Back to Journals » OncoTargets and Therapy » Volume 10
MTHFR C677T polymorphism and breast, ovarian cancer risk: a meta-analysis of 19,260 patients and 26,364 controls
Received 4 September 2016
Accepted for publication 13 November 2016
Published 6 January 2017 Volume 2017:10 Pages 227—238
DOI https://doi.org/10.2147/OTT.S121472
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Lilin He, Yongxiang Shen
Department of Oncology, The First People’s Hospital of Tianmen City, Tianmen, Hubei Province, People’s Republic of China
Objective: Previous studies have found that many gene variations can be detected in both breast cancer and ovarian cancer, which is beneficial for the elaboration of the molecular origin of breast and ovarian cancer. Furthermore, many studies have explored the association of methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism with the risk of breast cancer and/or ovarian cancer; however, the results remained inconclusive. Therefore, this study conducted a systematic review and meta-analysis to evaluate the association between MTHFR C677T polymorphism and the risk of breast and ovarian cancer.
Materials and methods: A total of 50 studies with 19,260 cases and 26,364 controls including 39 studies for breast cancer and 8 studies for ovarian cancer were identified on searching through PubMed, Embase, Web of Science, China National Knowledge Infrastructure, WanFang, and Database of Chinese Scientific and Technical Periodicals (VIP). Allele model, dominant model, recessive model, homozygous model, and co-dominant model were applied to evaluate the association of MTHFR C677T polymorphism with breast cancer and/or ovarian cancer risk. Moreover, the odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to assess the strength of the association between MTHFR C677T polymorphism and breast and ovarian cancer risk.
Results: A significantly increased breast cancer risk was observed in the overall analysis (for C vs T, OR =1.19, CI: 1.12–1.28, P<0.05; for CC vs TT, OR =1.20, CI: 1.10–1.23, P<0.05; for (CT+CC) vs TT, OR =1.19, CI: 1.11–1.27, P<0.05; for CC vs (CT+TT), OR =1.19, CI: 1.79–1.95, P<0.05), while no significantly increased ovarian cancer risk was detected. In the subgroup analysis based on ethnicity, a significant association of breast cancer and/or ovarian cancer risk with MTHFR C677T polymorphism was observed in Asians. Interestingly, there was no significant association between MTHFR C677T polymorphism and ovarian cancer risk in Caucasians, whereas a significantly increased risk of breast cancer was found in Caucasians.
Conclusion: This meta-analysis demonstrates that MTHFR C677T polymorphism may be a risk factor for breast and ovarian cancer, especially in Asians.
Keywords: MTHFR, C677T, polymorphism, breast cancer, ovarian cancer, meta-analysis
Introduction
Breast cancer is one of the most common cancers with an increasing mortality worldwide, while ovarian cancer is less frequent than breast cancer but is often fatal.1 Clinically, treatment of advanced breast cancer is often futile, and therefore, early diagnosis is critical to the therapy of breast cancer. In most cases, breast cancer occurs during the post-menopausal period, in which ovarian estrogen is no longer produced.2 It was reported that a number of novel genetic mutations were found in inherited breast and ovarian cancer patients.3 For example, mutations in BRCA1 and BRCA2 genes were often detected in the hereditary breast and ovarian cancer patients.4 Of hereditary breast and ovarian cancers, the familial hereditary variations accounted for only 10%.5 A previous study in American populations indicated that many molecular mutations were observed in both sporadic breast cancer and sporadic ovarian cancer.1 Six genetic techniques, including genomic DNA copy number arrays, DNA methylation, exome sequencing, messenger RNA arrays, microRNA sequencing, and reverse-phase protein arrays, were used to detect gene mutations in this study. The data, concerning genetic variations of breast cancer and ovarian cancer, were calculated using statistical methods. Obviously, similar molecule mutations were found in both sporadic breast cancer and sporadic ovarian cancer. In addition, some other studies, focusing on rare genes such as PALB2, ATM, CHEK2, BRIP1, RAD51C, and PPMID, were performed, and these studies have also found few common genetic mutations.6 These risk modifiers could be applied to the early treatments of cancers, which is important for intensive screening and prophylactic surgery of cancer patients. The elucidation of risk allele is also helpful for clarifying the pathogenic mechanisms of cancers.
The gene, encoding methylenetetrahydrofolate reductase (MTHFR), is located at 1p36.3 and is highly polymorphic, in which the C677T polymorphic variant is most commonly studied and it can lead to Ala222Val.7 The MTHFR C677T polymorphism could reduce the production of MTHFR and affect enzyme activity.8 MTHFR is a crucial enzyme which has an important role in the regulation of methionine and homocysteine concentrations in folate metabolism.9 Folate is a necessity in intracellular metabolic processes such as DNA and RNA synthesis, DNA repair, and DNA methylation.10 Folate could regulate the transfer of one carbon unit in various biochemical reactions, which is complicated in various pathological processes such as breast cancer, ovarian cancer, colorectal cancer, gastric cancer, and lung cancer.11,12 Although many studies are conducted to investigate the association between MTHFR C677T polymorphism and breast and ovarian cancer, there is no conclusive evidence that MTHFR C677T is a common risk factor for breast cancer and ovarian cancer due to the influences of many factors such as ethnicity, source of control, and sample size.
Therefore, this study has performed this meta-analysis based on published eligible case–control studies to evaluate the role of MTHFR C677T polymorphism in breast cancer and/or ovarian cancer risk.
Materials and methods
Publication search
PubMed, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), WanFang, and Database of Chinese Scientific and Technical Periodicals (VIP) were searched to identify the articles that investigate the association of MTHFR C677T polymorphism with breast and ovarian cancer risk. The retrieval was performed using the keywords: “breast neoplasms,” “breast cancer,” “breast carcinoma,” “ovarian carcinoma,” “ovarian neoplasms,” “ovarian cancer,” “MTHFR,” “Methylenetetrahydrofolate Reductase (NADPH2),” “C677T,” “rs1801133,” and the latest search was updated until June 2016. In addition, articles published only in English and Chinese were identified, while the full-text of the retrieved articles was scrutinized to confirm that these data were required for this study.
Inclusion and exclusion criteria
Studies were included if they met the following inclusion criteria: 1) case–control studies, 2) investigating the association of MTHFR C677T polymorphism with breast and ovarian cancer risk, 3) genotype data of cases and controls were complete, and 4) genotype distribution of control must comply with the Hardy–Weinberg equilibrium (HWE). The exclusion criteria were as follows: 1) duplicated studies, 2) no detailed information of genotype data, and 3) meta-analysis and reviews.
Data extraction
Two authors assessed the quality of the included studies independently and extracted the following information: the name of first author, year of publication, country of origin, ethnicity, sample size, and genotype data. In case of conflicting information, divergence was resolved through discussion with the team. The population was divided into the Asians and Caucasians.
Quality score assessment
The quality of the included studies was assessed with the Newcastle–Ottawa scale (NOS) (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) table. Two authors calculated the score of each study, respectively. The maximum score was 9, and a score ≥7 denoted that the study was of high quality.
Statistical analysis
HWE of the included studies was assessed with χ2 test. The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the association between MTHFR C677T and breast and ovarian cancer risk.13 Allele model, co-dominant model, dominant model, recessive model, and homozygous model were utilized to assess the association of the MTHFR C677T polymorphism with the risk of breast cancer and/or ovarian cancer. Moreover, a subgroup analysis based on ethnicity and source of control were conducted to reduce the heterogeneity. The chi-square-based Q test (P<0.05, the significant level of statistical heterogeneity) and I2 index (I2≥50%, the significant level of statistical heterogeneity) were used to evaluate the inconsistencies among studies, and the two values were shown on the forest plots.14,15 The fixed effect model, deriving from the Mantel-Haenszel method, was applied when heterogeneity did not exist, and the random effect model, depending on the DerSimonian and Laird method, was carried out in case of significant heterogeneity.16,17 Egger’s test and Begg’s test were conducted to examine the publication bias.18 Sensitivity analysis was performed by removing each study and was applied to observe stabilization of the results. All statistical analyses were conducted using Stata (version 12.0; StataCorp LP, College Station, TX, USA) software. In addition, the P-value is two sided, and P<0.05 was considered statistically significant.
Results
Literature selection
A total of 137 articles with 6 reviews and 22 meta-analyses were retrieved in initial search from PubMed, Embase, Web of Science, CNKI, WanFang, and VIP databases. About 28 articles were excluded as they were not case–control studies; 70 articles were included after analyzing the titles and abstracts; and 8 articles were excluded after reading the full-text. HWE was carried out to analyze the genetic equilibrium of the included studies, and 12 studies were excluded (P<0.05).19–26 Finally, 50 publications, involving 19,260 cases and 26,364 controls, were selected in this meta-analysis. The information of first author, ethnicity, nationality, cancer type, genotyping method, source of control, and genotype frequency was extracted (Table 1, Figure 1).
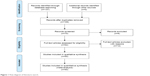  | Figure 1 Flow diagram of literature search. |
Quantitative analysis
In the overall and subgroup analysis, five genetic models were applied to evaluate the association of MHTFR C677T polymorphism with the risk of breast cancer and/or ovarian cancer. The results indicated that there was a significant correlation between MTHFR C677T polymorphism and breast cancer risk: allele model C vs T, OR =1.19, CI: 1.12–1.28, P<0.05; homozygous model CC vs TT, OR =1.20, CI: 1.12–1.28, P<0.05; recessive model (CT+CC) vs TT, OR =1.19, CI: 1.11–1.27, P<0.05; dominant model CC vs (CT+TT), OR =1.19, CI: 1.79–1.95, P<0.05. However, no significantly increased ovarian cancer risk was found (allele model C vs T, OR =1.03, CI: 0.98–1.09, P=0.26; homozygous model CC vs TT, OR =1.05, CI: 0.93–1.18, P=0.45; recessive model TT vs (CT+CC), OR =1.02, CI: 0.92–1.15, P=0.68; dominant model CC vs (CT+TT), OR =1.05, CI: 0.97–1.13, P=0.21; co-dominant model TT vs CT, OR =1.05, CI: 0.97–1.29, P=0.24). In the subgroup analysis by ethnicity, the results reflected that the MTHFR C677T mutation could significantly increase the breast cancer risk in both Caucasians and Asians (Table 2). None of the genetic models indicated a significant association between MTHFR C677T polymorphism and ovarian cancer risk in Caucasians, while significant ovarian cancer risk was observed in Asians: allele model C vs T, OR =1.19, CI: 1.13–1.25, P<0.05; homozygous model CC vs TT, OR =1.43, CI: 1.30–1.59, P<0.05; recessive model TT vs (CT+CC), OR =1.35, CI: 1.23–1.48, P<0.05; dominant model CC vs (CT+TT), OR =1.20, CI: 1.12–1.28, P<0.05; co-dominant model TT vs CT, OR =1.13, CI: 1.05–1.21, P<0.05 (Table 3). In addition, forest plots have been drawn to observe the weight of each included study and estimate the association of MTHFR C677T polymorphism with the relative risk of breast cancer and/or ovarian cancer using the homozygous genetic model (CC vs TT). In the meantime, the stratified analyses based on ethnicity, cancer type, and control type were conducted to eliminate the heterogeneity among studies (Figures 2 and 3).
Sensitivity analysis and publication bias
Sensitivity analysis indicated that the results were stable, and the summary ORs were not materially altered by excluding individual data set at each time. Moreover, no significant publication bias was shown according to Begg’s test and Egger’s test (Figures 4–6).
  | Figure 4 Funnel plot of homozygous comparison (CC vs TT) for breast cancer and/or ovarian cancer. |
  | Figure 5 Funnel plot of homozygous comparison (CC vs TT) for breast cancer. |
  | Figure 6 Funnel plot of homozygous comparison (CC vs TT) for ovarian cancer. |
Discussion
In previous studies, strong evidences show that genetic variations, involving DNA metabolism, existed in breast cancer and/or ovarian cancer.27,28 Because of the central roles of these genes in cell metabolism, the changes in the functions of these genes may increase the risk of cancers. As is well known, MTHFR C677T polymorphism could alter MTHFR enzyme activity, which affected the general balance in the process of DNA repair, DNA methylation, and DNA synthesis.29 Therefore, MTHFR might have a potential effect on the origin and progress of breast cancer and ovarian cancer.30 Several studies have been conducted to evaluate the contribution of MTHFR C677T polymorphism to breast and ovarian cancer, but the sample size, ethnicity, and the source of control were limited.31,32 In the study of Lu et al, the results suggested that MTHFR C677T polymorphism might be significantly associated with the risk and prognosis of breast cancer in Chinese population.33 Although the age has been corrected and the genotype data of control comply with the law of HWE in this study, the conclusion was still indeterminable because of small sample size and the influence of other environmental factors. The same results were also observed in other studies.34–36 In addition, there was a common problem in the studied Chinese populations, that is, the population of control often came from the hospital. This might reduce the persuasion of research results. Hence, in the meta-analysis, the subgroup analysis based on the source of control was conducted to increase the power of statistics and achieve a more accurate result. On the other hand, significant association between MTHFR C677T and breast cancer risk was also detected in Caucasians.37,38 However, contrasting results were described in Asians and Caucasians for breast cancer risk.39–43 In the studies for ovarian cancer, Gao et al found that the MTHFR C677T polymorphism was significantly associated with the susceptibility and the survival of ovarian cancer.31,45,46 Nevertheless, other results indicated that no association of MTHFR C677T polymorphism with ovarian cancer risk existed.47,48 The different results from these studies showed that the correlation between MTHFR C677T polymorphism and breast cancer and/or ovarian cancer risk were still inconclusive. Hence, the pooled analysis was carried out to analyze the correlation of MTHFR C677T polymorphism with breast cancer and/or ovarian cancer risk.
In the overall analysis, the results suggested that the MTHFR C677T polymorphism might significantly increase the breast cancer risk but not ovarian cancer risk. The CC genotype carriers had a higher breast cancer risk than that of TT genotype carriers in Asians. In the analysis of total population, the P-value and ORs revealed that breast cancer and/or ovarian cancer risk were significantly associated with MTHFR C677T polymorphism. Furthermore, the cumulative results indicated that TT allele carrier had a higher risk of breast cancer and/or ovarian cancer than the CC allele carrier. From the subgroup analysis, more significant risk of breast cancer and/or ovarian cancer was detected in Asians (for CC vs TT, P<0.05, OR =1.19, CI: 1.13–1.25).
According to subgroup analysis, source of control and ethnicity might have a great effect on the results. The results showed that the hospital-based case–control studies mainly contributed to the heterogeneity among ovarian cancer research studies. Based on the included studies for breast cancer, it could be mentioned that the main cause of heterogeneity might be ethnicity. In the stratified meta-analysis based on ethnicity for breast cancer, compared with C allele, a significantly increased breast cancer risk was significantly associated with T allele in Asians (C vs T, P<0.05, OR =1.12, CI: 1.06–1.18; CC vs TT, P<0.05, OR =1.29, CI: 1.16–1.44; CC vs (CT+TT), P<0.05, OR =1.10, CI: 1.03–1.18; (CC+CT) vs TT, P<0.05, OR =1.27, CI: 1.15–1.40). Under C vs T allele model, the polymorphism of MTHFR C677T could increase the risk of ovarian cancer in Asians (C vs T, P<0.05, OR =1.52, CI: 1.29–1.80). No statistical significance was detected between MTHFR C677T polymorphism and ovarian cancer risk in Caucasians. The T allele significantly increased ovarian cancer risk in the studies of hospital-based control (CC vs (CT+TT), P<0.05, OR =1.18, CI: 1.07–1.31). Subgroup analysis based on cancer type in Asians revealed that MTHFR C677T mutation could significantly increase the risk of ovarian cancer (allele model C vs T, OR =1.52, CI: 1.29–1.80, P<0.05; homozygous model CC vs TT, OR =2.74, CI: 1.85–4.06, P<0.05; recessive model TT vs (CT+CC), OR =2.46, CI: 1.68–3.59, P<0.05; dominant model CC vs (CT+TT), OR =1.49, CI: 1.21–1.84, P<0.05; co-dominant model TT vs CT, OR =1.30, CI: 1.04–1.63, P<0.05). The allele T carriers might have a higher breast cancer and/or ovarian cancer risk in Asians, which might result from the influence of the MTHFR enzyme in tumor cells.9
Several factors such as selection criteria of cases, age distribution, sample size, family history, ethnicity, source of control, and lifestyle might lead to the heterogeneity among studies. There was no significant publication bias based on Begg’s test and Egger’s test. In addition, no significant changing of the results was found in sensitivity analysis, which demonstrated the results were stable in the meta-analysis. And the studies that were not consistent with the HWE in the meta-analysis were excluded in order to improve the accuracy of the results.
According to the results, it was clear that the MTHFR C677T variant could increase the breast cancer and/or ovarian cancer risk in Asians. These results provided obvious evidence that metabolism genes could increase the risk of breast and ovarian cancer. Most notably, because of some genetic differences in Asians and Caucasians, the MTHFR C677T polymorphism might have a different effect on breast cancer and/or ovarian cancer in the two populations. But given the different role of gene variations in cell differentiation and proliferation, the function experiment and clinic trial were still needed to confirm the conclusions of this meta-analysis.49 Furthermore, environmental factors might have an important influence in breast cancer and/or ovarian cancer risk. Hence, it was expected that studies including environmental factors were carried out.
In summary, this meta-analysis demonstrated that the MTHFR C677T mutation might increase the risk of both breast cancer and ovarian cancer, especially in Asians. It provided a new insight into the molecular origin of breast cancer and ovarian cancer. Considering the limitations of the study, large well-designed studies including different ethnic populations should be conducted to further assess the association of the MTHFR C677T polymorphisms with increased susceptibility to breast cancer and/or ovarian cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. | ||
Lajin B, Alhaj SA, Ghabreau L, Alachkar A. Association of polymorphisms in one-carbon metabolizing genes with breast cancer risk in Syrian women. Tumour Biol. 2012;33:1133–1139. | ||
Ford D, Easton DF. The genetics of breast and ovarian cancer. Br J Cancer. 1995;72:805–812. | ||
Petrucelli N, Daly MB, Feldman GL. Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med. 2010;12:245–259. | ||
Marshall M, Solomon S. Hereditary breast-ovarian cancer: clinical findings and medical management. Plast Surg Nurs. 2007;27:124–127. | ||
Ruark E, Snape K, Humburg P, et al. Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature. 2013;493:406–410. | ||
Rosenberg N, Murata M, Ikeda Y, et al. The frequent 5,10-methylenetetrahydrofolate reductase C677T polymorphism is associated with a common haplotype in whites, Japanese, and Africans. Am J Hum Genet. 2002;70:758–762. | ||
Parle-McDermott A, Mills JL, Molloy AM, et al. The MTHFR 1298CC and 677TT genotypes have opposite associations with red cell folate levels. Mol Genet Metab. 2006;88:290–294. | ||
Nazki FH, Sameer AS, Ganaie BA. Folate: metabolism, genes, polymorphisms and the associated diseases. Gene. 2014;533:11–20. | ||
Qi X, Ma X, Yang X, et al. Methylenetetrahydrofolate reductase polymorphisms and breast cancer risk: a meta-analysis from 41 studies with 16,480 cases and 22,388 controls. Breast Cancer Res Treat. 2010;123:499–506. | ||
Panigrahi I, Chatterjee T, Biswas A, Behari M, Choudhry PV, Saxena R. Role of MTHFR C677T polymorphism in ischemic stroke. Neurol India. 2006;54:48–52. | ||
Sauer J, Mason JB, Choi SW. Too much folate: a risk factor for cancer and cardiovascular disease? Curr Opin Clin Nutr Metab Care. 2009;12:30–36. | ||
Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19:251–253. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. | ||
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. | ||
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. | ||
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. | ||
Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. | ||
Lissowska J, Gaudet MM, Brinton LA, et al. Genetic polymorphisms in the one-carbon metabolism pathway and breast cancer risk: a population-based case-control study and meta-analyses. Int J Cancer. 2007;120:2696–2703. | ||
Kan XX, Zou TN, Wu XY, Wang Xu. Association between mTHFR genotype polymorphism and breast cancer susceptibility in human population from Yunnan. Cancer Res Prev Treat. 2007;34:716–718. | ||
Stevens VL, McCullough ML, Pavluck AL, et al. Association of polymorphisms in one-carbon metabolism genes and postmenopausal breast cancer incidence. Cancer Epidemiol Biomarkers Prev. 2007;16:1140–1147. | ||
Le Marchand L, Haiman CA, Wilkens LR, Kolonel LN, Henderson BE. MTHFR polymorphisms, diet, HRT, and breast cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2004;13:2071–2077. | ||
Han H, Han LL, Gao HD, Hou L. Genetic polymorphisms of methylenetetrahydrofolate reductase, methionine synthase, methylation of NF2, and their association with breast cancer morbidity. Chin J Curr Adv Gen Surg. 2011;14:846–850. | ||
Jakubowska A, Gronwald J, Menkiszak J, et al. Methylenetetrahydrofolate reductase polymorphisms modify BRCA1-associated breast and ovarian cancer risks. Breast Cancer Res Treat. 2007;104:299–308. | ||
Hua ZP, Wang YB, Ni J, Ge F, Zou TN. Folic acid, Vitamin B12, MTHFR, MS gene polymorphisms associate with the risk of breast cancer. Mod Oncol. 2011;19:0428–0431. | ||
Wu YY, Wu L, Wang Y, Cao WH, Hou L. Relation between the SNPs in methylenetetrahydrofolate reductase gene C677T and G1793A and the susceptibility of sporadic breast cancer. Prog Mod Biomed. 2012;12:2609–2614. | ||
Renwick A, Thompson D, Seal S, et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet. 2006;38:873–875. | ||
Seal S, Thompson D, Renwick A, et al. Truncating mutations in the Fanconi anemia J gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nat Genet. 2006;38:1239–1241. | ||
Eroglu A, Akar N. Methylenetetrahydrofolate reductase C677T polymorphism in breast cancer risk. Breast Cancer Res Treat. 2010;122:897–898. | ||
Kim YI. 5,10-Methylenetetrahydrofolate reductase polymorphisms and pharmacogenetics: a new role of single nucleotide polymorphisms in the folate metabolic pathway in human health and disease. Nutr Rev. 2005;63:398–407. | ||
Jakubowska A, Rozkrut D, Antoniou A, et al. Association of PHB 1630 C>T and MTHFR 677 C>T polymorphisms with breast and ovarian cancer risk in BRCA1/2 mutation carriers: results from a multicenter study. Br J Cancer. 2012;106:2016–2024. | ||
Gershoni-Baruch R, Dagan E, Israeli D, Kasinetz L, Kadouri E, Friedman E. Association of the C677T polymorphism in the MTHFR gene with breast and/or ovarian cancer risk in Jewish women. Eur J Cancer. 2000;36:2313–2316. | ||
Lu Q, Jiang K, Li Q, Ji YJ, Chen WL, Xue XH. Polymorphisms in the MTHFR gene are associated with breast cancer risk and prognosis in a Chinese population. Tumour Biol. 2015;36:3757–3762. | ||
Qi J, Miao XP, Tan W, et al. 亚甲基四氢叶酸还原酶基因单核苷酸多态与乳腺癌风险 [Association between genetic polymorphisms in methylenetetrahydrofolate reductase and risk of breast cancer]. Chin J Oncol. 2004;26:287–289. Chinese. | ||
Yuan H, Xu XY, Wang ZL. The relation between polymorphisms of methylenetetrahydrofolate reductase C677T and the risk of breast cancer. J MuDanJiang Med Univ. 2009;30:2–4. | ||
Gao CM, Tang JH, Cao HX, et al. MTHFR polymorphisms, dietary folate intake and breast cancer risk in Chinese women. J Hum Genet. 2009;54:414–418. | ||
Langsenlehner U, Krippl P, Renner W, et al. The common 677C>T gene polymorphism of methylenetetrahydrofolate reductase gene is not associated with breast cancer risk. Breast Cancer Res Treat. 2003;81:169–172. | ||
Campbell IG, Baxter SW, Eccles DM, Choong DY. Methylenetetrahydrofolate reductase polymorphism and susceptibility to breast cancer. Breast Cancer Res. 2002;4:R14. | ||
Grieu F, Powell B, Beilby J, Iacopetta B. Methylenetetrahydrofolate reductase and thymidylate synthase polymorphisms are not associated with breast cancer risk or phenotype. Anticancer Res. 2004;24:3215–3219. | ||
Forsti A, Angelini S, Festa F, et al. Single nucleotide polymorphisms in breast cancer. Oncol Rep. 2004;11:917–922. | ||
Semenza JC, Delfino RJ, Ziogas A, Anton-Culver H. Breast cancer risk and methylenetetrahydrofolate reductase polymorphism. Breast Cancer Res Treat. 2003;77:217–223. | ||
Li WD, Chen SQ. Association of methylenetetrahydrofolate reductase C677T polymorphism and breast cancer risk. J Pract Med. 2009;25:2031–2033. | ||
Jin Z, Lu Q, Ge D, Zong M, Zhu Q. Effect of the methylenetetrahydrofolate reductase gene C677T polymorphism on C-erbB-2 methylation status and its association with cancer. Mol Med Rep. 2009;2:283–289. | ||
Pooja S, Carlus J, Sekhar D, et al. MTHFR 677C>T polymorphism and the risk of breast cancer: evidence from an original study and pooled data for 28031 cases and 31880 controls. PLoS One. 2015;10:e120654. | ||
Gao S, Liu N, Ma Y, Ying L. Methylenetetrahydrofolate reductase gene polymorphisms as predictive and prognostic biomarkers in ovarian cancer risk. Asian Pac J Cancer Prev. 2012;13:569–573. | ||
Wu Y, Zhang JY, Zuo WJ. Association between genetic polymorphisms of methylenetetrahydrofolate reductase C677T and susceptibility to ovarian cancer. Prog Obstet Gynecol. 2007;16:811–813. | ||
Prasad VV, Wilkhoo H. Association of the functional polymorphism C677T in the methylenetetrahydrofolate reductase gene with colorectal, thyroid, breast, ovarian, and cervical cancers. Onkologie. 2011;34:422–426. | ||
Terry KL, Tworoger SS, Goode EL, et al. MTHFR polymorphisms in relation to ovarian cancer risk. Gynecol Oncol. 2010;119:319–324. | ||
Schnekenburger M, Dicato M, Diederich M. Plant-derived epigenetic modulators for cancer treatment and prevention. Biotechnol Adv. 2014;32:1123–1132. | ||
Sharp L, Little J, Schofield AC, et al. Folate and breast cancer: the role of polymorphisms in methylenetetrahydrofolate reductase (MTHFR). Cancer Lett. 2002;181:65–71. | ||
Ergul E, Sazci A, Utkan Z, Canturk NZ. Polymorphisms in the MTHFR gene are associated with breast cancer. Tumour Biol. 2003;24:286–290. | ||
Lee SA, Kang D, Nishio H, et al. Methylenetetrahydrofolate reductase polymorphism, diet, and breast cancer in Korean women. Exp Mol Med. 2004;36:116–121. | ||
Lin WY, Chou YC, Wu MH, et al. The MTHFR C677T polymorphism, estrogen exposure and breast cancer risk: a nested case-control study in Taiwan. Anticancer Res. 2004;24:3863–3868. | ||
Shrubsole MJ, Gao YT, Cai Q, et al. MTHFR polymorphisms, dietary folate intake, and breast cancer risk: results from the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2004;13:190–196. | ||
Justenhoven C, Hamann U, Pierl CB, et al. One-carbon metabolism and breast cancer risk: no association of MTHFR, MTR, and TYMS polymorphisms in the GENICA study from Germany. Cancer Epidemiol Biomarkers Prev. 2005;14:3015–3018. | ||
Kalemi TG, Lambropoulos AF, Gueorguiev M, Chrisafi S, Papazisis KT, Kotsis A. The association of p53 mutations and p53 codon 72, Her 2 codon 655 and MTHFR C677T polymorphisms with breast cancer in Northern Greece. Cancer Lett. 2005;222:57–65. | ||
Deligezer U, Akisik EE, Dalay N. Homozygosity at the C677T of the MTHFR gene is associated with increased breast cancer risk in the Turkish population. In Vivo. 2005;19:889–893. | ||
Chou YC, Wu MH, Yu JC, et al. Genetic polymorphisms of the methylenetetrahydrofolate reductase gene, plasma folate levels and breast cancer susceptibility: a case-control study in Taiwan. Carcinogenesis. 2006;27:2295–2300. | ||
Reljic A, Simundic AM, Topic E, Nikolac N, Justinic D, Stefanovic M. The methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and cancer risk: the Croatian case-control study. Clin Biochem. 2007;40:981–985. | ||
Hekim N, Ergen A, Yaylim I, et al. No association between methylenetetrahydrofolate reductase C677T polymorphism and breast cancer. Cell Biochem Funct. 2007;25:115–117. | ||
Xu X, Gammon MD, Zhang H, et al. Polymorphisms of one-carbon-metabolizing genes and risk of breast cancer in a population-based study. Carcinogenesis. 2007;28:1504–1509. | ||
Macis D, Maisonneuve P, Johansson H, et al. Methylenetetrahydrofolate reductase (MTHFR) and breast cancer risk: a nested-case-control study and a pooled meta-analysis. Breast Cancer Res Treat. 2007;106:263–271. | ||
Yu CP, Wu MH, Chou YC, et al. Breast cancer risk associated with multigenotypic polymorphisms in folate-metabolizing genes: a nested case-control study in Taiwan. Anticancer Res. 2007;27:1727–1732. | ||
Kotsopoulos J, Zhang WW, Zhang S, et al. Polymorphisms in folate metabolizing enzymes and transport proteins and the risk of breast cancer. Breast Cancer Res Treat. 2008;112:585–593. | ||
Langsenlehner T, Renner W, Yazdani-Biuki B, Langsenlehner U. Methylenetetrahydrofolate reductase (MTHFR) and breast cancer risk: a nested-case-control study and a pooled meta-analysis. Breast Cancer Res Treat. 2008;107:459–460. | ||
Cheng CW, Yu JC, Huang CS, et al. Polymorphism of cytosolic serine hydroxymethyltransferase, estrogen and breast cancer risk among Chinese women in Taiwan. Breast Cancer Res Treat. 2008;111:145–155. | ||
Inoue M, Robien K, Wang R, Van Den Berg DJ, Koh WP, Yu MC. Green tea intake, MTHFR/TYMS genotype and breast cancer risk: the Singapore Chinese Health Study. Carcinogenesis. 2008;29:1967–1972. | ||
Suzuki T, Matsuo K, Hirose K, et al. One-carbon metabolism-related gene polymorphisms and risk of breast cancer. Carcinogenesis. 2008;29:356–362. | ||
Cam R, Eroglu A, Egin Y, Akar N. Dihydrofolate reductase (DHRF) 19-bp intron-1 deletion and methylenetetrahydrofolate reductase (MTHFR) C677T polymorphisms in breast cancer. Breast Cancer Res Treat. 2009;115:431–432. | ||
Henriquez-Hernandez LA, Murias-Rosales A, Hernandez GA, et al. Gene polymorphisms in TYMS, MTHFR, p53 and MDR1 as risk factors for breast cancer: a case-control study. Oncol Rep. 2009;22:1425–1433. | ||
Platek ME, Shields PG, Marian C, et al. Alcohol consumption and genetic variation in methylenetetrahydrofolate reductase and 5-methyltetrahydrofolate-homocysteine methyltransferase in relation to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:2453–2459. | ||
Ericson U, Sonestedt E, Ivarsson MI, et al. Folate intake, methylenetetrahydrofolate reductase polymorphisms, and breast cancer risk in women from the Malmo Diet and Cancer cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:1101–1110. | ||
Maruti SS, Ulrich CM, Jupe ER, White E. MTHFR C677T and postmenopausal breast cancer risk by intakes of one-carbon metabolism nutrients: a nested case-control study. Breast Cancer Res. 2009;11:R91. | ||
Ma E, Iwasaki M, Junko I, et al. Dietary intake of folate, vitamin B6, and vitamin B12, genetic polymorphism of related enzymes, and risk of breast cancer: a case-control study in Brazilian women. BMC Cancer. 2009;9:122. | ||
Ma E, Iwasaki M, Kobayashi M, et al. Dietary intake of folate, vitamin B2, vitamin B6, vitamin B12, genetic polymorphism of related enzymes, and risk of breast cancer: a case-control study in Japan. Nutr Cancer. 2009;61:447–456. | ||
Bentley AR, Raiszadeh F, Stover PJ, Hunter DJ, Hankinson SE, Cassano PA. No association between cSHMT genotypes and the risk of breast cancer in the Nurses’ Health Study. Eur J Clin Nutr. 2010;64:108–110. | ||
Wu XY, Ni J, Xu WJ, Zhou T, Wang X. Interactions between MTHFR C677T-A1298C variants and folic acid deficiency affect breast cancer risk in a Chinese population. Asian Pac J Cancer Prev. 2012;13:2199–2206. | ||
Akilzhanova A, Nurkina Z, Momynaliev K, et al. Genetic profile and determinants of homocysteine levels in Kazakhstan patients with breast cancer. Anticancer Res. 2013;33:4049–4059. | ||
Awwad N, Yousef AM, Abuhaliema A, Abdalla I, Yousef M. Relationship between genetic polymorphisms in MTHFR (C677T, A1298C and their haplotypes) and the incidence of breast cancer among Jordanian females – case-control study. Asian Pac J Cancer Prev. 2015;16:5007–5011. | ||
Webb PM, Ibiebele TI, Hughes MC, et al. Folate and related micronutrients, folate-metabolising genes and risk of ovarian cancer. Eur J Clin Nutr. 2011;65:1133–1140. | ||
Pawlik P, Mostowska A, Lianeri M, Sajdak S, Kedzia H, Jagodzinski PP. Folate and choline metabolism gene variants in relation to ovarian cancer risk in the Polish population. Mol Biol Rep. 2012;39:5553–5560. | ||
Zhang L, Liu W, Hao Q, Bao L, Wang K. Folate intake and methylenetetrahydrofolate reductase gene polymorphisms as predictive and prognostic biomarkers for ovarian cancer risk. Int J Mol Sci. 2012;13:4009–4020. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





