Back to Journals » Journal of Inflammation Research » Volume 15
MSC Transplantation Attenuates Inflammation, Prevents Endothelial Damage and Enhances the Angiogenic Potency of Endogenous MSCs in a Model of Pulmonary Arterial Hypertension
Authors Shao F, Liu R, Tan X , Zhang Q, Ye L, Yan B, Zhuang Y, Xu J
Received 7 January 2022
Accepted for publication 18 March 2022
Published 30 March 2022 Volume 2022:15 Pages 2087—2101
DOI https://doi.org/10.2147/JIR.S355479
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Fengjin Shao,1– 3 Rui Liu,1 Xun Tan,1– 4 Qiaoyan Zhang,1– 3 Lujie Ye,1– 3 Bingxuan Yan,1– 3 Ying Zhuang,1,2,4 Jiaxue Xu1,2,4
1Department of Veterinary Medicine, Zhejiang University, Hangzhou, Zhejiang Province, People’s Republic of China; 2Veterinary Medical Center, Zhejiang University, Hangzhou, Zhejiang Province, People’s Republic of China; 3Institute of Preventive Veterinary Sciences, Zhejiang University, Hangzhou, Zhejiang Province, People’s Republic of China; 4Hainan Institute of Zhejiang University, Sanya, Hainan Province, People’s Republic of China
Correspondence: Xun Tan, Department of Veterinary Medicine, Zhejiang University, Yuhangtang Road 866, Hangzhou, 310058, People’s Republic of China, Tel +86 571 8898 2393, Fax +86 571 8898 2310, Email [email protected]
Purpose: Pulmonary arterial hypertension (PAH) is a progressive and fatal pulmonary vascular disease initiated by endothelial dysfunction. Mesenchymal stromal cells (MSCs) have been shown to ameliorate PAH in various rodent models; however, these models do not recapitulate all the histopathological alterations observed in human PAH. Broiler chickens (Gallus gallus) can develop PAH spontaneously with neointimal and plexogenic arteriopathy strikingly similar to that in human patients. Herein, we examined the protective effects of MSC transplantation on the development of PAH in this avian model.
Methods: Mixed-sex broilers at 15 d of age were received 2× 106 MSCs or PBS intravenously. One day later, birds were exposed to cool temperature with excessive salt in their drinking water to induce PAH. Cumulative morbidity from PAH and right-to-left ventricle ratio were recorded. Lung histologic features were evaluated for the presence of endothelial damage, endothelial proliferation and plexiform lesions. Expression of proinflammatory mediators and angiogenic factors in the lung was detected. Matrigel tube formation assay was performed to determine the angiogenic potential of endogenous MSCs.
Results: MSC administration reduced cumulative PAH morbidity and attenuated endothelial damage, plexiform lesions and production of inflammatory mediators in the lungs. No significant difference in the expression of paracrine angiogenic factors including VEGF-A and TGF-β was determined between groups, suggesting that they are not essential for the beneficial effect of MSC transplantation. Interestingly, the endogenous MSCs from birds receiving MSC transplantation demonstrated endothelial differentiatial capacity in vitro whereas those from the mock birds did not.
Conclusion: Our results support the therapeutic use of MSC transplantation for PAH treatment and suggest that exogenous MSCs produce beneficial effects through modulating inflammation and endogenous MSC-mediated vascular repair.
Keywords: plexiform lesion, right ventricular hypertrophy, VEGF-A, TGF-β, angiogenesis, bone marrow
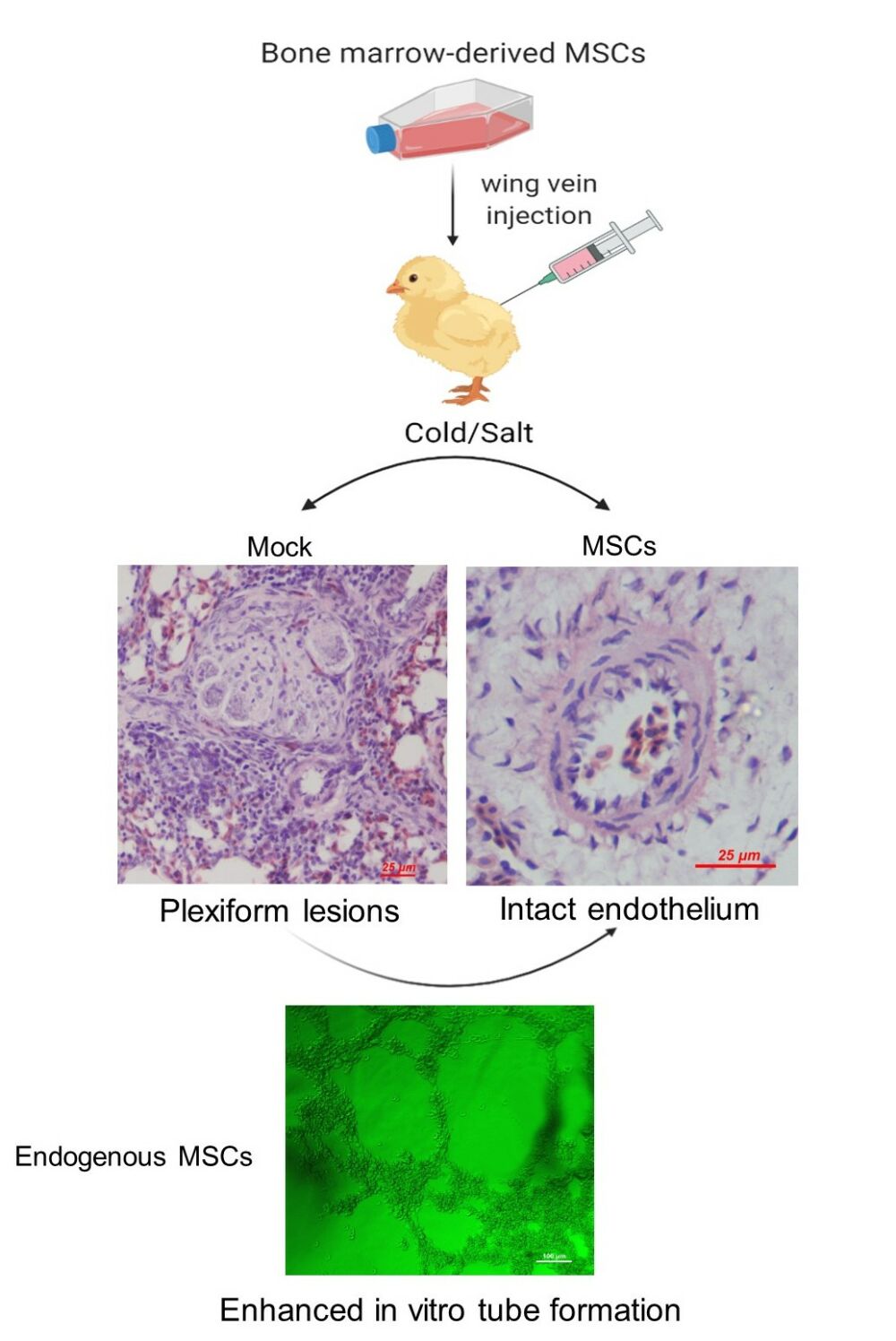
Graphical Abstract:
Introduction
Pulmonary arterial hypertension (PAH) is a devastating disease in humans characterized by sustained elevations in pulmonary vascular pressure, resulting in right heart failure and death. The histological hallmark of PAH is the structural and functional remodeling of small pulmonary arteries.1,2 Disordered angiogenesis causes the formation of glomeruloid structures called plexiform lesions, resulting in complete obliteration of pulmonary arteries.3–5 Although the detailed mechanisms underlying vascular pathology in PAH remain to be elucidated, accumulating evidence suggests that endothelial dysfunction acts as first trigger in driving this process.6
Despite major advances in pharmacological treatments, PAH remains a fatal disease.7 Over the last decades, stem cell-based therapies have attracted great interest in the field of PAH. One of the cell types currently undergoing preclinical trials is the mesenchymal stem cells (MSCs, also known as mesenchymal stromal cells). MSCs are one of the most well-characterized stem cells that can be obtained from various tissues involving bone marrow, peripheral blood and adipose tissue, are easily cultivated, expand extensively in vitro, have intrinsic differentiation potentials, and produce an abundance of bioactive factors mediating beneficial angiogenic effects as well as immunosuppression.8–11 Numerous preclinical studies demonstrated promising therapeutic potential of MSC therapy for PAH; however, the majority of the studies was conducted by using the monocrotaline (MCT) model.12 Notably, this model does not recapitulate all the histopathological alterations observed in human PAH, such as neointimal and plexogenic arteriopathy.13,14 Furthermore, many drugs that have demonstrated efficacy in the MCT models have failed to show clinical benefit in human trial.13,15 Thus, further preclinical data from more clinically-relevant models are needed.
Fast-growing meat-type broiler chickens (Gallus gallus) can spontaneously develop PAH (also known as ascites or pulmonary hypertension syndrome), with an estimated incidence of 3% in all broiler chickens reared under conditions that promote maximal growth. Increased oxygen demand (cold stress), hypobaric hypoxia (high altitude)16 or sodium chloride toxicity17 predisposes the birds to develop PAH. Broilers with PAH exhibit histological features closely resembling that of human idiopathic PAH, including medial hypertrophy, intimal hyperplasia and plexiform lesions in lung vasculature.18–23 We have recently proposed a concept that the formation of plexiform lesions is associated with local immune/inflammatory response resulting from hemodynamic stress.21,24 It is now well established that, like that in humans, pulmonary artery endothelial cell damage plays a major role in the pathogenesis of PAH in broilers.25–27 Thus, broilers offer a particularly useful model for the study of PAH pathogenesis and development of new therapies for PAH.
The aim of the present study was to provide additional information regarding the application of MSCs for PAH treatment by administrating MSCs to broiler chickens with PAH induced by cold stress combined with sodium chloride toxicity. Here, we used the chicken model to investigate the effects of MSC transplantation on PAH. We confirmed that intravenous-infused MSCs reduced PAH incidence and attenuated endothelial damage, plexogenic arteriopathy and inflammation. We demonstrated, for the first time, that MSC transplantation activated endogenous MSCs to differentiation to endothelial cells, which may thus contribute to the beneficial effects produced by MSC transplantation.
Materials and Methods
The animal experiments followed the guidelines for the ethical review of laboratory animal welfare in Zhejiang University and were approved by the Ethics Committee of the Zhejiang University (Approved No. ZJU2015-445-12).
Isolation and Culture of MSCs
MSCs were isolated from the bone marrow of healthy broilers (Ross 308) at 1-week old. In brief, the birds were killed by cervical dislocation and femurs and tibia bones were removed, cleaned of all soft tissues. The bones were soaked in 70% (v/v) ethanol for 10 minutes. Bone marrow cells were extracted as previously described.28 Mononuclear cell (MNC) fraction was enriched by density gradient centrifugation using Ficoll-Paque, density of 1.078 g/mL (Haoyang Biological Manufacture Co., Ltd, Tianjin, China) and cultured in DMEM supplemented with 10% FBS, 100 IU/mL penicillin and 100 μg/mL streptomycin at 39 °C with 5% CO2. After 48 hours, hematopoietic cells and other non-adherent cells were removed. Medium was replaced every 2–3 days. The remaining cells were further expanded in culture until ∽80% confluence. Afterward, the cells were detached with 0.25% trypsin-EDTA (Sigma-Aldrich, Shanghai, China) and replated in other flasks at 1:6 ratios. Cultures up to passage 2 were used for the experiments.
Phenotypical Characterization of MSCs
At the end of passage 2, cells were harvested for evaluating mRNA expression of cell surface markers CD44, CD90, CD105, CD31, CD34 and CD45 by using reverse transcription (RT)-PCR. Briefly, total RNA was extracted from harvested cells with TRIzol reagent (TaKaRa, Dalian, China), and cDNA was synthesized from 1 μg of total RNA using PrimeScript RT Reagent Kit (TaKara, Dalian, China). The cDNA was subjected to PCR amplification as previously described.29 The oligonucleotide primers are presented in Table S1. PCR products were separated by electrophoresis with 1.2% agarose gel and visualized with GoldView (Yeasen Biotechnology, Shanghai, China).
The phenotypic expression of α-SMA and CD133 in MSCs was examined by immunofluorescence. Cells were fixed in ice-cold methanol and stained as previously described.30 Briefly, cells were incubated with either monoclonal mouse anti-human α-SMA (Bostar Bio, Wuhan, China) or polyclonal mouse anti-chicken CD133 (self-prepared) overnight at 4°C, followed by incubation with a FITC-labeled secondary antibody for 60 min at 37°C in dark. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Oil Red O stain was performed to determine adipocyte differentiation using our previously described protocol.31 In brief, cells in 6-well plates were fixed in 10% formalin and washed in 60% isopropanol. Lipid droplets were then visualized by fresh oil red O solution. To characterize osteogenic differentiation, cells were stained by alizarin red S as previously described.31 Orange/red calcific deposits were observed under a microscope.
Cell Proliferation Assay
Cell proliferation was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Briefly, cells at passage 2 were seeded in 96-well plates at an initial density of 100 or 1000 cell per well. At the indicated time points, 20 μL of MTT (5 mg/mL; Sigma-Aldrich, USA) solution was added into each well, followed by incubation of the plates at 39°C in 5% CO2 for 4 h. The supernatant was then discarded and 150 µL of dimethyl sulfoxide (DMSO) was added to each well to dissolve formazan precipitation. The optical density of each well was measured at 570 nm using a microplate reader.
Animal Model and Study Design
Mixed-sex 1-day-old broilers (Ross 308) were purchased from a local commercial hatchery. Birds were fed a commercial corn-soybean meal diet formulated to meet or exceed the NRC (1994) standards for all ingredients. Thermoneutral temperatures were applied to the birds until 15 d of age when the birds were randomly divided into 2 groups (body weight 300–400 g) and received either 2×106 MSCs suspended in 200 μL PBS (MSC group) or PBS alone (mock group) through the wing vein. On d 16, birds were exposed to cool environmental temperatures in combination with excess salt in their drinking water to induce PAH as we have previously described.25 Briefly, when starting on d 16, the brooding temperature was gradually decreased by 1°C per day until a final temperature of 17 °C was reached. Along with the exposure to cold stress, sodium chloride (0.3%, w/v) was given in the drinking water to further accelerate the development of PAH.23 Birds had free access to full feed and water. Bird mortality was recorded daily and necropsies were performed to identify PAH-related death from d 16 onward. Birds that died with a right-to-total ventricle ratio (RV/TV) above 0.25 were included in the PAH mortality.32 Six birds were randomly selected from each group at weekly intervals for 4 weeks after MSC implantation and subjected to the following experiments (Figure 1).
 |
Figure 1 Experimental design. Abbreviation: MSCs, mesenchymal stem cells. |
Lung Histological Analyses
The birds were humanly killed and the lungs were perfused immediately with PBS through right ventricle. Thereafter, the right lungs were removed and cut in the transverse plane at the major rib indentations (costal sulci). One inter-rib division was collected and fixed in 4% (w/v) formaldehyde solution for 24 h. The paraffin-embedded tissues were subsequently serially cut in the transverse plane at 5 to 7 μm thickness and stained with hematoxylin–eosin. Morphological changes of the pulmonary arterioles were observed under an optical microscope. To evaluate endothelial damage, at least 20 arterioles with an external diameter of <50 μm in each slide were photographed, and the histological alterations of vascular endothelium were assessed by an expert. The percentage of the arterioles with normal endothelium was calculated. Plexiform lesions in each slide were also counted and lesion density (number of lesions per section/cm2 per section) was calculated as described previously.24,33
Right-to-Total Ventricle Ratio (RV/TV)
Hearts were removed and carefully dissected. Weights of the free-wall of the right ventricle (RV) and the total ventricle (TV) were measured, and the ratio of RV/TV was calculated as the RV hypertrophy index.34
Real-Time Quantitative PCR (qPCR)
Total RNA was extracted from frozen lung tissue by using RNA-Quick Purification Kit according to the manufacturer’s instructions (Yishan, Shanghai, China). First-strand cDNA was synthesized from 1000 ng total RNA using HiScript II Q RT SuperMix for qPCR with genomic DNA wiper (Vazyme, Nanjing, China). Gene expression was quantified by qPCR using Roche LightCycler 480 II system (Roche Diagnostics GmbH, Mannheim, Germany) with SYBR Green Realtime PCR Master Mix Plus (Vazyme, Nanjing, China). The primer sets used and the amplification program have been described previously.24 The relative expression of the target genes was corrected to reference genes B2M and RPL19 using efficiency corrected method (Pfaffl).
Western Blot
Lung tissue was homogenized in radioimmunoprecipitation assay (RIPA) buffer (Fudebio, Hangzhou, China) containing protease inhibitors (PMSF, Beyotime Technology, Nanjing, China). Protein concentration was quantified by using a bicinchoninic acid (BCA) method (Beyotime Technology, Nanjing, China). For Western blot, the samples were separated by sodium dodecyl sulfate (SDS)-12% polyacrylamide gel electrophoresis (PAGE) and electroblotted onto polyvinylidene difluoride (PVDF) membrane (Millipore, USA). The membranes were subsequently blocked in 5% non-fat milk in TBST and probed with primary antibodies against IL-1β (Abclonal, Wuhan, China), IL-6 (Huabio, Hangzhou, China) and TNF-ɑ (Santa Cruz, Texas, USA) at a final dilution of 1:1000 overnight at 4°C, then immunoblotted with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Fudebio, Hangzhou, China). The immunoreactive bands were visualized by electrochemiluminescent (ECL).
In vitro Tubular Formation Assay
Tubular formation assay was conducted as previously described.21 In brief, Matrigel matrix (BD Biosciences, San Jose, CA, USA) diluted in DMEM (1:1, v/v) was added into 96-well plates and allowed to solidify at 39 °C for 30 min. MSCs suspended in DMEM were plated onto the surface of the matrix at 5×104 cells/well with triplicate. After 4 h cells were visualized by a phase-contrast microscope (Nikon, Japan) and representative fields (×20 magnification) were photographed.
Statistical Analysis
Data are presented as mean ± SEM. PAH morbidity was analyzed using independent chi‐squared test. Normality was assessed for other data sets by Shapiro–Wilk test. RV/TV ratio, plexiform lesion density and the mRNA level of angiogenic factors were analyzed by using non-parametric Mann–Whitney U-test due to non-normal distribution or small sample size (n < 5). Other data were analyzed using an unpaired Student’s t-test. Significance was set at P < 0.05, and all P values are listed. Analysis was performed using the SPSS 22.0 (IBM Corp., Armonk, NY, USA).
Results
Cell Characterization
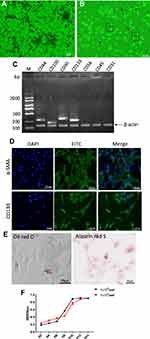
In the present study, MNCs were isolated from chicken bone marrow and cultured in DMEM to obtain MSCs. The MSCs displayed spindle-shaped morphology, gradually grew into small colonies during the initial days of incubation (Figure 2A), and reached ~80% confluence by d 10 after plating. After passage, three subpopulations, ie, triangular or star-like cells, spindle-shaped cells, and large, flattened cells, could be observed (Figure 2B), in line with previous studies on human MSCs.35,36 Cells at Passage 2 were subjected to PCR for the determination of cell surface markers, which demonstrated that the cells were positive for MSC markers (CD44, CD90, and CD105) and negative for hematopoietic surface markers (CD34 and CD45) and hematopoietic/endothelial marker CD3137 (Figure 2C). Almost all of the cells expressed α-SMA and CD133, a typical stem cell marker of mesenchymal origin (Figure 2D). The Alizarin Red and Oil Red O staining results confirmed the potential of the cells to differentiate into the osteogenic lineage and adipocytes (Figure 2E). Growth curves showed active proliferation of these cells (Figure 2F). All these results are inductive of the MSC phenotype of the cultured cells.
Identification of Transplanted MSCs in the Lung
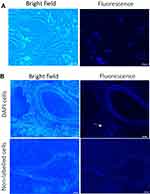
To identify MSCs in the lung after transplantation, MSCs were pre-labeled with 4’,6-diamidino-2-phenylindole (DAPI). Approximately 90% of MSCs showed blue fluorescence after labeling (Figure 3A). One week after intravenous injection, DAPI-positive MSCs were sparsely present in the lung, which were mainly clustered in the parenchyma near to blood vessels (Figure 3B).
Transplantation of MSCs Confers Protection Against PAH
In order to determine whether MSC transplantation has a preventive effect on PAH, we delivered MSCs through wing vein injection to birds 1 d before PAH induction and measured PAH-related morbidity and RV/TV ratio as an indicator of increased pulmonary arterial pressure. Cases of PAH found at processing and cumulative PAH incidence are shown in Table 1. MSC transplantation markedly reduced the cumulative PAH morbidity as compared to mock treatment, coincident with reduced RV/TV ratio at weeks 2 and 3 post transplantation (Figure 4) when a peak incidence in the mock group was observed (Table 1). These results indicate that MSC transplantation prevents the development of PAH.
 |
Table 1 Cases of PAH Found at Processing and Cumulative PAH Incidence |
Transplantation of MSCs Attenuates Inflammatory Response in the Lung
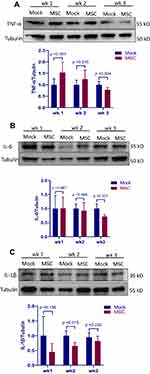
MSC treatment has been shown to attenuate inflammatory response in a MCT-induced PAH rat model.12 To determine if the protective effect of MSCs on the development of PAH is associated with attenuated inflammation in our avian model, production of major inflammatory mediators in the lung tissue was measured by Western blot. As shown in Figure 5, there were no significant differences in TNF-α and IL-6 production between groups during the first 2 weeks post transplantation. However, MSC group had lower TNF-α and IL-6 production than mock group at week 3. MSC transplantation led to a consistent reduction in IL-1β production as compared to mock treatment and a significant difference was observed at week 2. Taken together, the data suggest that MSC transplantation attenuates PAH-induced inflammatory response in the lung.
Transplantation of MSCs Protects the Lung Endothelium from Damage
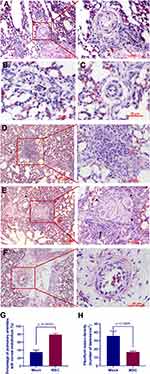
Endothelial cell damage is thought to be the first trigger of PAH.6 Since a peak incidence of PAH in mock group was observed between weeks 2 and 3, lung samples collected at weeks 3 were subjected to histological analyses of endothelial damage. While detachment of endothelial cells from the underlying basement membrane (Figure 6A), intimal thickening (Figure 6B), endothelial cell proliferation (Figure 6C), and plexiform lesions with perivascular inflammatory infiltrates (Figure 6D and E) were frequently observed in the lung of birds in mock group, the majority of the pulmonary vessels in MSC group displayed intact endothelium (Figure 6F and G). Particularly, the density of plexiform lesions in MSC group was significantly lower than that in mock group (Figure 6H).
The Effect of MSC Transplantation on the mRNA Levels of Angiogenic Factors
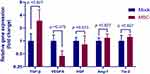
Dysregulation of angiogenic factors has been implicated in the development and progression of PAH in both humans38 and broilers.20 To determine if MSCs exert protective effects by modulating angiogenesis in our avian model of PAH, we next assessed by qPCR the mRNA levels of vascular endothelial growth factor (VEGF), transforming growth factor β (TGF-β), hepatocyte growth factor (HGF), angiopoietin (Ang)-1 and angiopoietin receptor Tie-2 in the lung tissues. As shown in Figure 7, there were no significant differences in the mRNA levels of these factors between groups at weeks 3 post transplantation. However, the birds treated with MSCs exhibited a 2.95-fold increase in TGF-β level in respect to their counterparts. In contrast, and in line with a previous study,39 MSC transplantation led to a downregulation in VEGF mRNA expression (0.44-fold relative to mock control). It is worthy to note that the expression levels of the genes investigated varied extremely between individuals.
Transplantation of MSCs Enhances the Angiogenic Potency of Endogenous MSCs
MSCs are capable of differentiating into endothelial lineage cells upon the stimulation of proangiogenic factors, forming capillary-like network structures on Matrigel matrix.40 We thus performed a Matrigel tube formation assay to evaluate the endothelial differentiation of peripheral blood-derived MSCs from normal, mock- and MSC-treated birds at weeks 3 post PAH induction. While the peripheral MSCs from normal birds exhibited individual round cells on the Matrigel surface with the formation of few cords (Figure 8A), these from mock-treated animals gave rise to clusters (Figure 8B). By contrast, MSCs from birds that received exogenous MSCs developed well-organized tube-like network structures (Figure 8C). It is noteworthy that the cells were not treated with proangiogenic factors. The data suggest that MSC transplantation improves the endothelial differentiation of endogenous MSCs.
Discussion
In the present study, intravenous administration of MSCs efficiently prevented the development of cold temperature/salt-induced PAH in birds, as evidenced by markedly reduced morbidity and right ventricular hypertrophy. This effect was associated with attenuated endothelial damage, plexogenic arteriopathy and local inflammation. In vitro angiogenic assay provided clear evidence that MSC transplantation potentiates the differentiation of endogenous MSCs into endothelial lineage cells, suggesting that endogenous MSCs are actively involved in endothelial repair or regeneration after MSC transplantation. To the best of our knowledge, functional modulation of transplanted MSCs on endogenous MSCs has never been investigated before.
MSCs are first discovered in mammalian bone marrow,41 which is currently the most studied source of MSCs for both experimental and clinical studies. MSCs from chicken bone marrow have also been previously characterized.42 In the present study, the chicken bone marrow-derived MSCs exhibited features consistent with those of mammalian MSCs in terms of morphology, rapid proliferation, adherence to plastic, multilineage differentiation, and immunophenotype.43,44 In this regard, we believed that the bioactive of avian MSCs would be comparable to that of mammals.
PAH in broilers can be induced by either cold temperature22 or excessive salt in drinking water.23 In this work, we challenged the birds with a combination of cold temperature and excessive salt in drinking water. Under this condition, 50% (15/30) of birds in the mock group developed PAH as assessed by RV/TV ratio. In contrast, and as expected, MSC transplantation reduced PAH morbidity by ~44% and delayed the occurrence of PAH by 2 weeks as compared to mock treatment. This finding is in line with a previous study where MSCs have been shown to confer a protective role in both chronic hypoxia- and Sugen5416/hypoxia/normoxia-exposed PAH in rat.45 Since birds with PAH were usually found dead or quickly died in response to capture stress during the experiment, our attempt to conduct MSC transplantation in PAH birds was hampered. Thus, the beneficial effect of MSCs on established PAH was not evaluated.
Accumulating evidence suggests that the efficacy of MSC therapies is mainly attributed to paracrine action.11 MSCs are found to produce a plenty of paracrine factors including angiogenic cytokines such as VEGF-A, HGF, Ang-1, and TGF-β.46 However, it still remains uncertain whether the angiogenic factors play a role in the therapeutic mechanisms of MSCs for PAH, as conflicting results have been reported regarding the production of angiogenic cytokines in the lungs of MCT models after MSC therapies.39,47 After determining the protective effect of MSC transplantation on the progression of PAH in our avian model, we analyzed if the angiogenic factors are involved. Unexpectedly, we did not determine a remarkable difference in the expression of the tested angiogenic factors in the lung tissues between MSC and mock groups. Indeed, the expression of VEGF-A, which is known to play a predominant role in angiogenesis,48 tended to be downregulated in MSC group, which is in line with a previous study demonstrating decreased VEGF-A production in MCT model after MSC therapy.39 Thus, it is likely that the paracrine angiogenic factors are not essential for the protective and therapeutic effects of MSC transplantation. Indeed, it is suggested that the paracrine activity of MSCs for tissue repair is mainly ascribed to their immunomodulatory properties.49 In this work, MSC transplantation reduced the production of proinflammatory factors TNF-α, IL-6 and IL-1β as compared to the mock treatment at different time points, coincident with a moderate increase in the expression of anti-inflammatory cytokine TGF-β. Taken together, our observations suggest that the immunosuppressive cytokines released by the transplanted MSCs might play a more important role than paracrine angiogenic factors in pulmonary repair.
It is generally accepted that endothelial injury resulting in endothelial dysfunction and proliferation plays a central role in the initiation and progression of vascular pathology in PAH including vascular remodeling, inflammation and the formation of plexiform lesions.6,50–52 MSCs have been shown to improve MCT-induced pulmonary endothelial injury.53 Similarly, the present study revealed that MSC transplantation significantly attenuated PAH-induced endothelial abnormalities seen in the mock group, ie, endothelial cell detachment and proliferation. Thus, our findings together with the observations in MCT models suggest that the implanted MSCs induce functional repair or regeneration of endothelial cells.
A growing body of literature suggests that differentiation into vascular cells in the arterial wall is not involved in the endothelial repair mechanisms of the transplanted MSCs, although they were found to be trapped in the lung after intravenous injection or intratracheal administration.45,47,53,54 Indeed, despite extensive research, the origin of the newly developed endothelial cells involved in vascular repair is still controversial and has been a subject of continued interest.55 Considering the fact that MSC population is selectively mobilized from bone marrow in response to hypoxia and endothelial injury,56,57 and that those cells are capable of differentiating into endothelial cells,58 we next determined whether MSC transplantation affect the angiogenic potential of endogenous MSCs. As a result, MSC transplantation significantly potentiated the endothelial differentiation of endogenous MSCs, as indicated by a robust enhancement of in vitro tubular formation of peripheral blood-derived MSCs in birds from MSC group. Thus, our results allow us to argue that the observed effects of MSC transplantation in our animal model are associated with endogenous MSC-mediated endothelial repair or regeneration. No previous studies have been conducted to address the proangiogenic effects of transplanted MSCs on endogenous MSCs. The mechanisms by which the transplanted MSCs regulate endothelial differentiation of endogenous MSCs remain unclear, but might be associated with their paracrine effects. It is also possible that the transplanted MSCs may directly contact and transfer cellular components to endogenous MSCs, thereby enhancing their angiogenic potential. Indeed, a “contact-dependent” mechanism of action by which MSCs ameliorate acute lung injury has been previously proposed.59 Further studies are warranted to determine the exact mechanisms underlying this process.
Some limitations of this work should be considered. Although PAH birds share all the reported histological features observed in human PAH, the plexiform lesions, which are considered as a hallmark of severe PAH in humans,14 can be found in birds without signs of PAH.20,21,33 Nevertheless, we recently provided clear evidence that the development of plexiform lesions in birds is associated with increased pulmonary arterial pressure.24 In addition, we evaluated the angiogenic potential of blood-derived MSCs after MSC transplantation. However, this population of MSCs might differ from the tissue-resident lung MSCs, as that observed between lung-derived and bone marrow-derived MSCs.60 Therefore, further studies should be carried out to determine the beneficial effects of exogenous MSCs on lung-resident MSCs. Finally, in this work, only a single dose of MSCs was performed. Whether two or more doses of MSCs yield enhanced endothelial repair during the development of PAH warrants further research.
Conclusion
In summary, this work demonstrates that MSC transplantation attenuates inflammation and prevents the progression of PAH. We show clear evidence that MSC transplantation potentiates the angiogenic potency of endogenous MSCs, providing a novel insight into the mechanisms accounting for the beneficial effects of MSC transplantation on PAH. Our findings encourage the development of MSC therapy for treating PAH.
Ethical Approval
All the operation was approved by the Ethics Committee of the Zhejiang University (Approved No. ZJU2015-445-12).
Acknowledgments
The study was supported by the National Natural Science Foundation of China (Project No. 31872444 to X.T.) and Zhejiang Provincial Natural Science Foundation of China (Project No. LR12C18001 to X.T.).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Frost A, Badesch D, Gibbs J, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53(1):1801904. doi:10.1183/13993003.01904-2018
2. Tuder RM. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res. 2017;367(3):643–649. doi:10.1007/s00441-016-2539-y
3. Tuder RM, Chacon M, Alger L, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol. 2001;195(3):367–374. doi:10.1002/path.953
4. Jonigk D, Golpon H, Bockmeyer CL, et al. Plexiform lesions in pulmonary arterial hypertension composition, architecture, and microenvironment. Am J Pathol. 2011;179(1):167–179. doi:10.1016/j.ajpath.2011.03.040
5. Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144(2):275–285.
6. Kurakula K, Smolders V, Tura-Ceide O, et al. Endothelial dysfunction in pulmonary hypertension: cause or consequence? BioMedicines. 2021;9(1):57. doi:10.3390/biomedicines9010057
7. Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360:j5492. doi:10.1136/bmj.j5492
8. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi:10.1126/science.276.5309.71
9. Pittenger MF, Discher DE, Peault BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. doi:10.1038/s41536-019-0083-6
10. Maacha S, Sidahmed H, Jacob S, et al. Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int. 2020;2020:4356359. doi:10.1155/2020/4356359
11. Fukumitsu M, Suzuki K. Mesenchymal stem/stromal cell therapy for pulmonary arterial hypertension: comprehensive review of preclinical studies. J Cardiol. 2019;74(4):304–312. doi:10.1016/j.jjcc.2019.04.006
12. Oh S, Jang AY, Chae S, et al. Comparative analysis on the anti-inflammatory/immune effect of mesenchymal stem cell therapy for the treatment of pulmonary arterial hypertension. Sci Rep. 2021;11(1):2012. doi:10.1038/s41598-021-81244-1
13. Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009;297(6):L1013–L1032. doi:10.1152/ajplung.00217.2009
14. St. Croix CC, Steinhorn RH. New thoughts about the origin of plexiform lesions. Am J Respir Crit Care Med. 2016;193(5):484–485. doi:10.1164/rccm.201510-1959ED
15. Hill NS, Gillespie MN, McMurtry IF. Fifty years of monocrotaline-induced pulmonary hypertension: what has it meant to the field? Chest. 2017;152(6):1106–1108. doi:10.1016/j.chest.2017.10.007
16. Baghbanzadeh A, Decuypere E. Ascites syndrome in broilers: physiological and nutritional perspectives. Avian Pathol. 2008;37(2):117–126. doi:10.1080/03079450801902062
17. Xiang RP, Sun WD, Zhang KC, et al. Sodium chloride-induced acute and chronic pulmonary hypertension syndrome in broiler chickens. Poult Sci. 2004;83(5):732–736. doi:10.1093/ps/83.5.732
18. Kluess HA, Stafford J, Evanson KW, et al. Intrapulmonary arteries respond to serotonin and adenosine triphosphate in broiler chickens susceptible to idiopathic pulmonary arterial hypertension. Poult Sci. 2012;91(6):1432–1440. doi:10.3382/ps.2011-01919
19. Wideman RJ, Hamal KR. Idiopathic pulmonary arterial hypertension: an avian model for plexogenic arteriopathy and serotonergic vasoconstriction. J Pharmacol Toxicol Methods. 2011;63(3):283–295. doi:10.1016/j.vascn.2011.01.002
20. Hamal KR, Erf GF, Anthony NB, Wideman RF. Immunohistochemical examination of plexiform-like complex vascular lesions in the lungs of broiler chickens selected for susceptibility to idiopathic pulmonary arterial hypertension. Avian Pathol. 2012;41(2):211–219. doi:10.1080/03079457.2012.663077
21. Tan X, Juan FG, Shah AQ. Involvement of endothelial progenitor cells in the formation of plexiform lesions in broiler chickens: possible role of local immune/inflammatory response. J Zhejiang Univ Sci B. 2017;18(1):59–69. doi:10.1631/jzus.B1600500
22. Tan X, Chai J, Bi SC, et al. Involvement of matrix metalloproteinase-2 in medial hypertrophy of pulmonary arterioles in broiler chickens with pulmonary arterial hypertension. Vet J. 2012;193(2):420–425. doi:10.1016/j.tvjl.2012.01.017
23. Shao FJ, Ying YT, Tan X, Zhang QY, Liao WT. Metabonomics profiling reveals biochemical pathways associated with pulmonary arterial hypertension in broiler chickens. J Proteome Res. 2018;17(10):3445–3453. doi:10.1021/acs.jproteome.8b00316
24. Tan X, Shao FJ, Fan GJ, Ying YT. Expression of angiogenic factors and plexiform lesions in the lungs of broiler and layer chickens: a comparison. Poult Sci. 2018;97(5):1526–1535. doi:10.3382/ps/pey008
25. Tan X, Pan JQ, Li JC, et al. L-arginine inhibiting pulmonary vascular remodelling is associated with promotion of apoptosis in pulmonary arterioles smooth muscle cells in broilers. Res Vet Sci. 2005;79(3):203–209. doi:10.1016/j.rvsc.2004.12.004
26. Pan JQ, Li JC, Tan X, et al. The injury effect of oxygen free radicals in vitro on cultured pulmonary artery endothelial cells from broilers. Res Vet Sci. 2007;82(3):382–387. doi:10.1016/j.rvsc.2006.08.001
27. Bautista-Ortega J, Stallone JN, Ruiz-Feria CA. Effects of arginine and antioxidant vitamins on pulmonary artery reactivity to phenylephrine in the broiler chicken. Poult Sci. 2013;92(4):1062–1072. doi:10.3382/ps.2012-02472
28. Khatri M, O’Brien TD, Sharma JM. Isolation and differentiation of chicken mesenchymal stem cells from bone marrow. Stem Cells Dev. 2009;18(10):1485–1492. doi:10.1089/scd.2008.0223
29. Bi S, Tan X, Ali SQ, Wei L. Isolation and characterization of peripheral blood-derived endothelial progenitor cells from broiler chickens. Vet J. 2014;202(2):396–399. doi:10.1016/j.tvjl.2014.08.017
30. Karaoz E, Aksoy A, Ayhan S, et al. Characterization of mesenchymal stem cells from rat bone marrow: ultrastructural properties, differentiation potential and immunophenotypic markers. Histochem Cell Biol. 2009;132(5):533–546. doi:10.1007/s00418-009-0629-6
31. Shah QA, Tan X, Bi S, Liu X, Hu S. Differential characteristics and in vitro angiogenesis of bone marrow- and peripheral blood-derived endothelial progenitor cells: evidence from avian species. Cell Prolif. 2014;47(4):290–298. doi:10.1111/cpr.12111
32. Julian RJ, McMillan I, Quinton M. The effect of cold and dietary energy on right ventricular hypertrophy, right ventricular failure and ascites in meat-type chickens. Avian Pathol. 1989;18(4):675–684. doi:10.1080/03079458908418641
33. Wideman RF, Hamal KR, Bayona MT, et al. Plexiform lesions in the lungs of domestic fowl selected for susceptibility to pulmonary arterial hypertension: incidence and histology. Anat Rec. 2011;294(5):739–755. doi:10.1002/ar.21369
34. Tan X, Hu SH, Wang XL. The effect of dietary l-carnitine supplementation on pulmonary hypertension syndrome mortality in broilers exposed to low temperatures. J Anim Physiol Anim Nutr (Berl). 2008;92(2):203–210. doi:10.1111/j.1439-0396.2007.00727.x
35. Nancarrow-Lei R, Mafi P, Mafi R, Khan W. A systemic review of adult mesenchymal stem cell sources and their multilineage differentiation potential relevant to musculoskeletal tissue repair and regeneration. Curr Stem Cell Res Ther. 2017;12(8):601–610. doi:10.2174/1574888X12666170608124303
36. Haasters F, Prall WC, Anz D, et al. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J Anat. 2009;214(5):759–767. doi:10.1111/j.1469-7580.2009.01065.x
37. Roubelakis MG, Tsaknakis G, Pappa KI, Anagnou NP, Watt SM. Spindle shaped human mesenchymal stem/stromal cells from amniotic fluid promote neovascularization. PLoS One. 2013;8(1):e54747. doi:10.1371/journal.pone.0054747
38. Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8(8):443–455. doi:10.1038/nrcardio.2011.87
39. de Mendonca L, Felix NS, Blanco NG, et al. Mesenchymal stromal cell therapy reduces lung inflammation and vascular remodeling and improves hemodynamics in experimental pulmonary arterial hypertension. Stem Cell Res Ther. 2017;8(1):220. doi:10.1186/s13287-017-0669-0
40. Wang C, Li Y, Yang M, et al. Efficient differentiation of bone marrow mesenchymal stem cells into endothelial cells in vitro. Eur J Vasc Endovasc Surg. 2018;55(2):257–265. doi:10.1016/j.ejvs.2017.10.012
41. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of Guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi:10.1111/j.1365-2184.1970.tb00347.x
42. Bai C, Hou L, Ma Y, et al. Isolation and characterization of mesenchymal stem cells from chicken bone marrow. Cell Tissue Bank. 2013;14(3):437–451. doi:10.1007/s10561-012-9347-8
43. Lotfy A, El-Sherbiny YM, Cuthbert R, Jones E, Badawy A. Comparative study of biological characteristics of mesenchymal stem cells isolated from mouse bone marrow and peripheral blood. Biomed Rep. 2019;11(4):165–170. doi:10.3892/br.2019.1236
44. Muniz C, Teodosio C, Mayado A, et al. Ex vivo identification and characterization of a population of cd13(high) cd105(+) cd45(-) mesenchymal stem cells in human bone marrow. Stem Cell Res Ther. 2015;6:169. doi:10.1186/s13287-015-0152-8
45. Huang J, Lu W, Ouyang H, et al. Transplantation of mesenchymal stem cells attenuates pulmonary hypertension by normalizing the endothelial-to-mesenchymal transition. Am J Respir Cell Mol Biol. 2020;62(1):49–60. doi:10.1165/rcmb.2018-0165OC
46. Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017;26(9):617–631. doi:10.1089/scd.2016.0349
47. Zhang Y, Liao S, Yang M, et al. Improved cell survival and paracrine capacity of human embryonic stem cell-derived mesenchymal stem cells promote therapeutic potential for pulmonary arterial hypertension. Cell Transplant. 2012;21(10):2225–2239. doi:10.3727/096368912X653020
48. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi:10.1038/nm0603-669
49. Mokhber DM, Jabbari FM, Sadeghian CS, et al. Intrapulmonary autologous transplant of bone marrow-derived mesenchymal stromal cells improves lipopolysaccharide-induced acute respiratory distress syndrome in rabbit. Crit Care. 2018;22(1):353. doi:10.1186/s13054-018-2272-x
50. Mathew R. Pathogenesis of pulmonary hypertension: a case for caveolin-1 and cell membrane integrity. Am J Physiol Heart Circ Physiol. 2014;306(1):H15–H25. doi:10.1152/ajpheart.00266.2013
51. Yang JX, Pan YY, Zhao YY, Wang XX. Endothelial progenitor cell-based therapy for pulmonary arterial hypertension. Cell Transplant. 2013;22(8):1325–1336. doi:10.3727/096368912X659899
52. Ghouleh IA, Sahoo S, Meijles DN, et al. Endothelial nox1 oxidase assembly in human pulmonary arterial hypertension; Driver of gremlin1-mediated proliferation. Clin Sci (Lond). 2017;131(15):2019–2035. doi:10.1042/CS20160812
53. Baber SR, Deng W, Master RG, et al. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292(2):H1120–H1128. doi:10.1152/ajpheart.00173.2006
54. Ionescu L, Byrne RN, van Haaften T, et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303(11):L967–L977. doi:10.1152/ajplung.00144.2011
55. Tang J, Zhu H, Liu S, et al. Sca1 marks a reserve endothelial progenitor population that preferentially expand after injury. Cell Discov. 2021;7(1):88. doi:10.1038/s41421-021-00303-z
56. Rochefort GY, Delorme B, Lopez A, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24(10):2202–2208. doi:10.1634/stemcells.2006-0164
57. Patry C, Doniga T, Lenz F, et al. Increased mobilization of mesenchymal stem cells in patients with acute respiratory distress syndrome undergoing extracorporeal membrane oxygenation. PLoS One. 2020;15(1):e227460. doi:10.1371/journal.pone.0227460
58. Wang C, Liu H, Yang M, et al. RNA-seq based transcriptome analysis of endothelial differentiation of bone marrow mesenchymal stem cells. Eur J Vasc Endovasc Surg. 2020;59(5):834–842. doi:10.1016/j.ejvs.2019.11.003
59. Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–765. doi:10.1038/nm.2736
60. Rolandsson ES, Andersson SA, Skog I, et al. MSC from fetal and adult lungs possess lung-specific properties compared to bone marrow-derived MSC. Sci Rep. 2016;6:29160. doi:10.1038/srep29160
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.