neoplasms of the Pancreas. World Health Organization Classification of Tumors. Lyon: IARC Press; 2000.
11. Bosman FTCF, Hruban RH, Theise ND. Mucinous Cystic Neoplasms of the Liver. Lyon: IARC Press; 2010.
12. Gao J, Zheng J, Cai J, et al. Differentiation and management of hepatobiliary mucinous cystic neoplasms: a single centre experience for 8 years. BMC Surg. 2021;21(1):146. doi:10.1186/s12893-021-01110-9
13. Buetow PC, Buck JL, Pantongrag-Brown L, et al. Biliary cystadenoma and cystadenocarcinoma: clinical-imaging-pathologic correlations with emphasis on the importance of ovarian stroma. Radiology. 1995;196(3):805–810. doi:10.1148/radiology.196.3.7644647
14. Soares KC, Arnaoutakis DJ, Kamel I, et al. Cystic neoplasms of the liver: biliary cystadenoma and cystadenocarcinoma. J Am Coll Surg. 2014;218(1):119–128. doi:10.1016/j.jamcollsurg.2013.08.014
15. Sanfelippo PM, Beahrs OH, Weiland LH. Cystic disease of the liver. Ann Surg. 1974;179(6):922–925. doi:10.1097/00000658-197406000-00018
16. Ishak KG, Willis GW, Cummins SD, Bullock AA. Biliary cystadenoma and cystadenocarcinoma: report of 14 cases and review of the literature. Cancer. 1977;39(1):322–338. doi:10.1002/1097-0142(197701)39:1<322::AID-CNCR2820390149>3.0.CO;2-P
17. Lewis WD, Jenkins RL, Rossi RL, et al. Surgical treatment of biliary cystadenoma. A report of 15 cases. Arch Surg. 1988;123(5):563–568. doi:10.1001/archsurg.1988.01400290045007
18. Woods GL. Biliary cystadenocarcinoma: case report of hepatic malignancy originating in benign cystadenoma. Cancer. 1981;47(12):2936–2940. doi:10.1002/1097-0142(19810615)47:12<2936::AID-CNCR2820471234>3.0.CO;2-4
19. Matsuoka Y, Hayashi K, Yano M. Case report: malignant transformation of biliary cystadenoma with mesenchymal stroma: documentation by CT. Clin Radiol. 1997;52(4):318–321. doi:10.1016/S0009-9260(97)80066-9
20. Daniels JA, Coad JE, Payne WD, Kosari K, Sielaff TD. Biliary cystadenomas: hormone receptor expression and clinical management. Dig Dis Sci. 2006;51(3):623–628. doi:10.1007/s10620-006-3181-4
21. Quigley B, Reid MD, Pehlivanoglu B, et al. Hepatobiliary Mucinous Cystic Neoplasms With Ovarian Type Stroma (So-Called “Hepatobiliary Cystadenoma/Cystadenocarcinoma”): clinicopathologic Analysis of 36 Cases Illustrates Rarity of Carcinomatous Change. Am J Surg Pathol. 2018;42(1):95–102. doi:10.1097/PAS.0000000000000963
22. Lee CW, Tsai HI, Lin YS, Wu TH, Yu MC, Chen MF. Intrahepatic biliary mucinous cystic neoplasms: clinicoradiological characteristics and surgical results. BMC Gastroenterol. 2015;15:67. doi:10.1186/s12876-015-0293-3
23. Armutlu A, Quigley B, Choi H, et al. Hepatic Cysts: reappraisal of the Classification, Terminology, Differential Diagnosis, and Clinicopathologic Characteristics in 258 Cases. Am J Surg Pathol. 2022;46(9):1219–1233. doi:10.1097/PAS.0000000000001930
24. Zen Y, Jang KT, Ahn S, et al. Intraductal papillary neoplasms and mucinous cystic neoplasms of the hepatobiliary system: demographic differences between Asian and Western populations, and comparison with pancreatic counterparts. Histopathology. 2014;65(2):164–173. doi:10.1111/his.12378
25. Arnaoutakis DJ, Kim Y, Pulitano C, et al. Management of biliary cystic tumors: a multi-institutional analysis of a rare liver tumor. Ann Surg. 2015;261(2):361–367. doi:10.1097/SLA.0000000000000543
26. Zen Y, Pedica F, Patcha VR, et al. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod Pathol. 2011;24(8):1079–1089. doi:10.1038/modpathol.2011.71
27. Van Treeck BJ, Lotfalla M, Czeczok TW, et al. Molecular and Immunohistochemical Analysis of Mucinous Cystic Neoplasm of the Liver. Am J Clin Pathol. 2020;154(6):837–847. doi:10.1093/ajcp/aqaa115
28. Zhelnin K, Xue Y, Quigley B, et al. Nonmucinous Biliary Epithelium Is a Frequent Finding and Is Often the Predominant Epithelial Type in Mucinous Cystic Neoplasms of the Pancreas and Liver. Am J Surg Pathol. 2017;41(1):116–120. doi:10.1097/PAS.0000000000000745
29. Albores-Saavedra J, Córdova-Ramón JC, Chablé-Montero F, Dorantes-Heredia R, Henson DE. Cystadenomas of the liver and extrahepatic bile ducts: morphologic and immunohistochemical characterization of the biliary and intestinal variants. Ann Diagn Pathol. 2015;19(3):124–129. doi:10.1016/j.anndiagpath.2015.03.001
30. Koffron A, Rao S, Ferrario M, Abecassis M. Intrahepatic biliary cystadenoma: role of cyst fluid analysis and surgical management in the laparoscopic era. Surgery. 2004;136(4):926–936. doi:10.1016/j.surg.2004.06.031
31. Choi HK, Lee JK, Lee KH, et al. Differential diagnosis for intrahepatic biliary cystadenoma and hepatic simple cyst: significance of cystic fluid analysis and radiologic findings. J Clin Gastroenterol. 2010;44(4):289–293. doi:10.1097/MCG.0b013e3181b5c789
32. Kovacs MD, Sheafor DH, Burchett PF, Picard MM, Hardie AD. Differentiating biliary cystadenomas from benign hepatic cysts: preliminary analysis of new predictive imaging features. Clin Imaging. 2018;49:44–47. doi:10.1016/j.clinimag.2017.10.022
33. Boyum JH, Sheedy SP, Graham RP, et al. Hepatic Mucinous Cystic Neoplasm Versus Simple Biliary Cyst: assessment of Distinguishing Imaging Features Using CT and MRI. AJR Am J Roentgenol. 2021;216(2):403–411. doi:10.2214/AJR.20.22768
34. Hardie AD, Chamberlin JH, Boyum JH, et al. Multi-Center Follow-up Study to Develop a Classification System Which Differentiates Mucinous Cystic Neoplasm of the Liver and Benign Hepatic Cyst Using Machine Learning. Acad Radiol. 2022;29(8):1149–1156. doi:10.1016/j.acra.2021.08.025
35. Logroño R, Rampy BA, Adegboyega PA. Fine needle aspiration cytology of hepatobiliary cystadenoma with mesenchymal stroma. Cancer. 2002;96(1):37–42. doi:10.1002/cncr.10315
36. Fujikura K, Akita M, Abe-Suzuki S, Itoh T, Zen Y. Mucinous cystic neoplasms of the liver and pancreas: relationship between KRAS driver mutations and disease progression. Histopathology. 2017;71(4):591–600. doi:10.1111/his.13271
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.