Back to Journals » Clinical Ophthalmology » Volume 16
Morphological and Functional Assessment of the Optic Nerve Head and Retinal Ganglion Cells in Dry vs Chronically Treated Wet Age-Related Macular Degeneration
Authors Wichrowska M , Wichrowski P , Kocięcki J
Received 10 May 2022
Accepted for publication 4 July 2022
Published 28 July 2022 Volume 2022:16 Pages 2373—2384
DOI https://doi.org/10.2147/OPTH.S372626
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Małgorzata Wichrowska,1,2 Przemysław Wichrowski,1,3 Jarosław Kocięcki1
1Department of Ophthalmology, Poznan University of Medical Sciences, Poznań, Poland; 2Doctoral School, Poznan University of Medical Sciences, Poznań, Poland; 3I Clinic of Anesthesiology and Intensive Therapy, Poznan University of Medical Sciences, Poznań, Poland
Correspondence: Małgorzata Wichrowska, Department of Ophthalmology, Poznan University of Medical Sciences, Ul. Szamarzewskiego 84, Poznań, 60-569, Poland, Tel +48 61 101 98 00, Fax +48 61 101 98 13, Email [email protected]
Purpose: The aim of the study comprised the anatomical and functional assessment of the optic nerves and macular ganglion cells in the population of patients with unilateral wet age-related macular degeneration (wAMD) and comparison of its results to those obtained from the fellow eye with non-advanced dry age-related macular degeneration (dAMD). Furthermore, we aimed to determine if the number of administered injections contributed to the potential differences between the examined eyes.
Methods: The study was based on 104 eyes of 52 patients in a cross-sectional study. The eyes with chronically treated wAMD were the main subject of the study, while fellow eyes affected with non-advanced dAMD served as the control group. Primary morphological outcomes comprised differences in peripapillary retinal nerve fiber layer (RNFL) and GCC layers between the studied groups (treated and control). In turn, primary functional outcomes included differences in implicit times and amplitudes of the P100 wave of pattern visual evoked potentials (PVEP) and N95 wave of pattern electroretinogram (PERG) between treated and control groups.
Results: We did not find any differences in total RNFL thickness between wAMD and dAMD groups. The number of injections only affected the RNFL in the nasal quadrant of the optic disc (p = 0.023). We did not find any differences regarding GCL thickness in both groups. In the study group, a longer implicit time of the P100 of PVEP (p = 0.014) and a shorter amplitude of the P50-N95 wave of PERG (p = 0.005) were detected. The total number of injections had no significant effect on these two values.
Conclusion: We detected worse functional parameters of the optic nerve and retinal ganglion cells in eyes with wAMD, with lack of significant differences in anatomical (RNFL, GCL) parameters compared to the control group. However, the number of injections did not contribute to the differences found.
Keywords: age-related macular degeneration, anti-VEGF, retinal nerve fiber layer, RNFL, PVEP, PERG
Introduction
Due to the aging of the worldwide population, age-related macular degeneration (AMD) appears to be a problem of growing significance. According to the World Health Organisation, one-sixth of the people in the world will be at least 60 years old by 2030. Among those people, 243.4 million will be suffering from age-related macular degeneration.1,2 Unfortunately, we still lack effective treatment methods for the dry form of this disease (dAMD), characterized by macular pathologies such as drusen, retinal pigment epithelium abnormalities (hipo- or hyperpigmentation), or atrophy. Nonetheless, modern ophthalmology managed to make a significant breakthrough in the treatment of wet or exudative AMD (wAMD). As macular neovascularization is a characteristic component of this disease, introduction of intravitreal anti-VEGF injections allows many patients to maintain functional visual acuity for many years after disease onset. Due to their effectiveness, intravitreal anti-VEGFs became a method of treatment widely applied by ophthalmologists worldwide. Among the generally known drugs, ranibizumab, aflibercept, bevacizumab (as an off-label therapy), and recently brolucizumab, are currently used to treat this disease in Poland. While the effectiveness of these medications in the treatment of macular disease has been confirmed in numerous studies and publications, there are still discussions about their effects on other structures of the eyeball, including the optic nerve.3
The literature mentions three mechanisms through which the optic nerve could be susceptible to damage resulting from intravitreal anti-VEGF therapy. The first is an increase in intraocular pressure induced by the injection of additional liquid volume (50 µL- 0.05 mL) into the vitreous chamber.4,5 It is also believed that the increase in pressure can be caused by damage to the trabecular meshwork, both through mechanical blockage of aqueous humor outflow by microparticles derived from drug contaminants, and direct induction of trabecular inflammation.4
As VEGF activates endothelial nitric oxide synthase, anti-VEGF therapy may theoretically reduce nitric oxide (NO) levels, which play a crucial role in vasodilatation. Thus, the second potential optic nerve damage mechanism indicates impaired perfusion in the optic nerve head as a direct pharmacological effect of anti-VEGF drugs, unrelated to increased intraocular pressure.6,7
Finally, the third mechanism is directly related to the deactivation of the endogenous action of vascular endothelial growth factor (VEGF), and hence the prevention of its neuroprotective and anti-apoptotic activity.8–11
Nerve damage may be associated with conduction disturbances and nerve fiber layer thinning. Diagnostic tests used in ophthalmology allow us to perform anatomical and functional assessments of the optic nerve. Optical coherence tomography (OCT) seems especially rewarding, enabling anatomical assessment of both retina and optic disc. Electrophysiological examinations, including pattern visual evoked potentials (PVEP), with proper recording electrode placement (The ISCEV standard VEP protocols), allow to objectively assess conduction within the anterior segment of the visual pathway, including the optic nerve, indirectly defining its functional status.12–15 For this purpose, we evaluated the implicit time (measured in milliseconds, ms) and amplitude (measured in microvolts, µV) of the positive VEP deflection (P100). We also analyzed the pattern-generated electroretinogram (PERG), particularly the implicit time (ms) and amplitude (µV) of N95 (measured from the peak of P50 to the maximum N95 deflection). This examination allowed for selective assessment of the retinal ganglion cell function.13–19 The aim of the study comprised anatomical and functional assessment of the optic nerves and retinal ganglion cells. Furthermore, we aimed to evaluate whether there are significant differences in the assessed parameters between the eyes with wAMD and non-advanced dAMD, with the latter not subjected to treatment. Additionally, a secondary aim was to examine if the number of intravitreal injections given contributed to the possible differences.
In the literature, assumed nerve injury is examined mainly through morphological evaluation using optical coherence tomography imaging.5,20–26 According to our knowledge, this work is the first to assess the functioning of the nerve and retinal ganglion cells (which are histologically continuous with the nerve fibers), using electrophysiological PVEP and PERG examinations, in a population of patients undergoing chronic wAMD therapy with intravitreal anti-VEGFs.
Materials and Methods
Patients
We selected a group of patients with unilateral wAMD at various stages of the treatment of the disease (both treatment-naive and already treated with intravitreal injections; with previous treatment being accounted for when considering the number of injections) and with dAMD in the fellow eye (but no end-stage dAMD such as geographic atrophy, as the patient needed to be able to fixate with each eye- The patient had to confirm that he could see the fixation mark) from all patients treated for wAMD at the Ophthalmology Clinic of the Medical University of Poznań in the period from November 2020 to January 2022. We excluded those with macular comorbidities such as vitreoretinal traction, macular holes (both full or lamellar), epiretinal membrane, any stage of diabetic retinopathy, history of any optic nerve disease, including a history of glaucoma or ocular hypertension, and glaucoma development factors such as pseudo-exfoliating lens epithelial syndrome (PEX). Moreover, patients who underwent intraocular surgery other than uncomplicated cataract surgery at least six months before study enrollment, and those who did not consent to participate in the study, were also excluded. Inclusion criteria also comprised a spherical equivalent of −4.00 to +4.50 diopters, no disease other than drug-controlled hypertension, and no history of ischemic events in the central nervous system. Ultimately, 31 females (mean age 73.23; SD 7.60) and 21 males (mean age 71.71; SD 7.13) were enrolled in the study. All patients signed an informed consent form. The study protocol was approved by the Bioethics Committee of the Medical University of Karol Marcinkowski in Poznań (resolution No. 560/21 of June 24, 2021) and followed the tenets of the Declaration of Helsinki.
Morphological Assessment
Each participant underwent a comprehensive ophthalmological examination, including an assessment of visual acuity using the Snellen chart and dilated fundus (induced by 1% tropicamide) slit-lamp examination. Macular and optic nerve head optical coherence tomography (OCT) scans were performed using a TOPCON DRI OCT Triton (Version 10.16.003.02). The 3D Macula (H) 7.0x 7.0 mm macular protocol was used to evaluate ganglion cell layer thickness (GCL), with automated division into individual layers of the retina performed by the device software. Peripapillary retinal nerve fiber layer (RNFL) thickness was assessed using the 3D Disc 6.0 x 6.0 mm protocol, which allowed to obtain an average of the total and each quadrant RNFL thickness of the optic disc.
The quality of each performed macular and optic disc scan, ie TopQ Image Quality index above 55 and lack of motion artifacts, was personally verified by the leading author (MW).
Functional Assessment
Electrophysiological examinations were performed on a separate visit using the RETI-port (Roland Consult) system (V1021.3.0.SBC03.05). The test was conducted following the International Society for Clinical Electrophysiology of Vision (ISCEV) guidelines. Patients were awaiting examination in normally illuminated room without dark adaptation. Tests were performed under semi-dim lighting (we avoided strong light sources in the examination room) and without dilation of the pupils, with appropriate optical correction for a viewing distance of one meter from the screen. To assess visual evoked potentials, separate stimulation for each eye with a full-field pattern of a black and white checkerboard (PVEP), with an angular size of one side of the square 1.0° = 60’ and 1,53,872 pattern reversals per second (rps), was used. The contrast between black and white squares was 97%, with 80 cd/m2 luminance for the white elements. Gold cup skin electrodes were positioned according to the international 10/20 system: active midline occipital (Oz), mid-frontal reference (Fz), and vertex (Cz) ground electrodes were used.
To obtain pattern electroretinogram (PERG), the examination was performed simultaneously for both eyes using a full-field checkboard pattern stimulus at 4,28,643 rps, under the same conditions as PVEP. Sterile fiber ERG thread electrode (Roland Consult RC-1000-510-420) was embedded near the free margin of the lower eyelid, in contact with the bulbar conjunctiva of each eye. In turn, reference skin electrodes were attached near each eye’s outer canthus, and one ground skin electrode was affixed on the forehead (Fpz). Two recorded waveforms for each eye were offline analyzed for repeatability, proper automatic cursor placement was ensured (if not, manual correction by the author was performed), and the results of the two evaluated parameters (implicit time and amplitude of N95) were averaged.
Statistical Analysis
Statistical analyses were conducted on Windows 10 Pro 64 (build 19044), using the R programming language (version 4.1.1; R Core Team, 2021), with report (version 0.5.1; Makowski et al 2020), psych (version 2.1.6; Revelle, 2021), effectsize (version 0.6.0.1; Ben-Shachar et al 2020), and base (R Core Team, 2021) packages. The statistical significance level of statistical test results was assumed at α=0.05.
For the variables on the interval scale, description of the studied set and drawing basic conclusions and generalizations about the samples, grouped descriptive statistics were used. To do this, we employed the built-in describeBy () method of the {psych} package.
Additionally, the normality of data distribution was evaluated using the Shapiro–Wilk test, with an indication of the significance of p (the shapiro.test () method of the {stats} package was used). In addition, the selection of statistical tests considered the measures of shape and symmetry of the distribution. When the threshold value was exceeded (2.0 for skewness or/and 7.0 for kurtosis), distribution was considered different than normal.
Differences between the means of two related groups with nonnormal distributions were examined using the Wilcoxon rank sum test for the correlated data. The rank biserial correlation coefficient was calculated as effect size. The effect size was interpreted according to the convention of Funder (Funder, 2019).
Differences between the means of two related groups of normal distribution were examined using the t-test for paired data with Hedges g effect size estimation. Effect size was interpreted according to the convention of Cohen (Cohen, 1988).
Analysis of multivariate effects on the dependent variable was examined through a linear regression model. Since BCVA may indirectly indicate the degree of maculopathy, which may have a secondary effect on the measured anatomical parameters, we also included this parameter into regression modeling.
Results
Statistical Description of Demographic Data (BCVA, ie, the Best-Corrected Visual Acuity; S.E., ie, the Spherical Equivalent of the Refractive Error, the Number of Injections in Total and Broken Down into Individual Drugs (Aflibercept, Ranibizumab, Bevacizumab))
The descriptive statistics describing visual acuity and the total number of injections are presented in Table 1.
The Shapiro–Wilk test only showed a normal distribution for the S.E. variable (W = 0.99, p = 0.346). The distributions of the rest of the variables presented in Table 1 were non-normal (p < 0.05).
STAGE 1 - Optic Nerve
Anatomical Assessment
The multivariate linear regression model results for RNFL (retinal nerve fiber layer) total, superior, nasal, temporal, and inferior dependent variables were presented in Tables 2–6, respectively.
 |
Table 2 The Coefficients of a Multivariate Linear Regression Model for the RNFL Total Dependent Variable |
 |
Table 3 The Coefficients of a Multivariate Linear Regression Model for the RNFL Superior Dependent Variable |
 |
Table 4 The Coefficients of a Multivariate Linear Regression Model for the RNFL Temporal Dependent Variable |
 |
Table 5 The Coefficients of a Multivariate Linear Regression Model for the RNFL Inferior Dependent Variable |
 |
Table 6 The Coefficients of a Multivariate Linear Regression Model for the RNFL Nasal Dependent Variable |
Table 2 shows that the intercept of the model corresponding to the female sex, control group and the remaining predictors with zero values was 139.24 (95% CI [114.49, 163.99], t(97) = 11.17, p < 0.001).
In the study group, the value of the dependent variable was 6.5 RNFL units lower than in the control group. However, the difference did not reach statistical significance (p = 0.461). The only factor that had a positive effect on the dependent variable was S.E. (+2.74 on the RNFL total for each additional S.E. unit).
Other factors that decreased the dependent variable were BCVA (−1277 on the RNFL total for a 0.1 increase in BCVA) and patient age (−0.34 on the RNFL total for each additional patient-year).
The total number of anti-VEGF injections had no significant effect on the dependent variable. The same was true for the predictor gender, whose effect on total RNFL was only evident at the trend level (0.05 < p ≤ 0.10).
Table 3 shows that neither the group factor nor the total number of anti-VEGF injections had a significant effect on the dependent variable. The positive impact was demonstrated for S.E. (+3.96 RNFL superior for each additional S.E.unit). In turn, patient age negatively affected the dependent variable (−0.44 for each patient’s year).
Table 4 shows that neither the group factor nor the total number of anti-VEGF injections had a significant effect on the dependent variable. The BCVA factor significantly affected the dependent variable (−17.29 units of RNFL temporal for each additional BCVA unit). In men, RNFL temporal was significantly lower (−6.16 units) than in women.
Table 5 shows that neither the group factor nor the total number of anti-VEGF injections had a significant effect on the dependent variable. Only S.E. significantly affected the dependent variable (+3.25 RNFL inferior for each additional S.E.unit).
The data from Table 6 demonstrate a significant effect of the total number of anti-VEGF injections on the dependent variable (+0.536 RFNL nasal for each additional injection). Group factor, BCVA, patient age and gender exhibited no significant effects on RNFL nasal.
Functional Assessment
A two-factor linear regression model was used to estimate the significance of the impact of the group and number of injections on PVEP P100 implicit time factor (mean) and PVEP N75-P100 (mean) dependent variables, with the results reported in Tables 7 and 8.
 |
Table 7 The Coefficients of a Two-Factor Linear Regression Model with the PVEP P100 Implicit Time Factor (Mean) Dependent Variable |
 |
Table 8 The Coefficients of a Two-Factor Linear Regression Model for the PVEP P100 Amplitude Factor (Mean) Dependent Variable |
In Table 7, it can be seen that the group had a significant impact on the dependent variable. The positive effect indicated that the study group was characterized by PVEP P100 implicit time factor (mean) 10.06 units higher than the control group (provided that the other factors remained unchanged). However, no significant influence of the number of injections on the dependent variable was demonstrated.
Table 8 shows that neither group nor the total number of anti-VEGF injections had a significant effect on the PVEP P100 amplitude factor (mean).
STAGE 2 – Retinal Ganglion Cells
Anatomical Assessment
GCL Thickness
The study of differences in mean GCL variables between the study and control correlated groups showed no significance with small effect size: tStudent(51) = 1.55, p = 0.127, gHedges = 0.21.
Adding additional factors (age, gender, and total number of anti-VEGF injections), we can see that none of them had a statistically significant effect (p > 0.05) on ganglion cell layer (GCL) thickness (Table 9).
 |
Table 9 The Coefficients of a Three-Factor Linear Regression Model for the Ganglion Cell Layer (GCL) Dependent Variable |
Functional Assessment
The Shapiro–Wilk test demonstrated that the distribution of PERG N95 implicit time averages and PERG P50-N95 amplitude [averages] differed from normality.
PERG N95 Implicit Time
The Wilcoxon rank sum test for the correlated data showed no significant difference in mean measures for the PERG N95 implicit time variable between the treatment group (Mdn = 93.25, IQR = 15.39) and the control group (Mdn = 92.65, IQR = 10.94) with very small effect size, VWilcoxon = 704, p = 0.895, 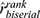 = 0.02; CI95% [−0.28; 0.32], nobs = 52.
= 0.02; CI95% [−0.28; 0.32], nobs = 52.
The total number of anti-VEGF injections had no significant effect (p > 0.05) on the PERG N95 implicit time in both the two- (see Table 10) and one-factor linear model (see Table 11).
 |
Table 10 The Coefficients of a Two-Factor Linear Regression Model for the PERG N95 Implicit Time (Mean) Dependent Variable |
 |
Table 11 The Coefficients of a One-Factor Linear Regression Model for the PERG N95 Implicit Time Factor (Mean) Dependent Variable |
PERG P50-N95 Amplitude
The Wilcoxon rank sum test for correlated data showed a significant difference in mean measures for PERG P50-N95 amplitude between the study group (Mdn = 1.64, IQR = 1.57) and the control group (Mdn = 2.56, IQR = 1.56) with a very large effect size, VWilcoxon= 1000, p = 0.004, 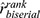 = 0.45, CI95% [0.18; 0.67], nobs = 52.
= 0.45, CI95% [0.18; 0.67], nobs = 52.
Again, the total number of anti-VEGF injections had no significant effect (p > 0.05) on the PERG P50-N95 amplitude for both the two- (see Table 12) and one-factor linear model (see Table 13).
 |
Table 12 The Coefficients of a Two-Factor Linear Regression Model for the PERG P50-N95 Amplitude Factor (Mean) Dependent Variable |
 |
Table 13 The Coefficients of a One-Factor Linear Regression Model for the PERG P50-N95 Amplitude Factor (Mean) Dependent Variable |
Discussion
The main aim of our study was to determine the morphological and functional differences in retinal nerve fibers and retinal ganglion cells between eyes with wAMD and non-advanced dAMD. The second purpose was to detect if the number of injections given contributed to these outcomes.
Morphological Outcomes
Although RNFL total was thinner by 6.16 µm compared to the control group in our study, the result did not reach statistical significance (p = 0.461). Interestingly, the number of injections only affected the RNFL in the nasal quadrant of the optic disc (an increase of the RNFL by 0.536 um for each additional injection, p = 0.023).
The findings from similar studies vary. Sobacı et al and El Ashry et al also declared no statistically significant changes in RNFL thickness during anti-VEGF wAMD treatment.5,11 On the contrary, Yau et al demonstrated a significantly greater mean RNFL in chronically treated eyes with wAMD compared to the fellow eyes with dAMD.20 Whereas, the loss of the RNFL layer in the wAMD eyes treated with anti-VEGF injections was described by Martinez-de-la-casa and Lee et al23,24 In turn, while the findings of Ahn et al also described significantly decreased total RNFL thickness after twelve months of treatment, they found no significant correlation between the number of injections and overall RNFL changes. However, Wang L et al documented that the relationship between RNFL thinning and the number of injections becomes apparent after 30 injections.21 As they rightly noted, the effects of such treatment on the nerve fiber layer in most of the previously cited works were observed in research groups consisting of patients that received less than thirty injections.21 In our study, the maximum number of injections received by the patient was 31. However, we did not find RNFL thinning in the group with wAMD versus dAMD.
As mentioned by Lee EK, dAMD also contributes to the loss of GCL and RNFL,27 with the smallest changes in RNFL thickness observed in the nasal quadrant. In our study, the only statistically significant increase in RNFL thickness was observed in this quadrant of the treated eyes (+0.536 µm for each additional injection, p = 0.023). In the literature, RNFL thickness increase during the therapy was already reported in the temporal quadrant, which the authors explained as a shift of fiber swelling from the macular area.20 However, as nerve fibers (retinal ganglion cell axons) from the theoretically unaffected area of the nasal retina converge within the nasal sector of the disc, this mechanism does not explain the observed phenomenon. Perhaps, due to the direct mechanism of impaired perfusion described by Mursch-Edlmayr AS and Barash A, we may be dealing with fiber swelling, as is observed in ischemic neuropathies.6,7 Nonetheless, additional research is needed to confirm this hypothesis.
Since nerve fibers are de facto axons of the retinal ganglion cells, we considered it reasonable to evaluate this retinal layer. Although we detected a decrease in GCL thickness by 2.61 µm in the study group, the correlation did not reach statistical significance (p = 0.092). Similarly, Cheng et al reported a lack of retinal ganglion cell loss.28 On the contrary, Beck et al, Kim et al, and Lee et al demonstrated a decrease in the retinal ganglion cell layer in the eyes of patients undergoing anti-VEGF wAMD treatment compared to untreated eyes.24–26
Overall, we are cautious in drawing conclusions from the results obtained, as the study protocol covered the macular area. It is known that age-related macular degeneration probably affects the outer layer of the retina. Still, as the disease progresses, it is suggested that it may also affect the inner layer, including retinal vasculature, a region that we did not focus on in this study.24,29–31 However, scanning extramacular areas seem to be a solution to this dilemma and could be an interesting direction for further research.
We also found that age impacted the obtained results regarding both RNFL (β= −0.34, p = 0.019) and GCL (β= −2.61, p = 0.004). Hence, this factor should be considered in statistical analysis, especially in the case of age-related diseases.
Gender also turned out to be a factor in the thickness of nerve fibers in our study - men had thinner peripapillary nerve fibers than women in the temporal sector of the optic disc (β −6.16, p = 0.012). Literature data vary in this regard, although the results of a large study by Li et al on 5646 healthy participants seem to confirm our finding.32
Functional Outcomes
It is the first study focused on the functional assessment of the retina and optic nerve using pattern electroretinogram and visual evoked potentials in patients with age-related macular degeneration chronically treated with intravitreal anti-VEGF injections. We considered these tests safe for patients due to their non-invasive nature and relatively short duration resulting from a lack of pharmacologically induced mydriasis (which allowed to fulfill a goal of maintaining maximum patients safety during the COVID-19 pandemic). At the same time, these examinations enabled a functional assessment of the nervous system at almost every layer of the retina.12–16
In the optic nerve conduction assessment, we found a statistically significant difference in P100 implicit time between the two analyzed groups (p = 0.014), with the time 10.06 ms longer in the treatment group. However, the number of injections did not result in a difference in the P100 implicit time (p = 0.071). These outcomes may indicate a dysfunction of the innermost retinal layers or neural conduction.12,15 It is possible that P100 PVEP could result from worse BCVA in the wAMD group (0.58; SD 0.20 versus 0.85; SD 0.16 in the control group), which could indicate more severe macular lesions in the study versus the control group. However, to minimize the impact of maculopathy on P100, we used checkboard stimuli with large checks (1 degree), allowing us to elicit a parafoveal response (conduction along large axons).17,18 This technique has been shown to improve the evaluation of paracentral ganglion cells (more eccentric retinal area or the magnocellular pathway).34,35 It was reported in the literature that the use of this type of pattern reduces the potential influence of pathologies of the central part of the retina on the examination results.34
P100 values are relatively constant in the population and have minimal inter-ocular variability. However, they may be affected by some clinical parameters, including age.15,18 According to ISCEV, VEP waveform or PERG changes are relatively small over 18–60 and 18–55 years of age, respectively.12,13,16 AMD, in its very definition, mainly affects older patients. Hence, as the mean age of our study participants was 72.62 years (SD 7.34), we did not relate the results to the usual standards and instead focused on comparing the treated and control groups.
PVEP abnormalities may also result from pathologies of earlier visual pathway structures, including retinal ganglion cells, that can be captured using the PERG test.13,36 Two main parameters in electroretinogram can be investigated to evaluate retinal ganglion cell function: the photopic negative responses (PhNRs), the evaluation of which requires a full-field electroretinogram (ERG), or the N95 amplitude in pattern electroretinogram (PERG).13,18 Although we did not focus on the PhNRs measurement, we detected worse N95 amplitudes in wAMD eyes compared to fellow eyes with dAMD. Nishimura et al confirmed the detrimental effect of multiple injections on the more peripheral retinal ganglion cells or the absence of this effect, depending on the anti-VEGF preparation used. However, they focused on evaluating PhNRs, so the outcomes of their work cannot be simply compared to our results.33,37 The reduced amplitude of the N95 wave may be associated with a loss in the number or function of ganglion cells. As mentioned before, we did not detect GCL thinning in our study in relation to the fellow eye. However, electrophysiological tests may precede morphological changes or patient complaints, which is why we considered it appropriate to focus not only on the morphological assessment.17,36
PERG N95 amplitude reduction was also observed in glaucoma or ischemic neuropathies.15,38 As retinal and optic nerve perfusion abnormalities have been reported in the literature during anti-VEGF treatment, this mechanism may have influenced our results.6,7
The main limitation of our research was the relatively small study group, and the use of automatic segmentation by our device software for GCL and RNFL. Furthermore, the patients were not divided into groups based on the type of anti-VEGF medication administered. We could not consider the treatment time due to the lack of data, as some patients had been previously treated outside our clinic. We are also aware that we did not take the physiological asymmetries between the eyes and the influence of the axial length of the eyeball on the assessed parameters into account, which may have an impact on the results. Nonetheless, we tried to select patients with a specific range of spherical equivalent of the refractive error (−4- (+4.5) D sph).39,40
In conclusion, our study demonstrates that wAMD is characterized by worse functional parameters (delayed VEP P100 and reduced PERG P50-N95 amplitude) than non-advanced dAMD, with lack of significant differences in anatomical (RNFL, GCL) parameters between both groups. Moreover, the number of anti-VEGF injections administered does not contribute to these differences. Furthermore, electrophysiological tests appear to be a rewarding tool in assessing the effect of intravitreal injections on the structures of the retina and the optic nerve, with functional changes potentially preceding morphological ones.
Abbreviations
n, nobs, a sample of the population; M, mean;SD, standard deviation; Mdn, median; W, Shapiro–Wilk test statistics; p, p-value; df, degrees of freedom; 95% CI, confidence interval 95% 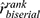 , rank biserial correlation coefficient; VWilcoxon, The Wilcoxon rank sum test statistics; IQR, interquartile range; Min, minimal value; Max, maximum value; Sk, skew of the distribution; Kurt, kurtosis of the distribution; W, Shapiro–Wilk test statistics; t, t-test statistics; SE, standard error; β, linear regression coefficient; tWelch, t-Welch statistics; g, hedges g effect size.
, rank biserial correlation coefficient; VWilcoxon, The Wilcoxon rank sum test statistics; IQR, interquartile range; Min, minimal value; Max, maximum value; Sk, skew of the distribution; Kurt, kurtosis of the distribution; W, Shapiro–Wilk test statistics; t, t-test statistics; SE, standard error; β, linear regression coefficient; tWelch, t-Welch statistics; g, hedges g effect size.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization [homepage on the Internet]. Fact Sheets: Ageing and Health; 4 October 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
2. World Health Organization. World Report on Vision. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO.
3. Bakri SJ, Thorne JE, Ho AC, et al. Safety and efficacy of anti-vascular endothelial growth factor therapies for neovascular age-related macular degeneration: a report by the American Academy of Ophthalmology. Ophthalmology. 2019;126(1):55–63. doi:10.1016/j.ophtha.2018.07.028
4. Bracha P, Moore NA, Ciulla TA, WuDunn D, Cantor LB. The acute and chronic effects of intravitreal anti-vascular endothelial growth factor injections on intraocular pressure: a review. Surv Ophthalmol. 2018;63(3):281–295. doi:10.1016/j.survophthal.2017.08.008
5. Sobacı G, Güngör R, Ozge G. Effects of multiple intravitreal anti-VEGF injections on retinal nerve fiber layer and intraocular pressure: a comparative clinical study. Int J Ophthalmol. 2013;6(2):211–215. doi:10.3980/j.issn.2222-3959.2013.02.20
6. Mursch-Edlmayr AS, Luft N, Podkowinski D, Ring M, Schmetterer L, Bolz M. Short-term effect on the ocular circulation induced by unilateral intravitreal injection of aflibercept in age-related maculopathy. Acta Ophthalmol. 2019;97(6):e927–e932. doi:10.1111/aos.14098
7. Barash A, Chui TYP, Garcia P, Rosen RB. Acute macular and peripapillary angiographic changes with intravitreal injections. Retina. 2020;40(4):648–656. doi:10.1097/IAE.0000000000002433
8. Nishijima K, Ng YS, Zhong L, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171(1):53–67. doi:10.2353/ajpath.2007.061237
9. Silva-Hucha S, Pastor AM, Morcuende S. Neuroprotective effect of vascular endothelial growth factor on motoneurons of the oculomotor system. Int J Mol Sci. 2021;22(2):814. doi:10.3390/ijms22020814
10. Foxton RH, Finkelstein A, Vijay S, et al. VEGF-A is necessary and sufficient for retinal neuroprotection in models of experimental glaucoma. Am J Pathol. 2013;182(4):1379–1390. doi:10.1016/j.ajpath.2012.12.032
11. El-Ashry MF, Lascaratos G, Dhillon B. Evaluation of the effect of intravitreal ranibizumab injections in patients with neovascular age related macular degeneration on retinal nerve fiber layer thickness using optical coherence tomography. Clin Ophthalmol. 2015;9:1269–1274. doi:10.2147/OPTH.S80704
12. Odom JV, Bach M, Brigell M, et al.; International Society for Clinical Electrophysiology of Vision. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc Ophthalmol. 2016;133(1):1–9. doi:10.1007/s10633-016-9553-y
13. Robson AG, Nilsson J, Li S, et al. ISCEV guide to visual electrodiagnostic procedures. Doc Ophthalmol. 2018;136(1):1–26. doi:10.1007/s10633-017-9621-y
14. Marmoy OR, Viswanathan S. Clinical electrophysiology of the optic nerve and retinal ganglion cells. Eye. 2021;35(9):2386–2405. doi:10.1038/s41433-021-01614-x
15. Jurkute N, Robson AG. Electrophysiology in neuro-ophthalmology. Handb Clin Neurol. 2021;178:79–96. doi:10.1016/B978-0-12-821377-3.00019-2
16. Bach M, Brigell MG, Hawlina M, et al. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc Ophthalmol. 2013;126(1):1–7. doi:10.1007/s10633-012-9353-y
17. Krasodomska K, Lubiński W, Potemkowski A, Honczarenko K. Pattern electroretinogram (PERG) and pattern visual evoked potential (PVEP) in the early stages of Alzheimer’s disease. Doc Ophthalmol Adv Ophthalmol. 2010;121(2):111–121. doi:10.1007/s10633-010-9238-x
18. Parisi V, Ziccardi L, Tanga L, et al. Neural conduction along postretinal visual pathways in glaucoma. Front Aging Neurosci. 2021;13:697425. doi:10.3389/fnagi.2021.697425
19. Parisi V, Manni G, Spadaro M, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40(11):2520–2527.
20. Yau GL, Campbell RJ, Li C, Sharma S. Peripapillary RNFL thickness in nonexudative versus chronically treated exudative age-related macular degeneration. Can J Ophthalmol. 2015;50(5):345–349. doi:10.1016/j.jcjo.2015.01.008
21. Wang L, Swaminathan SS, Yang J, et al. Dose-response relationship between intravitreal injections and retinal nerve fiber layer thinning in age-related macular degeneration. Ophthalmol Retina. 2021;5(7):648–654. doi:10.1016/j.oret.2020.10.004
22. Ahn J, Jang K, Sohn J, Park JI, Hwang DD. Effect of intravitreal ranibizumab and aflibercept injections on retinal nerve fiber layer thickness. Sci Rep. 2021;11(1):5010. doi:10.1038/s41598-021-84648-1
23. Martinez-de-la-Casa JM, Ruiz-Calvo A, Saenz-Frances F, et al. Retinal nerve fiber layer thickness changes in patients with age-related macular degeneration treated with intravitreal ranibizumab. Invest Ophthalmol Vis Sci. 2012;53(10):6214–6218. doi:10.1167/iovs.12-9875
24. Lee SW, Sim HE, Park JY, et al. Changes in inner retinal layer thickness in patients with exudative age-related macular degeneration during treatment with anti-vascular endothelial growth factor. Medicine. 2020;99(17):e19955. doi:10.1097/MD.0000000000019955
25. Beck M, Munk MR, Ebneter A, Wolf S, Zinkernagel MS. Retinal ganglion cell layer change in patients treated with anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Am J Ophthalmol. 2016;167:10–17. doi:10.1016/j.ajo.2016.04.003
26. Kim SY, Yoon MH, Chin HS. Changes in the ganglion cell-inner plexiform layer after consecutive intravitreal injections of anti-vascular endothelial growth factor in age-related macular degeneration patients. Korean J Ophthalmol. 2020;34(1):11–18. doi:10.3341/kjo.2019.0081
27. Lee EK, Yu HG. Ganglion cell-inner plexiform layer and peripapillary retinal nerve fiber layer thicknesses in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56(6):3976–3983. doi:10.1167/iovs.15-17013
28. Cheng CK, Peng PH, Tien LT, Cai YJ, Chen CF, Lee YJ. Bevacizumab is not toxic to retinal ganglion cells after repeated intravitreal injection. Retina. 2009;29(3):306–312. doi:10.1097/IAE.0b013e3181909404
29. Demir N, Sevincli S, Kayhan B, Sonmez M. Anatomical effects of intravitreal anti-vascular endothelial growth factor injections on inner layers of the lesion-free retina. Cutan Ocul Toxicol. 2021;40(2):135–139. doi:10.1080/15569527.2021.1919136
30. Toto L, Borrelli E, Mastropasqua R, et al. Association between outer retinal alterations and microvascular changes in intermediate stage age-related macular degeneration: an optical coherence tomography angiography study. Br J Ophthalmol. 2017;101(6):774–779. doi:10.1136/bjophthalmol-2016-309160
31. Trinh M, Kalloniatis M, Nivison-Smith L. Radial peripapillary capillary plexus sparing and underlying retinal vascular impairment in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2021;62(4):2. doi:10.1167/iovs.62.4.2
32. Li D, Rauscher FG, Choi EY, et al. Sex-specific differences in circumpapillary retinal nerve fiber layer thickness. Ophthalmology. 2020;127(3):357–368. doi:10.1016/j.ophtha.2019.09.019
33. Nishimura T, Machida S, Harada T, Kurosaka D. Retinal ganglion cell function after repeated intravitreal injections of ranibizumab in patients with age-related macular degeneration. Clin Ophthalmol. 2012;6:1073–1082. doi:10.2147/OPTH.S31674
34. Parisi V, Scarale ME, Balducci N, Fresina M, Campos EC. Electrophysiological detection of delayed postretinal neural conduction in human amblyopia. Invest Ophthalmol Vis Sci. 2010;51(10):5041–5048. doi:10.1167/iovs.10-5412
35. Sponsel WE, Johnson SL, Trevino R, et al. Pattern electroretinography and visual evoked potentials provide clinical evidence of CNS modulation of high- and low-contrast VEP latency in glaucoma. Transl Vis Sci Technol. 2017;6(6):6. doi:10.1167/tvst.6.6.6
36. Parisi V, Miglior S, Manni G, Centofanti M, Bucci MG. Clinical ability of pattern electroretinograms and visual evoked potentials in detecting visual dysfunction in ocular hypertension and glaucoma. Ophthalmology. 2006;113(2):216–228. doi:10.1016/j.ophtha.2005.10.044
37. Nishimura T, Machida S, Hara Y. Changes in cone-driven functions after intravitreal aflibercept injections in patients with age-related macular degeneration. Doc Ophthalmol. 2020;141(2):137–147. doi:10.1007/s10633-020-09758-z
38. Parisi V, Manni G, Centofanti M, Gandolfi SA, Olzi D, Bucci MG. Correlation between optical coherence tomography, pattern electroretinogram, and visual evoked potentials in open-angle glaucoma patients. Ophthalmology. 2001;108(5):905–912. doi:10.1016/s0161-6420(00)00644-8
39. Hirasawa K, Shoji N, Yoshii Y, Haraguchi S. Determination of axial length requiring adjustment of measured circumpapillary retinal nerve fiber layer thickness for ocular magnification. PLoS One. 2014;9(9):e107553. doi:10.1371/journal.pone.0107553
40. Dalgliesh JD, Tariq YM, Burlutsky G, Mitchell P. Symmetry of retinal parameters measured by spectral-domain OCT in normal young adults. J Glaucoma. 2015;24(1):20–24. doi:10.1097/IJG.0b013e318287ac2f
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

