Back to Journals » Journal of Blood Medicine » Volume 11
Monocentric Analysis of the Effectiveness and Financial Consequences of the Use of Lenograstim versus Filgrastim for Mobilization of Peripheral Blood Progenitor Cells in Patients with Lymphoma and Myeloma Receiving Chemotherapy and Autologous Stem Cell Transplantation
Authors Restelli U , Croce D , Bonizzoni E, Marzanatti M, Andreini A , Sorio M , Tecchio C, Barison E, Benedetti F
Received 22 July 2019
Accepted for publication 9 March 2020
Published 2 April 2020 Volume 2020:11 Pages 123—130
DOI https://doi.org/10.2147/JBM.S224173
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Umberto Restelli,1,2 Davide Croce,1,2 Erminio Bonizzoni,3 Mario Marzanatti,3 Angelo Andreini,4 Marco Sorio,4 Cristina Tecchio,4 Erika Barison,4 Fabio Benedetti4
1School of Public Health, Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa; 2Center for Health Economics, Social and Health Care Management, LIUC Università Cattaneo, Castellanza, VA, Italy; 3Section of Medical Statistics and Biometry “GA Maccacaro”, Department of Clinica Science and Community, University of Milan, Milan, Italy; 4Bone Marrow Transplant Unit- Hematology, University of Verona, Verona, Italy
Correspondence: Fabio Benedetti
Bone Marrow Transplant Unit- Hematology, University of Verona, Piazza L.A. Scuro, 10, Verona 37134, Italy
Tel + 39 45 812 4647
Fax + 39 45 8027488
Email [email protected]
Purpose: Granulocyte-colony stimulating factors (G-CSFs) are widely used to mobilize CD34+ stem cells and to support the engraftment after hematopoietic stem cell transplantation (HSCT). A budget impact analysis and an incremental cost-effectiveness study of two G-CSFs (Lenograstim and Filgrastim biosimilar), considering engraftment, number of hospitalization days and number of G-CSF vials administered were performed.
Patients and Methods: Between 2009 and 2016, 248 patients undergoing autologous HSCT have been evaluated and divided into three groups (100 Leno-Leno, 93 Leno-Fil, 55 Fil-Fil) according to the type of G-CSF used for hematopoietic stem cell mobilization and hematopoietic stem cell recovery after transplant.
Results: The following statistically significant differences have been observed between Leno-Leno, Leno-Fil, Fil-Fil groups: a higher number of harvested CD34+ cells (10.56 vs 8.00 vs 7.20; p=0.0003) and a lower number of G-CSF vials (8 vs 8 vs 9; p=0.00020) used for full bone marrow recovery favoring Lenograstim. No statistically significant differences were found regarding the number of G-CSF vials used for mobilization, apheresis number and CD34+ cell peak. The post-transplant hematological recovery was faster in Lenograstim group than Filgrastim group: median time to neutrophil count engraftment (> 500/mmc) was 12 vs 13 days; median time for platelets recovery (> 20.000/mmc) was 12 vs 15 days (p=0.0001). The use of Lenograstim achieved cost savings of € 566/patient over Filgrastim biosimilar, related to a decreased number of days of hospitalization (16 vs 17 days; p=0.00012), a lower overall incidence of adverse events, laboratory tests, transfusions for platelet recovery following discharge.
Conclusion: In our experience, Lenograstim outperforms Filgrastim in terms of effectiveness and lower cost. This study shows a clinical superiority of Lenograstim over Filgrastim suggesting a potential cost savings favoring Lenograstim.
Keywords: lenograstim, filgrastim, mobilization, cost-effectiveness, G-CSF, HSCT
Introduction
In the current clinical practice, dose-intensive chemotherapy followed by peripheral blood stem cell transplantation (PBSCT) is a procedure commonly performed in the treatment of a variety of hematological malignancies.1,2
Use of granulocyte-colony stimulating factor (G-CSF)-mobilized peripheral blood stem cells (PBSCs) has replaced bone marrow as a source of stem cells for both autologous and allogeneic hematopoietic stem cell transplantation (HSCT).1 G-CSFs remain the most commonly used agent for hematopoietic stem cells (HSCs) mobilization in the clinic. G-CSFs are biological growth factors that promote the proliferation, differentiation and activation of neutrophils in the bone marrow.3 Currently, four chemically different formulations of recombinant human (rh) G-CSF are commercially available in Europe: glycosylated (Lenograstim), non-glycosylated (Filgrastim), pegylated (Pegfilgrastim) and glycopegylated (Lipegfilgrastim).4–7 All different formulations are indicated to reduce duration of neutropenia and incidence of febrile neutropenia in patients with non-myeloid malignancies receiving myelosuppressive chemotherapy.7–9
Lenograstim and Filgrastim are short-acting G-CSFs commonly administered in the transplant setting, either for mobilization of HSCs out of bone marrow into peripheral blood or as supportive care after autologous or allogeneic transplantation. Lenograstim, derived from Chinese hamster ovary cells, is indistinguishable from the human endogenous glycoprotein, while Filgrastim is produced in Escherichia coli bacterial cells, therefore is non-glycosylated and carries an extra Methionine at the N-terminal end of the peptide chain as compared to Lenograstim. Both are used to treat chemotherapy-induced febrile neutropenia, in PBSCs mobilization and in promoting recovery after autologous HSCT.3,8,9 In vitro and ex vivo studies have shown biological differences between Lenograstim and Filgrastim with particular emphasis on the quality of activated neutrophils. In clinical practice, these differences translate into a lower incidence of febrile episodes and increased CD34+ mobilization efficacy upon Lenograstim administration compared with Filgrastim, and therefore, a clinical superiority of Lenograstim has been postulated.10,11
Biosimilars are non-identical version of the biopharmaceutical originator that may vary in terms of size of the active substance, complexity and nature of the manufacturing process. Numerous biosimilar G-CSFs have been approved in Europe and introduced in clinical practice.12 Although the paucity of data supporting clinical equivalence in terms of efficacy, safety and immunogenicity, the European Medicines Agency (EMA) declared the therapeutic equivalence of biosimilars for PBSC mobilization and recovery after autologous HSCT.3,8,9
Considering that biological differences can translate into different clinical outcomes, the objective of this analysis was assessing the effectiveness of Lenograstim versus Filgrastim for PBSC mobilization and hematopoietic recovery after transplantation and the related costs in a real clinical setting.
Patients and Methods
At Azienda Ospedaliera Universitaria Integrata in Verona (Italy), a retrospective comparative study has been carried out involving 248 consecutive patients with multiple myeloma, non-Hodgkin lymphoma or Hodgkin lymphoma undergoing mobilization and subsequent autologous HSCT between January 2009 and June 2016. Our retrospective database analysis has been conducted in accordance with the Declaration of Helsinki, and informed consent was not required due to the anonymous and retrospective design of the data collection. The analysis has been notified to the ethical committee of Azienda Ospedaliera Universitaria Integrata di Verona that received and approved the study. And it was approved by the local institutional review board. Eligible patients were assigned to three groups based on availability of G-CSF used for HSC mobilization and post-transplantation recovery: 100 patients treated with Lenograstim for both mobilization and hematological recovery (Leno-Leno group); 93 patients initially treated with Lenograstim and then with Filgrastim biosimilar (Leno-Fil group); 55 patients treated with Filgrastim biosimilar for both mobilization and post-transplantation support (Fil-Fil group). Patient's characteristics in terms of age, sex and type of tumor were well balanced between the three treatment groups. Baseline patient characteristics are summarized in Table 1.
 |
Table 1 Characteristics of the Study Population |
Patients were mobilized with 5 µg/kg G-CSF (Lenograstim or Filgrastim biosimilar) for nine days per standard procedure, regardless of leukocyte and CD34+ count, and the collection of PBSCs was performed by apheresis at the ninth day. In the case of mobilization failure, patients underwent G-CSF stimulation for two additional days followed by subsequent apheresis until mobilization has reached an optimal level, defined as more than 2.5 x 106 CD34+ cells/kg. All the patients received also chemotherapy for mobilization: all patients with multiple myeloma and Hodgkin lymphoma received cytoxan 3g/sqm, while patients with Non-Hodgkin lymphoma received either cytoxan 3g/sqm or DHAP regimen. Malignancies and different regimens were balanced among the three groups.
The CD34+ cell collection was followed by the conditioning regimen that reduces tumor burden and suppresses the recipient immune system, allowing stem cell engraftment. As conditioning regimes, all myeloma patients received Melphalan 200 mg/sqm i.v. in 4 doses on day −3 from transplant, while lymphoma patients were conditioned with Mitoxantrone 60 mg/sqm i.v. on day −5 and Melphalan 160 mg/sqm i.v. on day −4. The conditioning was followed by infusion of autologous CD34+ stem cells. G-CSF was started on day +4 after transplant and continued until hematological recovery. Patients were then discharged from the hospital after reaching a complete recovery of their neutrophils number (>500/mmc). Once discharged, patients received a follow-up visit every two days (with a maximum of three visits per week) to control their platelet level and receive, if necessary, a blood transfusion, until complete recovery of platelets (>20,000/µL).
The clinical variables collected in the analysis to assess the effectiveness of each G-CSF were the number of G-CSF vials used for the recovery, the number of days from the first G-CSF administration to neutrophil recovery (>500/mmc), the number of days from the first G-CSF administration to platelet recovery (>20,000/µL) and the percentage of patients developing pneumonia, fever and sepsis.
The economic analysis was conducted according to the Regional Health Service policies and procedures and considering the sole differential costs between the three groups of patients (ie, Leno-Leno, Leno-Fil, Fil-Fil). The costs taken into account, referred to year 2016, were those related to aphereses, inpatient stay, adverse events management and G-CSF administration. The cost reported in Table 2 was then multiplied by the median number of aphereses, inpatient day, adverse events, platelet transfusions and G-CSF vial used in each group, to assess the differential economic impact of the use of different G-CSFs.
 |
Table 2 Cost Data Considered in the Analysis |
Statistical Analysis
All statistical analyses were performed using the procedures of SAS version 9.4. A two-tailed p-value of 0·05 or less was chosen to define statistically significant results. Statistical analyses were carried out by comparing the three groups against each other (pairwise comparisons) and by looking for the presence of a linear trend between Leno-Leno, Leno-Fil, Fil-Fil groups in this order.
Means, medians, standard deviations, minimum and maximum values were used to summarize continuous and count variables (number of harvested CD34+ cells/number of aphereses, CD34+ cell peak, number of G-CSF vials, etc.). Statistical comparison across the three groups was performed using a Non-Parametric ANOVA following rank transformation of data.
Times to event variables (time to WBC > 500, time to PTLS > 20,000, hospitalization days, days of fever, etc.) were described using the Kaplan–Meier method and compared using the Peto Log-rank test. Percentages were used to summarize categorical variables (number of aphereses, pneumonia, sepsis, etc.), while statistical comparison across the three groups was carried out using Fisher’s Exact Test.
Results
Two-hundred forty-eight patients have been included in the analysis: 100 patients in the Leno-Leno group, 93 patients in the Leno-Fil group and 55 patients in the Fil-Fil group. Effectiveness of HSC mobilization was evaluated considering the following variables: number of G-CSF vials administered, number of aphereses performed, number of harvested CD34+ cells and CD34+ cell peak. As reported in Table 3, a significantly higher number of harvested CD34+ cells were observed in patients treated with Lenograstim (Leno-Leno and Leno-Fil groups) compared to patients treated with Filgrastim (Fil-Fil group): the mean number of harvested CD34+ cells was 10.10 ± 5.87 x106 cells/kg for Leno-Leno/Leno-Fil groups and 7.92 ± 3.9 x106 cells/kg for Fil-Fil group (p=0.0101). No differences were observed between Lenograstim and Filgrastim in terms of number of vials needed to obtain CD34+ cell mobilization, number of aphereses and CD34+ cell peak. The mean number of G-CSF vials administered was 10.34 ± 1.58 for the Leno-Leno/Leno-Fil groups and 10.13 ± 1.32 for the Fil-Fil group without statistically significant differences (p=0.3691); the mean number of aphereses was 1.66 ± 0.78 for the Leno-Leno/Leno-Fil groups and 1.56 ± 0.66 for the Fil-Fil group (p=0.3872); the mean of CD34+ cell peak was 112.86 ± 123.55/µL for the Leno-Leno/Leno-Fil groups and 89.49 ± 87.21/µL for the Fil-Fil group (p=0.1908).
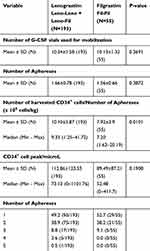 |
Table 3 Efficacy of the Mobilization Process |
A statistically significant difference has been detected in terms of days from chemotherapy required to obtain white blood cell (WBC) and platelet (PTL) recovery, defined as days after starting the treatment until stable hematological recovery. Engraftment was defined as ANC>500/mmc and platelet count >20,000/mmc. Particularly, the post-transplant hematological recovery was faster in the Leno-Leno group compared with the Fil-Fil group: median neutrophil >500/mmc (12 vs 13 days; p=0.00012), median platelets >20.000/mmc (12 vs 15 days; p=0.0001). The use of Lenograstim instead of Filgrastim led to a decreased number of days of hospitalization (median: 16 vs 17; p=0.00012) and number of G-CSF vials used for full bone marrow recovery (median: 8 vs 9; p=0.00020) (Table 4). Figure 1 shows the Kaplan–Meier curves of the three treatment groups in relation to days from chemotherapy necessary for WBC (>500/mmc) and PTL (>20.000/mmc) recovery and days of hospitalization. Regarding the adverse events (AEs), patients treated with Lenograstim showed a lower incidence of fever (p=0.01834) than Filgrastim group (Table 4).
 |
Table 4 Efficacy of the Post-Transplant Hematological Recovery |
 |
Figure 1 Kaplan–Meier curves of the 3 treatment groups in relation to days from chemotherapy necessary for WBCs (>500/mmc), PTLs (>20.000/mmc) recovery and days of hospitalization. |
When compared with Filgrastim-based treatment, the use of Lenograstim for both mobilization and bone marrow recovery (Leno-Leno group versus Fil-Fil group) resulted in a decrease of costs for the total patient’s management of −566€, considering the median values of clinical outcomes. This is mainly due to cost reduction in terms of median inpatient days (−822 €), median adverse event incidence (−182 €), median laboratory tests and median of transfusions for platelet recovery following discharge (-€115). The cost containment due to the aforementioned activities counterbalances the higher vial cost of Lenograstim (+302 € for mobilization and +251 € for bone marrow recovery).
The same economic analysis between the Leno-Leno group and the Leno-Fil group shows similar results. The use of Lenograstim for both mobilization and bone marrow recovery led to lower costs compared with the use of Lenograstim for mobilization and Filgrastim for bone marrow recovery, of −59 €. The reduction of costs is related to vials used for mobilization (−101 €) and to adverse event management (−226 €), while additional costs are due to vials used for bone marrow recovery (+268 €). This analysis revealed a clinical superiority of Lenograstim over Filgrastim that can translate into potential cost savings for the National Health Service.
Discussion
Recombinant human G-CSFs have become a clinical tool widely used by hematologists and oncologists for neutrophil recovery after myelotoxic chemotherapy in cancer patients, for PBSC mobilization in individuals with hematological malignancies and healthy donors and for potentiation of stem cell engraftment after HSCT.13,14
The current G-CSF scenario in Europe includes Lenograstim, Filgrastim and its biosimilars, Pegfilgrastim and Lipegfilgrastim, whose availability represents an important resource in cancer treatment. Since its patent expired in 2006, several Filgrastim biosimilars have been developed and approved, while no Lenograstim biosimilar is currently available on the market. At present, data regarding the use of G-CSF biosimilars in the context of autologous HSCT for mobilization have been published demonstrating a substantial equivalence in terms of stem cell collection. However, few data are still available about HSC mobilization from healthy donors.15,16 Moreover, in 2009 the Executive Committee of the European Bone Marrow Transplantation (EBMT) recommended against the use of G-CSF biosimilars for stem cell mobilization in healthy donors.17 The same concern about the use of biosimilars in normal donors was reported by World Marrow Donor Association in 2011.15
The current economic context, resulting from the financial crisis, led the National Health Service and the Health Organizations to implement cost-effectiveness criteria in decision-making processes of health technology application. The introduction of biosimilars into clinical practice has represented an important opportunity for cost reduction allowing significant cost advantages for transplant unit. However, although their development is strictly regulated, biosimilar approval is based on clinical data extrapolated from the originator. Hence, concerns have been raised on switching between biosimilars and their originator. Therefore, it is crucial to choose the most appropriate G-CSF for specific clinical settings. Indeed, economic criteria must be considered along with scientific and clinical evidence and, for these reasons, it is important to find a proper balance between cost, scientific data and medical needs.
We compared Lenograstim and Filgrastim in terms of economic impact and effectiveness in daily clinical practice in patients with hematologic malignancies undergoing PBSC mobilization and transplant. We analyzed differences in terms of number of G-CSF vials administered, number of aphereses performed, number of harvested CD34+ cells and CD34+ cell peak in response to Lenograstim or Filgrastim to determine which growth factor presented the best profile in terms of effectiveness and cost. For the measured variables, including number of harvested CD34+ cells, number of G-CSF vials administered for bone marrow recovery and time of engraftment, Lenograstim stimulation obtained better results with a consequent positive effect on direct medical cost. Similarly, earlier bone marrow recovery decreased hospitalization time (16 vs 17 days) in patients mobilized with Lenograstim. Our results are consistent with other clinical trials comparing the different G-CSFs. Orciuolo and colleagues demonstrated a lower incidence of febrile episodes, a much more effective stem cell mobilization and a higher count of mobilized stem cells upon Lenograstim administration compared with Filgrastim.2 Ria and colleagues compared the use of Lenograstim, Filgrastim and Pegfilgrastim in 146 patients undergoing autologous HSCT for lymphoproliferative disorders. Their results showed that Lenograstim achieved an adequate mobilization and a target collection faster and with fewer leukaphereses as compared to Filgrastim and Pegfilgrastim.16
In our study, although the price of Lenograstim is higher compared to Filgrastim biosimilar, the total medical cost related to inpatient stay, management of adverse events, laboratory tests, transfusions for for platelets and recovery after discharge was lower due to faster post-transplant hematological recovery, reduced use of G-CSF vials and days of hospitalization observed in the cohort of patients treated with Lenograstim (Table 5). The longer hospitalization due to delayed hematological recovery and complications (fever) counterbalances the cost of growth factor administration.
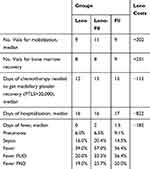 |
Table 5 Economic Results |
This study has some limitations related to the observational, retrospective and single-institution design that may confine the generalization of the results. Until results from multi-center randomized clinical trials comparing the different G-CSFs are reported, it is important to collect and describe individual clinical experience to support the scientific community to make decisions concerning the choice of G-CSF, especially regarding the transplant setting. In this regard, our findings suggest that Lenograstim administered for HSC mobilization and hematopoietic stem cell recovery, in patients with lymphoma and multiple myeloma undergoing chemotherapy and autologous HSCT, is associated with a greater health benefit and lower cost, likely representing a cost-effective option over Filgrastim.
Conclusions
Our study demonstrated that in patients with lymphoma and multiple myeloma undergoing chemotherapy and autologous HSCT, Lenograstim accelerated neutrophil and platelet recovery post-HSCT and shortened the duration of hospitalization. Lenograstim is an essential option in treatment of chemotherapy-induced neutropenia, PBSC mobilization and acceleration of hematological recovery. In conclusion, our experience identified Lenograstim as the preferable cost-saving G-CSF in comparison to Filgrastim.
Acknowledgments
The authors thank the nurses and the biologists of the Bone Marrow Transplant Unit of AOUI Verona.
Disclosure
Dr Umberto Restelli reports grants from Italfarmaco, during the conduct of the study; personal fees from Italfarmaco, outside the submitted work. Dr Erminio Bonizzoni reports personal fees from Italfarmaco, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Hopman RK, DiPersio JF. Advances in stem cell mobilization. Blood Rev. 2014;28(1):31–40. doi:10.1016/j.blre.2014.01.001
2. Orciuolo E, Buda G, Marturano E, et al. Lenograstim reduces the incidence of febrile episodes, when compared with Filgrastim, in multiple myeloma patients undergoing stem cell mobilization. Leuk Res. 2011;35(7):899–903. doi:10.1016/j.leukres.2010.10.029
3. Gardellini A, Gigli F, Babic A, et al. Filgrastim XM02 (Tevagrastim) after autologous stem cell transplantation compared to lenograstim: favorable cost-efficacy analysis. Ecancermedicalscience. 2013;7:327.
4. Rogers KM. Topics in clinical pharmacology: filgrastim, a myeloid colony stimulating factor. Am J Med Sci. 1992;303(6):429–431. doi:10.1097/00000441-199206000-00015
5. Curran MP, Goa KL. Pegfilgrastim. Drugs. 2002;62(8):1207–1213. doi:10.2165/00003495-200262080-00012
6. Baumann I, Testa NG, Lange C, et al. Haemopoietic cells mobilised into the circulation by lenograstim as alternative to bone marrow for allogeneic transplants. Lancet. 1993;341(8841):369. doi:10.1016/0140-6736(93)90166-E
7. Guariglia R, Martorelli MC, Telesca D, Milella M, Musto P. Lipegfilgrastim in the management of chemotherapy –induced neutropenia of cancer patients. Biol Targets Ther. 2016;10:1–8.
8. Trotta F, Mayer F, Mecozzi A, Amato L, Addis A. Impact of guidance on the prescription patterns of G-CSF for the prevention of febrile neutropenia following anticancer chemotherapy: a population based utilization study in the Lazio Region. BioDrugs. 2017;31(2):117–124. doi:10.1007/s40259-017-0214-9
9. Sourgens H, Lefrére F. A systematic review of available clinical evidence filgrastim compared with lenograstim. J Clin Pharmacol Ther. 2011;49(8):510–518. doi:10.5414/CP201537
10. Innocenti R, Rigacci L, Restelli U, et al. Lenograstim and Filgrastim in the febrile neutropenia prophylaxis of hospitalized patients: efficacy and cost of the prophylaxis in a retrospective survey. J Blood Med. 2019;10:21–27. doi:10.2147/JBM.S186786
11. Wang L, Baser O, Kutikova L, Page JH, Barron R. The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: a systematic review and meta-analysis of randomized trials. SuppCareCancer. 2015;23:3131–3140.
12. Gascon P. Presently available biosimilars in hematology-oncology: G.CSF. Targ Oncol. 2012;7(suppl 1):29–34. doi:10.1007/s11523-011-0190-9
13. Schmitt M, Publicover A, Orchard KH, et al. Biosimilar G-CSF based mobilization of peripheral blood hematopoietic stem cells for autologous and allogenic stem cell transplantation. Theranostics. 2014;4(3):280–289. doi:10.7150/thno.7752
14. Farhan R, Urbanowska E, Zboroboska H, et al. Biosimilar G-CSF versus filgrastim and lenograstim in healthy unrelated volunteer hematopoietic stem cell donors. Ann Hematol. 2017;96:735–739. doi:10.1007/s00277-017-3060-4
15. Shaw B, Confer D, William Y, Hwang W, Pamphilon D, Pulsipher M. Concerns about the use of biosimilars granulocyte colony-stimulating factors for the mobilization of stem cells in normal donors position of the World Marrow Donor Association. Haematologica. 2011;96(7):942–947. doi:10.3324/haematol.2011.045740
16. Ria R, Reale A, Melaccio A, Racanelli V, Dammacco F, Vacca A. Filgrastim, Lenograstim and pegfilgrastim in the mobilization of peripheral blood progenitors cells in patients with lymphoproliferative malignancies. Clin Exp Med. 2015;15:145–150. doi:10.1007/s10238-014-0282-9
17. European Group for Blood and Marrow Transplantation (EBMT) 2009. Position statement: biosimilar granulocyte-colony stimulating factor (G-CSF) for stem cell mobilization in related and unrelated donors
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
