Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
MTA1 Expression Can Stratify the Risk of Patients with Multifocal Non-Small Cell Lung Cancers ≤3 cm
Authors Wang W, Hu Z, Ma M, Yin H, Huang Y, Zhao G, Cui X, Sun Q, Yang Y, Yang Y , Wang B, Ye L
Received 27 July 2021
Accepted for publication 18 November 2021
Published 3 December 2021 Volume 2021:17 Pages 1295—1304
DOI https://doi.org/10.2147/TCRM.S331317
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Deyun Wang
Wei Wang,1,2,* Zaoxiu Hu,3,* Mingsheng Ma,4 Haoyuan Yin,4 Yunchao Huang,1 Guangqiang Zhao,1 Xin Cui,1 Qinling Sun,1 Yantao Yang,1 Yichen Yang,1 Biying Wang,1 Lianhua Ye1
1Department of Thoracic Surgery, The Third Affiliated Hospital of Kunming Medical University, Kunming, People’s Republic of China; 2Department of Thoracic Surgery, Taihe Hospital (Hubei University of Medicine), Shiyan, People’s Republic of China; 3Department of Pathology, The Third Affiliated Hospital of Kunming Medical University, Kunming, People’s Republic of China; 4Department of Thoracic Surgery, The Sixth Affiliated Hospital of Kunming Medical University, Yuxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lianhua Ye
Department of Thoracic Surgery, The Third Affiliated Hospital of Kunming Medical University, No. 519 Kunzhou Road, Xishan District, Kunming City, Yunnan Province, People’s Republic of China
Email [email protected]
Purpose: Currently, there is no uniform standard to guide postoperative adjuvant chemotherapy for patients with multifocal non-small cell lung cancers (NSCLCs) ≤ 3 cm. Therefore, there is an urgent need to explore prognostic molecular markers to identify high-risk patients with multifocal NSCLCs ≤ 3 cm. We aimed to explore the potential value of metastasis-associated protein 1(MTA1) expression in risk stratification of patients with multifocal NSCLCs ≤ 3 cm.
Methods: We retrospectively analyzed the clinical data and postoperative survival data of patients with multifocal NSCLCs ≤ 3 cm. Paraffin-embedded tissue sections were used for immunohistochemistry. Semiquantitative immunoreactivity scoring (IRS) system was used to evaluate the nuclear expression of MTA1. SPSS software (version 23.0) was used to analyze the data.
Results: The IRS of MTA1 nuclear expression in 259 lesions of 119 patients ranged from 2.2 to 11.7 (median: 5.6). Our results showed that MTA1 expression was highest in high-risk pathological subtypes of lung adenocarcinoma. MTA1 expression in multiple primary lung cancers (MPLCs) was lower than that in intrapulmonary metastases (IPMs). The median follow-up duration was 25.97 months. The disease-free survival (DFS) of patients with MPLCs was significantly better than that of patients with IPMs, and the DFS of patients with high MTA1 expression was significantly worse than that of patients with low MTA1 expression. Multivariate Cox analysis showed that high MTA1 expression (hazard ratio: 7.937, 95% confidence interval: 2.433– 25.64, p =0.001) was a statistically significant predictor of worse DFS in patients with multifocal NSCLCs ≤ 3 cm.
Conclusion: MTA1 expression can stratify the risk in patients with multifocal NSCLCs ≤ 3 cm. Patients with MTA1 immunohistochemical score > 5.6 are at a high risk of postoperative recurrence, and these patients may benefit from postoperative adjuvant chemotherapy.
Keywords: multifocal, non-small cell lung cancer, MTA1, multiple primary lung cancers, intrapulmonary metastases, risk of postoperative recurrence
Introduction
The incidence of multifocal lung cancer has been increasing,1,2 with the reported incidence ranging from 0.2% to 20%. This might be attributed to the use of high-resolution chest imaging techniques and lung cancer screening procedures as well as to the monitoring of cancer patients who have been treated previously.3,4 The eighth edition of staging system for lung cancer described multifocal lung cancer as a separate entity for the first time. According to the clinicopathological characteristics, multifocal lung cancer is divided into four types, namely the second primary lung cancer and multifocal ground glass/lepidic adenocarcinoma, separate tumor nodules of the same cancer, and pneumonic-type adenocarcinoma, the former two are multiple primary lung cancers (MPLCs), and the latter two are intrapulmonary metastases (IPMs).5 In a multifocal non-small cell lung cancer (NSCLC) ≤3 cm classified as an MPLC, all lesions are T1-stage lesions and can be exempt from postoperative adjuvant chemotherapy.6 If it is classified as an IPM, the stage is T3, T4, or M1a according to the location of the lesions and postoperative adjuvant chemotherapy and close monitoring plan are required. Therefore, correct diagnosis and classification is the basis for TNM staging and subsequent treatment planning.
According to the eighth edition staging manual for lung cancer developed by the American Joint Committee on Cancer (AJCC), exact matching of the breakpoints identified by comparative genomic hybridization is the only evidence of IPM, a similar biomarker pattern is considered a relative argument in favor of a single tumor source.2 However, the complexity of bioinformatics calculations and methods related to probability statistics limits the widespread application of comparative genomic hybridization technology in clinical practice, leading to difficulties in making appropriate treatment decisions. Approximately 60% of the simultaneous MPLCs have the same histological type,7 making it difficult to correctly classify multifocal lung cancers with similar histological types. Studies have shown the value of biomarkers in monitoring lung cancer metastasis and recurrence,8,9 but there is still a lack of prognostic molecular markers to identify high-risk patients with multifocal NSCLCs ≤3 cm. Studies have shown that high expression of metastasis-associated protein 1(MTA1) in NSCLCs is closely associated with age ≥60 years, sex, histopathological type, T stage, and lymph node metastasis.10 MTA1 may distinguish high-risk patients with multifocal NSCLCs ≤3 cm and these patients may benefit from postoperative adjuvant chemotherapy. We studied MTA1 expression in multifocal NSCLCs ≤3 cm and explored the correlation of the level of MTA1 expression with clinicopathological characteristics and survival prognosis. Our results indicated the prognostic significance of MTA1 expression in patients with multifocal NSCLCs ≤3 cm and its potential value in risk stratification of these patients.
Patients and Methods
Patients
We retrospectively analyzed the clinical data and postoperative survival follow-up data of 119 patients with multifocal NSCLC ≤3 cm who underwent surgical treatment and whose diagnosis was confirmed with postoperative pathological examination in the Department of Thoracic and Cardiovascular Surgery of the Third Affiliated Hospital of Kunming Medical University from December 2013 to January 2019. The inclusion criteria were as follows: (1) lung lesions pathologically confirmed as NSCLCs after surgical resection, (2) number of lung lesions ≥2 and the diameter of a single lesion ≤3 cm on preoperative chest CT, (3) patients with complete clinical and follow-up data. The exclusion criteria were as follows: (1) lung lesions pathologically confirmed as metastases of malignant tumors of extrapulmonary origin, (2) patients who died during the perioperative period due to postoperative complications, (3) patients who received radiotherapy and chemotherapy before surgery, (4) patients who refused to participate in clinical research. The diagnostic criteria for MPLCs were based on the 2013 American College of Chest Physicians (ACCP) diagnostic recommendations.11 They were as follows: (1) the histological type of each cancer lesion was different, (2) each cancer lesion had different molecular genetic characteristics, (3) each cancer lesion originated from a different carcinoma in situ, (4) in case of cancer lesions with the same histological type, each cancer lesion was located in a different lung lobe with no mediastinal lymph node metastasis or systemic metastasis or the interval between the two lesions was not less than 4 years with no systemic metastasis. According to the World Health Organization classification of pathological subtypes of lung adenocarcinoma (2015),12 adenocarcinoma was divided into low-risk, moderate-risk and high-risk groups. Lepidic-predominant subtypes with no micropapillary or solid components were classified into the low-risk group, acinar or papillary-predominant subtypes with no micropapillary or solid components were classified into the moderate-risk group, and micropapillary and solid-predominant subtypes were classified into the high-risk group.13,14 The characteristics of the patients are presented in Table 1. This retrospective study was conducted in accordance with the declaration of Helsinki and was approved by the Institutional Review Committee and Ethics Committee of the Third Affiliated Hospital of Kunming Medical University (NO.KY2020160). Patient’s consent was waived as data was used in aggregate anonymously.
 |
Table 1 Characteristics of 119 Patients Included |
Immunohistochemistry
Paraffin-embedded tissue was cut into sections of 4μm-thickness, baked at 60 °C for 2 h, deparaffinized with xylene, hydrated twice in gradient ethanol (100%, 95%) for 10 min each, and rinsed with distilled water twice for 5 min each. Antigen repair was performed by adding a 1×citrate repair solution in a microwave oven. The samples were washed three times with distilled water for 5 min each. We added 3% hydrogen peroxide and incubated for 10 min to block peroxidase and washed the sections with phosphate-buffered saline (PBS) for 5 min. We used 200 ul of blocking solution to block each section for 1 hat room temperature. The blocking solution was removed and 300 µL of primary antibody (MTA1, diluted at 1:50, Catalog No. 5647S, CST) was added and incubated overnight at 4 °C. The primary antibody solution was removed and the sections were washed with PBS solution three times for 5 min each. Subsequently, they were incubated with the secondary antibody (1:50 dilution, Catalog No. 7074S, CST) at 20 °C for 30 min, streptomycin biotin-peroxidase solution was added, and the sections were stained using a diaminobenzidine kit. The sections were re-stained with hematoxylin for 2 min and washed with distilled water twice for 5 min each. The slides were incubated in gradient (95% and 100%) ethanol for twice dehydration cycles of 10 s each using cover glass and neutral-balsam dense slides. The negative control group was treated with PBS instead of the antibody.
Semiquantitative Immunoreactivity Scoring (IRS)
The semiquantitative IRS system15 was used to evaluate MTA1 nuclear expression in tumor tissues, and the accompanying cytoplasmic staining was not counted. Type A recorded the intensity of immunostaining: 0 (no immunostaining), 1 (weak immunostaining), 2 (medium immunostaining), 3 (strong immunostaining). Type B recorded the percentage of immune response cells: 0 (none), 1 (<10%), 2 (10–50%), 3 (51–80%) and 4 (>80%). The IRS obtained by multiplying types A and B was 0 to 12: negative (IRS =0), weak positive (0<IRS ≤4), moderate positive (4<IRS ≤8), strong positive (8<IRS ≤12). Both the positive percentage of cells and the intensity of staining were measured by two pathologists independently of each other. The inter-observer and intra-observer variability was negligible.
Follow Up
The patients were followed up by consulting the case data and telephone interviews. The deadline for follow-up was September 16, 2019. Disease-free survival (DFS) was defined as the time from the date of surgery to disease recurrence or death due to disease progression.16 At the last follow-up, no recurrence or death due to disease progression was observed, and the survival data were censored data. The follow-up duration was 4.27–76.53 months (median: 25.97 months). Data regarding lost to follow-up and deaths due to non-tumor reasons were regarded as “survival data” and treated as censored data.
Statistical Analysis
IBM SPSS Statistics version 23.0 (IBM Corp, Armonk, NY, USA) was used to analyze the data. Count data were described as frequency and percentage and measurement data were described as mean ± standard deviation (normal distribution) or median and range of variation (non-normal distribution) according to the type of distribution. MTA1 expression in patients with MPLCs and IPMs was compared using repeated measure analysis of variance. The rate of positive MTA1 expression in patients with positive or negative mediastinal lymph nodes was compared using the chi-square test. The median MTA1 score was calculated as the cut-off value and the patients were divided into two groups: high MTA1 expression and low MTA1 expression. One of the multiple lesions of a patient had a score greater than the median score, which was defined as high MTA1 expression. The survival curve was obtained by the Kaplan-Meier method and the Log rank test was used for analysis. A Cox proportional hazards regression model was used to conduct the univariate analysis. The stepwise forward method was used to conduct the multivariate analysis to determine the impact of each variable on DFS. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated and statistical significance was set at p <0.05.
Results
MTA1 Expression in the Tissues of 119 Patients with Multifocal NSCLCs ≤3 cm
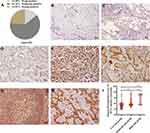
The IRS of MTA1 nuclear expression in 259 lesions of 119 patients ranged from 2.2 to 11.7, with a median value of 5.6. Altogether; 61, 161, and 37 lesions were strongly positive, moderately positive, and weakly positive for MTA1, respectively (Figure 1A). MTA1 was negatively expressed in para-carcinoma lung tissues (Figure 1B) and it was strongly positive, moderately positive and weakly positive in the tumor tissues of patients with multifocal NSCLC ≤3 cm (Figure 1C–E). MTA1 was mainly expressed in the nucleus of cancer tissues, but obvious cytoplasmic expression was observed in mucinous adenocarcinoma (Figure 1F). MTA1 was also positively expressed in lymphoid follicles of lymph nodes without tumor metastasis (Figure 1G). However, MTA1 immunostaining intensity was higher in lymph nodes with tumor metastasis (Figure 1H). The pathological classification of 259 lesions included: 244 adenocarcinomas, 7 squamous cell carcinomas, and 8 other types. 88 of the 244 adenocarcinomas were excluded due to their lepidic, acinar and papillary predominant pathological subtypes with solid or micropapillary components, and the remaining 156 adenocarcinomas were divided into low-risk (n =62), moderate-risk (n =73) and high-risk (n =21) according to the aggressiveness of pathological subtype. We studied the relationship between MTA1 expression and the risk of pathological subtypes of lung adenocarcinoma. The results showed that MTA1 expression was highest in the high-risk group and lowest in the low-risk group (Figure 1I).
MTA1 Expression in Patients with MPLCs and IPMs and Its Correlation with Prognosis
Among the 119 patients included in this group, 25 were N2-positive (N2 lymph node metastasis) and 94 were N2-negative. The rate of strongly positive MTA1 expression was 64% among N2-positive patients, which was significantly higher than that among N2-negative patients (Figure 2A). According to the 2013 ACCP diagnostic recommendations for MPLCs, 52 patients in this study were classified into the MPLC groups, 23 patients were classified into the IPM group, and the remaining 44 were classified as uncertain. 75 patients were clearly classified as MPLC patients or IPM patients, but 14 patients of them with nodules >2 were excluded, we compared MTA1 expression in the remaining 47 cases of double-lesion MPLCs and 14 cases of double-lesion IPMs. Statistical analysis was performed using repeated measures analysis of variance. The results suggested that MTA1 expression in patients with MPLCs was lower than that in patients with IPMs (Figure 2B). The follow-up duration was 4.27–76.53 months and the median follow-up duration was 25.97 months. We also observed that the DFS of the patients with MPLCs was significantly better than that of patients with IPMs (median DFS: 62.70 months vs 13.60 months and 5-year DFS rate: 66.37% vs 12.37%, respectively) (Figure 2C). The 119 patients in this study were divided into the high MTA1 expression group (n =79) and the low MTA1 expression group (n =40). The DFS of patients with high MTA1 expression was significantly worse than that of patients with low MTA1 expression (median DFS: 44.77 months vs 62.70 months and 5-year DFS rate: 18.66% vs 84.62%, respectively) (Figure 2D). Among the 44 patients with uncertain classification, the DFS of patients with high MTA1 expression (n =30) was significantly worse than that of patients with low MTA1 expression (n =14), (median DFS: 33.17 months vs >78 months and 5-years DFS rate: 37.01% vs 83.33%, respectively) (Figure 2E).
Effect of MTA1 Expression and Other Clinicopathological Factors on the DFS of Patients with Multifocal NSCLCs ≤3 cm
Altogether 14 variables were included in the Cox proportional hazards model for the univariate analysis to evaluate the impact on the prognosis of patients with multifocal NSCLCs ≤3 cm. These included: age, gender, whether the area was in Xuanwei, whether the lesions were on the ipsilateral lung, whether the lesions were in the same lung lobe, the number of lesions, whether all lesions were ground glass opacities (GGOs), the sum of maximum diameters of the lesions, mediastinal lymph node metastasis, pleural invasion, surgical resection method, MTA1 expression, severe postoperative complications, and postoperative adjuvant therapy. The results indicated that non-Xuanwei areas (HR: 1.867, 95% CI: 1.057–3.295, p =0.031), non-ipsilateral lung lesions (HR: 3.018, 95% CI: 1.263–7.215, p =0.013), mediastinal lymph node metastasis (HR: 3.058, 95% CI: 1.730–5.405, p <0.001), and high MTA1 expression (HR: 9.091, 95% CI: 2.833–29.412, p <0.001) were significant predictors of worse DFS (Table 2). In contrast, sex, age, whether the same lobe lesions, number of lesions, whether all lesions were GGOs, the sum of maximum diameters of the lesions, pleural invasion, surgical resection method, severe postoperative complications, and postoperative adjuvant therapy exhibited no significant correlation with the DFS of patients.
 |
Table 2 Univariate and Multivariate Analysis of Factors Affecting Disease-Free Survival |
To determine the best combination of parameters for predicting the recurrence of multifocal NSCLCs ≤3 cm, we used the stepwise forward method to perform a multivariate analysis using the Cox proportional hazards model. According to clinical experience, it was generally believed that same lobe lesions, whether all the lesions were GGOs, pleural invasion, surgical resection method and postoperative adjuvant therapy might be the factors affecting postoperative recurrence. Therefore, these five variables were included in the multivariate analysis together with the four variables that had a significant impact on the DFS in the univariate analysis (whether the area was in Xuanwei, whether the lesions were on the ipsilateral lung, mediastinal lymph node metastasis and MTA1 expression). The results indicated that non-ipsilateral lung lesions (HR: 3.445, 95% CI: 1.399–8.483, p =0.007), mediastinal lymph node metastasis (HR: 2.232, 95% CI: 1.253–3.968, p =0.006), and high MTA1 expression (HR: 7.937, 95% CI: 2.433–25.64, p =0.001) were statistically significant predictors of worse DFS in patients with multifocal NSCLCs ≤3 cm (Table 2).
Discussion
Of the 256 lesions in the 119 patients with multifocal NSCLC ≤ 3 cm in the study, 244 were lung adenocarcinoma, which means that most patients with multifocal lung cancer have the same pathological type, which undoubtedly increases the difficulty of correctly classifying these lesions as simultaneous MPLCs or IPMs. Although several studies2,17,18 have reported the value of comprehensive histological evaluation in differentiating simultaneous MPLCs and IPMs, a recent study reported that DNA extracted from tumor tissue for mate-pair sequencing could classify all tumors into lineages and histological evaluation seemed to have misclassified 27% of the same histological tumor types.19 According to the ACCP clinical practice guidelines for multifocal NSCLCs,11 it is difficult to accurately classify multifocal lung cancer as MPLCs or IPMs before surgery. However, surgical resection of the lesions is feasible as long as the patients do not exhibit mediastinal or distant metastases and have sufficient pulmonary functional reserve during the preoperative evaluation. For multifocal NSCLC ≤3 cm, distinguishing MPLCs from IPMs may not change the initial treatment of these patients. However, patients with IPMs require adjuvant chemotherapy and close monitoring programs, while patients with MPLCs may be exempt from adjuvant chemotherapy.20 Importantly, MPLCs have a better overall survival than IPMs.21 Thus, the key objective in multifocal NSCLCs ≤3 cm is the development of operable markers that can assess the prognosis for better guidance regarding postoperative treatment and monitoring plans.
Our results showed different degrees of MTA1 expression in NSCLC tumor tissues. MTA1 expression level in patients with MPLCs was lower than that in patients with IPMS, and the immunostaining intensity of MTA1 in tumor metastatic lymph nodes was higher than that in non-metastatic lymph nodes. Moreover, the rate of strongly positive MTA1 expression in N2-positive patients was significantly higher than that in N2-negative patients. Importantly, the results of both univariate and multivariate analyses suggested that high MTA1 expression was a statistically significant in predictor of worse DFS. Among patients with multifocal NSCLCs ≤3 cm whose classification was uncertain, the DFS of patients with high MTA1 expression was significantly worse than that of patients with low MTA1 expression. These results suggest that MTA1 expression is highly correlated with the DFS of patients with multifocal NSCLCs ≤3 cm. The results also indicate the potential value of MTA1 expression in predicting postoperative recurrence of multifocal NSCLCs ≤3 cm. In the present study, the median DFS of patients with MPLCs was 62.70 months and the 5-year DFS rate was 66.37%. These results were slightly worse than those reported by Li et al,16 indicating that some patients with MPLCs still exhibited recurrence after surgery, which may be related to the degree of tumor differentiation. We analyzed the relationship between MTA1 expression and the risk of pathological subtypes of lung adenocarcinoma. The results indicated that MTA1 expression was highest in the high-risk group and lowest in the low-risk group. Unfortunately, although the overall MTA1 expression level in patients with MPLCs was lower than that in patients with IPMs, we could not determine a perfect cut-off value that could distinguish MPLCs from IPMs. Thus, MTA1 expression can stratify the risk in patients with multifocal NSCLCs ≤3 cm. Moreover, patients with MTA1 immunohistochemical score >5.6 are at a high risk of postoperative recurrence and these patients may benefit from postoperative adjuvant chemotherapy. Further research is needed to confirm these findings.
The results of the multivariate analysis showed that non-ipsilateral lung lesions, mediastinal lymph node metastasis, and high MTA1 expression were statistically significant predictors of worse DFS. Surgical resection method, postoperative adjuvant therapy, whether the lesions were present in the same lung lobe lesions, whether all lesions were GGOs, and pleural invasion were not significantly related to the DFS. Guo et al22 concluded that there was no significant difference between the prognosis of patients with bilateral MPLCs and that of patients with MPLCs located in the ipsilateral lung. Moreover, there was no significant difference in the 5-year overall survival rate between patients with MPLCs located in the same lobe and those with MPLCs in different lobes. A Japanese study23 suggested that the prognosis of patients with bilateral lesions was poor, which was related to the late clinical stage of patients with bilateral lesions. The results of the present study are consistent with these findings. Studies have shown that the prognosis of patients having multifocal disease with all GGO lesions was significantly better than that of patients who had only some GGO lesions.24 In the present study, the DFS was not affected by whether all lesions were GGOs. This discrepancy might be due to the difference in the characteristics of enrolled patients. The present study and a previous French study25 concluded that there was no significant difference in the prognosis between radical resection and local resection in patients with multifocal NSCLCs. This might because the tumor diameter of the included patients was small and most of the patients were screened and treated early. The effect of postoperative adjuvant chemotherapy in patients with MPLCs remains controversial26–28 and it is difficult to accurately classify multifocal NSCLCs with the same pathological type as MPLCs or IPMs. Therefore, more extensive and in-depth clinical studies are needed to evaluate the effect of postoperative adjuvant therapy in patients with multifocal NSCLCs ≤3 cm.
The present study was the first to investigate MTA1 expression and its significance in multifocal NSCLCs. The results indicated that high MTA1 expression was associated with the DFS of patients with multifocal NSCLCs ≤3 cm. Among patients with uncertain classification, the expression level of MTA1 was related to the risk of recurrence, which indicated that MTA1 might have certain guiding significance for postoperative treatment and monitoring of patients with multifocal NSCLCs ≤ 3 cm. However, the present study has the following limitations. (1) The possible bias associated with retrospective study could not be avoided. (2) Immunohistochemical results were considered semi-quantitative. Although two pathologists evaluated the results of immunohistochemical staining independently, the semiquantitative analysis lacked the absolute quantitative accuracy of automated detection. (3) The sample size of the present study was small, especially while comparing MTA1 expression between patients with MPLCs and those with IPMs. Statistical analysis was conducted using repeated measures analysis of variance wherein patients with more than two lesions were excluded and some samples were lost. (4) We tried to define the cut-off value of the level of MTA1 expression according to the median value of MTA1 immunohistochemical IRS score, which was different from previous reports.29,30 If we had considered IRS ≤4 to indicate low MTA1 expression based on previous reports, 61 out of the 259 lesions would have shown low MTA1 expression. However, the number of patients with IRS ≤4 in all lesions was too small.
Conclusions
We used the semiquantitative immunohistochemical scoring of MTA1 expression in 119 patients with multifocal NSCLCs ≤3 cm to provide new insights into exploration of molecular markers for high-risk patients having NSCLCs with multiple lesions ≤3 cm. MTA1 expression was correlated with the risk of pathological subtypes of lung adenocarcinoma. Moreover, MTA1 expression in patients with MPLCs was lower than that in patients with IPMs and high MTA1 expression was statistically significant predictor of worse DFS. In conclusion, MTA1 expression can stratify the risk in patients with multifocal NSCLCs ≤3 cm. Patients with MTA1 immunohistochemical score >5.6 are at a high risk of postoperative recurrence and these patients may benefit from postoperative adjuvant chemotherapy. Further research is needed to confirm these finding.
Abbreviations
NSCLCs, non-small cell lung cancers; MTA1, metastasis-associated protein 1; IRS, immunoreactivity scoring; MPLCs, multiple primary lung cancers; IPMs, Intrapulmonary metastases; DFS, Disease-free survival; AJCC, American Joint Committee on Cancer; ACCP, American College of Chest Physicians; PBS, phosphate buffered saline; HR, hazard radios; CI, confidence intervals; GGOs, ground glass opacities.
Ethical Review Approval
This retrospective study was conducted in accordance with the declaration of Helsinki and was approved by the institutional review committee and ethics committee of the Third Affiliated Hospital of Kunming Medical University (NO.KY2020160). Patient’s consent was waived as data was used in aggregate anonymously.
Consent for Publication
All authors approved the manuscript for publication.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the following funds: National Natural Science Foundation of China (No.81860325); High-level health technical personnel of Yunnan Provincial Health Commission (No. L-2017006);Scientific Research Fund Project of Education Department of Yunnan Province (No. 2019J1282 and No. 2021J0307) and Yunnan Fundamental Research Projects (No.202101AY070001-172).
Disclosure
The authors declare that they have no competing interests.
References
1. Chen C, Huang X, Peng M, Liu W, Yu F, Wang X. Multiple primary lung cancer: a rising challenge. J Thorac Dis. 2019;11(Suppl 4):S523–523S536. doi:10.21037/jtd.2019.01.56
2. Schneider F, Dacic S. Histopathologic and molecular approach to staging of multiple lung nodules. Transl Lung Cancer Res. 2017;6(5):540–549. doi:10.21037/tlcr.2017.06.11
3. Park E, Ahn S, Kim H, et al. Targeted sequencing analysis of pulmonary adenocarcinoma with multiple synchronous ground-glass/lepidic nodules. J Thorac Oncol. 2018;13(11):1776–1783. doi:10.1016/j.jtho.2018.07.097
4. Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol. 2012;25(12):1574–1583. doi:10.1038/modpathol.2012.106
5. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203. doi:10.1016/j.chest.2016.10.010
6. Detterbeck FC, Nicholson AG, Franklin WA, et al. The IASLC lung cancer staging project: summary of proposals for revisions of the classification of lung cancers with multiple pulmonary sites of involvement in the forthcoming eighth edition of the TNM classification. J Thorac Oncol. 2016;11(5):639–650. doi:10.1016/j.jtho.2016.01.024
7. Lee JG, Lee CY, Kim DJ, Chung KY, Park IK. Non-small cell lung cancer with ipsilateral pulmonary metastases: prognosis analysis and staging assessment. Eur J Cardiothorac Surg. 2008;33(3):480–484. doi:10.1016/j.ejcts.2007.12.005
8. Naeli P, Yousefi F, Ghasemi Y, Savardashtaki A, Mirzaei H. The role of MicroRNAs in lung cancer: implications for diagnosis and therapy. Curr Mol Med. 2020;20(2):90–101. doi:10.2174/1566524019666191001113511
9. Amiri A, Pourhanifeh MH, Mirzaei HR, et al. Exosomes and lung cancer: roles in pathophysiology, diagnosis and therapeutic applications. Curr Med Chem. 2021;28(2):308–328. doi:10.2174/0929867327666200204141952
10. Zhu W, Li G, Guo H, et al. Clinicopathological significance of MTA 1 expression in patients with non-small cell lung cancer: a meta-analysis. Asian Pac J Cancer Prev. 2017;18(11):2903–2909. doi:10.22034/APJCP.2017.18.11.2903
11. Kozower BD, Larner JM, Detterbeck FC, Jones DR. Special treatment issues in non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e369S–e399S. doi:10.1378/chest.12-2362
12. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. doi:10.1097/JTO.0000000000000630
13. Westaway DD, Toon CW, Farzin M, et al. The International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society grading system has limited prognostic significance in advanced resected pulmonary adenocarcinoma. Pathology. 2013;45(6):553–558. doi:10.1097/PAT.0b013e32836532ae
14. Warth A, Muley T, Kossakowski C, et al. Prognostic impact and clinicopathological correlations of the cribriform pattern in pulmonary adenocarcinoma. J Thorac Oncol. 2015;10(4):638–644. doi:10.1097/JTO.0000000000000490
15. Zhu X, Guo Y, Li X, Ding Y, Chen L. Metastasis-associated protein 1 nuclear expression is associated with tumor progression and clinical outcome in patients with non-small cell lung cancer. J Thorac Oncol. 2010;5(8):1159–1166. doi:10.1097/JTO.0b013e3181e04d98
16. Li J, Yang X, Xia T, Guan Y, Zhong N. Stage I synchronous multiple primary non-small cell lung cancer: CT findings and the effect of TNM staging with the 7th and 8th editions on prognosis. J Thorac Dis. 2017;9(12):5335–5344. doi:10.21037/jtd.2017.12.101
17. Cheng H, Lei BF, Peng PJ, Lin YJ, Wang XJ. Histologic lung cancer subtype differentiates synchronous multiple primary lung adenocarcinomas from intrapulmonary metastases. J Surg Res. 2017;211:215–222. doi:10.1016/j.jss.2016.11.050
18. Schneider F, Derrick V, Davison JM, Strollo D, Incharoen P, Dacic S. Morphological and molecular approach to synchronous non-small cell lung carcinomas: impact on staging. Mod Pathol. 2016;29(7):735–742. doi:10.1038/modpathol.2016.66
19. Murphy SJ, Harris FR, Kosari F, et al. Using genomics to differentiate multiple primaries from metastatic lung cancer. J Thorac Oncol. 2019;14(9):1567–1582. doi:10.1016/j.jtho.2019.05.008
20. Girard N, Ostrovnaya I, Lau C, et al. Genomic and mutational profiling to assess clonal relationships between multiple non-small cell lung cancers. Clin Cancer Res. 2009;15(16):5184–5190. doi:10.1158/1078-0432.CCR-09-0594
21. Jiang L, He J, Shi X, et al. Prognosis of synchronous and metachronous multiple primary lung cancers: systematic review and meta-analysis. Lung Cancer. 2015;87(3):303–310. doi:10.1016/j.lungcan.2014.12.013
22. Guo H, Mao F, Zhang H, Qiu Y, Shen-Tu Y. [Analysis on the prognostic and survival factors of synchronous multiple primary lung cancer]. Zhongguo Fei Ai Za Zhi. 2017;20(1):21–27. Chinese. doi:10.3779/j.issn.1009-3419.2017.01.03
23. Ishikawa Y, Nakayama H, Ito H, et al. Surgical treatment for synchronous primary lung adenocarcinomas. Ann Thorac Surg. 2014;98(6):1983–1988. doi:10.1016/j.athoracsur.2014.07.006
24. Shimada Y, Saji H, Otani K, et al. Survival of a surgical series of lung cancer patients with synchronous multiple ground-glass opacities, and the management of their residual lesions. Lung Cancer. 2015;88(2):174–180. doi:10.1016/j.lungcan.2015.02.016
25. Trousse D, Barlesi F, Loundou A, et al. Synchronous multiple primary lung cancer: an increasing clinical occurrence requiring multidisciplinary management. J Thorac Cardiovasc Surg. 2007;133(5):1193–1200. doi:10.1016/j.jtcvs.2007.01.012
26. Murphy SJ, Aubry MC, Harris FR, et al. Identification of independent primary tumors and intrapulmonary metastases using DNA rearrangements in non-small-cell lung cancer. J Clin Oncol. 2014;32(36):4050–4058. doi:10.1200/JCO.2014.56.7644
27. Takamochi K, Oh S, Matsuoka J, Suzuki K. Clonality status of multifocal lung adenocarcinomas based on the mutation patterns of EGFR and K-ras. Lung Cancer. 2012;75(3):313–320. doi:10.1016/j.lungcan.2011.08.007
28. Yang H, Sun Y, Yao F, et al. Surgical therapy for bilateral multiple primary lung cancer. Ann Thorac Surg. 2016;101(3):1145–1152. doi:10.1016/j.athoracsur.2015.09.028
29. Shen J, Jin Z, Lv H, et al. PFKP is highly expressed in lung cancer and regulates glucose metabolism. Cell Oncol (Dordr). 2020;43(4):617–629. doi:10.1007/s13402-020-00508-6
30. Yang N, Li C, Han X, Feng Z, Qiu F, Han J. Associations of MTA1 expression with CT features, pathology and prognosis of elderly patients with non-small cell lung cancer. Oncol Lett. 2020;20(5):172. doi:10.3892/ol.2020.12034
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.