Back to Journals » Eye and Brain » Volume 15
Monitoring Eye Movement in Patients with Parkinson’s Disease: What Can It Tell Us?
Authors Sun YR, Beylergil SB, Gupta P, Ghasia FF, Shaikh AG
Received 20 February 2023
Accepted for publication 1 June 2023
Published 24 July 2023 Volume 2023:15 Pages 101—112
DOI https://doi.org/10.2147/EB.S384763
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Margaret Wong-Riley
Yue Ran Sun,1 Sinem B Beylergil,2 Palak Gupta,2,3 Fatema F Ghasia,4 Aasef G Shaikh1– 3
1Department of Neurology, University Hospitals, Case Western Reserve University, Cleveland, OH, USA; 2Neurology Service, Louis Stokes Cleveland VA Medical Center, Cleveland, OH, USA; 3Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, USA; 4Cole Eye Institute, Cleveland Clinic, Cleveland, OH, USA
Correspondence: Aasef G Shaikh, Department of Neurology, University Hospitals, Case Western Reserve University, Cleveland, OH, 44022, USA, Tel +1 2167856981, Email [email protected]
Abstract: Parkinson’s disease (PD) affects approximately 10 million individuals worldwide. Visual impairments are a common feature of PD. Patients report difficulties with visual scanning, impaired depth perception and spatial navigation, and blurry and double vision. Examination of PD patients reveals abnormal fixational saccades, strabismus, impaired convergence, and abnormal visually-guided saccades. This review aims to describe objective features of abnormal eye movements in PD and to discuss the structures and pathways through which these abnormalities may manifest.
Keywords: Parkinson’s disease, eye movement, gaze holding, strabismus, vergence, saccades
Introduction
The second most common neurodegenerative condition, Parkinson’s disease (PD), affects about 10 million individuals worldwide.1 Visual impairments are far more common in PD than appreciated. Composite visual function questionnaire (VFQ) score in PD patients is significantly reduced compared to healthy controls; the impairment is much more robust for the ocular motor functions necessary for visual scanning.2 The visual deficits in PD can be due to multiple etiologies. The inability to rapidly shift gaze (ie saccades) affects the ability to read and scan the visual scene.2–6 Impairment in the simultaneous movement of both eyes in opposite directions (ie vergence) results in compromised depth perception and impaired spatial navigation in PD.7,8 Vergence abnormality also leads to misalignment of the two eyes, causing strabismus, and abnormal fusion of the visual signal from each eye, causing blurred or double vision (ie diplopia). Diplopia is prevalent in up to one-third of PD patients, and tired eyes or blurred vision while reading was reported in 23%.5,6 Vergence abnormalities in PD include increased latency, decreased speed, decreased gain, and a need to recruit an alternate class of eye movements to make a three-dimensional gaze shift.9–11 This review aims to summarize objective features of abnormal eye movements in PD and examine their mechanistic underpinning. The subsequent sections are organized according to the individual eye movement subclasses, the objective assessment of their abnormality, and corresponding mechanistic underpinning.
Gaze Holding
Gaze-holding abnormalities include abnormally excessive fixational eye movements (such as microsaccades), drifts following microsaccades, and somewhat controversial pervasive pendular movements of the eyes. While the pathophysiology of microsaccades was directly linked to abnormal basal ganglia physiology, the pervasive pendular eye movements are thought to be a physiological response (ie the vestibulo-ocular reflex) following transmitted oscillations due to tremor.12–18
The fixational saccades are yoked miniature eye movements critical to counteract fading.19–21 Square wave jerks are a continuum of fixational saccades and, when too numerous, disrupt visual clarity.22 Squarewaves are prominent in PD, but they are also seen in other Parkinsonian disorders.13,23–27
Figure 1 depicts abnormal gaze holding in a PD patient compared with a healthy subject. The plot shows the angular position vector of the horizontal eye position on the y-axis and a corresponding time on the x-axis. The example from the healthy control is depicted in Figure 1A, while Figure 1B illustrates the PD patient. The excursion of the traces (arrows Figure 1) depicts fixational saccades, and some of these excursions are followed by a returning saccade, forming a squarewave jerk (closed arrow, Figure 1). The amplitudes of fixational saccades are larger in PD compared to healthy controls. As a result, fixational saccades in PD are often paired with a return saccade forming a squarewave jerk.
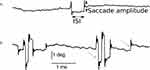 |
Figure 1 Eye position traces from a healthy control participant (A), and a PD patient (B). Composite vectors of horizontal and vertical eye positions are shown. Saccade amplitude (closed arrows, (A)) and intersaccadic interval (ISI, closed arrows, (A)) are the two metrics we used to characterize microsaccades including squarewaves. Microsaccades are depicted by an open arrow in (B); in several instances the microsaccades are followed by return eye movement comprising squarewave (closed arrow, (B)). ISI: Intersaccadic interval, deg: degrees, ms: millisecond. Adapted from Beylergil SB, Murray J, Noecker AM et al. Effects of subthalamic deep brain stimulation on fixational eye movements in Parkinson’s disease.J Comput Neurosci. 49(3):345–356, Springer Nature, 2021, permission from SNCSC.13 |
The fixational saccades form important markers of PD pathophysiology. Basal ganglia are believed to be at the epicenter of abnormal fixational saccades and squarewave jerks in PD. However, their effects manifest through their superior colliculus and cerebellar connections. The proposal is based on the premise that the substantia nigra pars reticulata (SNr) in PD exerts abnormally high inhibitory control over the superior colliculus.28–34 The superior colliculus modulates the downstream burst generators critical for the saccade generation. The subthalamus, connected to the cerebellum via precerebellar neurons in the pons, can modulate the saccade matrix; abnormal activity in the subthalamus can change the microsaccades and squarewave via its effects on the cerebellum. The effects of subthalamic (STN) deep brain stimulation (DBS) on microsaccades are directly probed in this proposal by demonstrating the effects of reversible interventions on the kinematic properties of fixational saccades and squarewaves.
Figure 2 depicts the comparison of fixational saccade amplitude in healthy controls, PD with STN DBS on and DBS off. The average age was 69±8.1 years (controls were age-matched), and the average disease duration was 13.1±4.9 years. The effects of DBS on the same individual are depicted in Figure 2A. In general, healthy controls’ fixational saccade amplitudes are smaller than PD’s (Figure 2B). The effects of STN DBS on fixational saccade amplitude are diverse in PD. In some patients, DBS significantly decreases the fixational saccade amplitude, while in others, there is a significant increase (Figure 2B and C). PD patients with relatively smaller fixational saccade amplitude and variance in gaze holding at baseline (ie when DBS is off) demonstrate increased amplitude with subthalamic DBS.
 |
Figure 2 Summary of microsaccade analyses. (A) Median saccade amplitudes of the individuals in DBS-off and DBS-on conditions. Asterisks indicate 6 patients showing a significant change due to DBS. (B) Normalized composite amplitude histograms of microsaccades of PD subjects who showed a significant decrease in saccade amplitude due to DBS. Histograms in DBS-on (red) and DBS-off (blue with black edges) conditions are plotted with the normalized frequency histogram of amplitude in healthy controls (HC; gray line). (C) Normalized composite amplitude histograms of microsaccades of PD subjects who showed a significant increase in saccade amplitude due to DBS. Histograms in DBS-on (red) and DBS-off (blue with no edge color) conditions are plotted with the normalized frequency histogram of amplitude in HC (gray line). (D) Normalized baseline (DBS-off) amplitudes of the two response groups that were shown in (B) (with black edge color) and (C) (no edge color). The asterisk indicates that the two distributions are statistically different. (E) Median intersaccadic interval (ISI) durations of the individuals in DBS-off and DBS-on conditions. Asterisks indicate the six patients who show a significant change in ISI due to DBS. (F) Normalized ISI histograms of PD subjects who showed a DBS-related decrease. Histograms in DBS-on (red) and DBS-off (blue with black edges) conditions are plotted with the normalized frequency histogram of amplitude in HC (gray line). (G) Normalized intersaccadic interval duration histograms of saccades of PD subjects who showed a DBS-related increase. Histograms in DBS-on (red) and DBS-off (blue with no edge color) conditions are plotted with the normalized frequency histogram of amplitude in HC (gray line). (H) Normalized baseline (DBS-off) ISI of the two response groups that were shown in (F) (with black edge color) and (G) (no edge color). The asterisk indicates that the two distributions are statistically different. Adapted from Beylergil SB, Murray J, Noecker AM et al. Effects of subthalamic deep brain stimulation on fixational eye movements in Parkinson’s disease. J Comput Neurosci. 49(3):345–356, Springer Nature, 2021, adapted with permission from SNCSC.13 |
In contrast, DBS decreases the fixational saccade amplitude when the saccade amplitude is smaller at baseline. Figure 2D depicts the comparison of baseline microsaccade amplitude. While there is a significant difference in baseline saccade amplitude between the two PD groups, the difference between fixational saccade amplitude disappears when the DBS is turned on. In other words, subthalamic DBS eliminates the intersubject disparity in the range of fixational saccade amplitude, increasing the amplitude in those with baseline decrease and vice versa.
The intersaccadic intervals (ISIs) between adjacent fixational saccades are also affected by PD and modified by STN DBS (Figure 2E). As a group, ISIs are shorter in PD than healthy controls, and DBS further modulates the intervals. Again, there are two discrete groups of PD patients: in one group, subthalamic DBS decreases ISI (Figure 2F), while ISIs are increased in the other group (Figure 2G). The PD patients where DBS increases ISIs have more frequent microsaccades at baseline, and vice versa when DBS decreases the ISIs (Figure 2H). PD patients with DBS have frequent oblique saccades compared to the same group of patients when their DBS is turned off; this is the same group of PD patients where DBS increases the microsaccade amplitude. Such differences are, however, absent in the group where DBS decreases the microsaccade amplitude. Fixational saccades, as their amplitude increases, become part of a squarewave jerk. The number of squarewave jerks to fixational saccade ratio changes with DBS. Subthalamic DBS increases the ratio in nearly 70% of PD patients, it is decreased in remaining 30%. The relationship between the fixational saccade to squarewaves is described by a coupling mechanism.25,35,36 There is a robust coupling in healthy controls and PD with DBS on, but not so in PD with DBS off.
Strabismus
Strabismus in PD presents with non-specific complaints, sometimes diplopia, blurred vision, or reading difficulty. For example, in one study of 39 PD patients, about one-fourth of the cohort reported tired eyes or blurred vision while reading, but only 7% reported diplopia (tired eyes or blurred vision 9/37, diplopia 3/37). Other studies reported diplopia in up to 20% of PD patients, and all subjects in a study of 44 Parkinson’s disease patients with diplopia also had convergence insufficiency.5,6 The prevalence of strabismus in PD could be due to the critical role of dopamine in the vergence pathway, with disruption of the such mechanism leading to vergence impairment and strabismus.
The convergence insufficiency in PD improves with levodopa or DBS in conjunction with levodopa.2,37 In this study of 27 PD patients, there was an improvement in convergence amplitude in the “on” medication phase compared to “off” medication phase. Of note, although the “on” medication phase improved, the amplitude was worse compared to healthy controls. Many PD patients had substantial exotropia at near, but there was no difference in the mean exodeviation or ocular ductions with medication on/off periods.2 The fluctuation in convergence ability through the day poses a significant challenge in the ophthalmic management of PD patients, significantly affecting their quality of life. Such fluctuation also underscores the importance of considering the medication timing while performing ophthalmological examination in PD patients.
Eye deviation is a known side effect of subthalamic DBS.38 Transient diplopia is common in patients undergoing DBS surgery, but the deficit usually resolves by changing the stimulation parameters.39,40 The mechanistic underpinning of DBS-induced diplopia is related to electrical overflow into the structures next to the STN, such as the corticospinal and corticobulbar tracts, as they pass through the internal capsule, lateral to the STN. It is not unlikely for typically placed STN DBS to affect the supra bulbar fibers of the extraocular motor nerve or nuclei, as these fibers pass along the border of the red nucleus and are affected by implants placed too far medially.38 A case of hypertropia resulting in vertical diplopia was also reported as a side effect of DBS implantation in PD. However, it was due to the hemorrhage at the implantation site, not the stimulation itself.41 In summary, strabismus and diplopia are established side effects of DBS. They should be monitored closely in the postoperative course to ensure that such iatrogenic symptoms do not impact the quality of life in PD.
Examining the qualities of strabismus and vergence insufficiency in PD offers an important insight into the disease pathophysiology, providing possible biomarkers of the disease progression. Although strabismus and vergence insufficiency in isolation are neither necessary nor sufficient for the disease diagnosis, their correlation with the disease severity may offer an important progression marker. For example, in a study of 39 PD patients, a correlation was found between binocular abnormalities and disease severity.42 The response of convergence insufficiency to conventional PD treatment supports its correlation with overall disease pathophysiology and symptomatology.
Vergence
PD significantly affects the perception of dimension and depth. Two types of synchronous eye movements in the opposite direction (ie vergence) facilitate such a task. These two types of vergence eye movements, convergence (looking from far to near) and divergence (looking from near to far) are paired with accommodation to assure proper focal length of the intraocular lens so that the image projected on the retina is sharp (ie blur prevention). Therefore, there are two drives for triggering vergence: disparity in the image depth, and a blur on the retina.42–46 Vergence and accommodation are cross-coupled (Figure 3), and as a result, accommodation is triggered by the change in disparity alone or the change in blur alone.47 Several neural substrates participate in accommodation and vergence, some transmitting motor information, generally downstream after the cross-coupling between accommodation and vergence has occurred (eg nodes “A” and “V” in Figure 3). The downstream motor signal equally responds to blur or disparity-driven vergence. On the contrary, the sensory or sensorimotor convergent signal (ie the blur or disparity controllers, Figure 3) is upstream and differently influenced from blur versus disparity-driven signals. PD patients seem to have blur-driven vergence function similar to age-matched healthy controls.9,48 In contrast, disparity-driven vergence is significantly different in PD compared to healthy controls.10,11
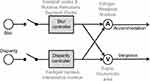 |
Figure 3 Schematic model of disparity and blur-driven vergence system. Each arm, one leading to vergence and the other leading to accommodation, is coupled to the other. There are four nodes of controllers. Blur controller and disparity controllers are upstream before cross-coupling occurs, while nodes “A” and “V” are downstream after cross-coupling has occurred. The upstream controllers are sensory while downstream nodes are motor. Known anatomical and physiological organization suggests that cerebral cortex and nucleus reticularis tegmenti pontis act as blur controller; fastigial and interpositus nucleus are disparity controller, node A is the Edinger-Westphal nucleus while node V is the supraoculomotor nucleus. Adapted with permission from Wolters Kluwer Health, Inc.: Gupta P, Beylergil S, Murray J et al. Effects of Parkinson Disease on Blur-Driven and Disparity-Driven Vergence Eye Movements. J Neuroophthalmol. 2021;41(4):442–451. Available from: https://journals.lww.com/jneuro-ophthalmology/Fulltext/2021/12000/Effects_of_Parkinson_Disease_on_Blur_Driven_and.4.aspx.10 |
Figure 4A illustrates an example of eye position trajectory and standard deviation around the mean in PD patients and healthy control making convergence eye movements while viewing binocularly, hence triggering disparity-driven vergence. Unlike healthy controls, PD patients had substantial limitations in generating disparity-driven vergence. The same task of making convergence eye movement is illustrated in Figure 4B, but done in monocular viewing, triggering blur-driven vergence. It is noteworthy that blur-driven vergence is impaired in PD and healthy controls, suggesting its impairment is not exclusive to the pathophysiology of PD.
 |
Figure 4 Comparison of disparity- and blur-driven vergence measured from PD patients and healthy controls. (A and B) The differences between right and left eyes (vergence) eye positions are plotted on the y-axis, corresponding time is plotted on the x-axis. The black line depicts the mean position in PD patients, while the blue line depicts the control subjects. The light shades (grey and light blue) depict the standard deviation of spread around the mean. (A) Depicts gaze shift in binocular viewing condition depicting disparity-driven vergence. (B) Depicts gaze shift in monocular viewing condition depicting blur-driven vergence. The overlapping values depict the lack of difference between healthy controls and PD patients in the case of blur-driven vergence. Readers are referred to Gupta et al 202110 for detailed patient demographics. Adapted with permission from Wolters Kluwer Health, Inc.: Gupta P, Beylergil S, Murray J et al. Effects of Parkinson Disease on Blur-Driven and Disparity-Driven Vergence Eye Movements. J Neuroophthalmol. 2021;41(4):442–451. Available from: https://journals.lww.com/jneuro-ophthalmology/Fulltext/2021/12000/Effects_of_Parkinson_Disease_on_Blur_Driven_and.4.aspx.10 |
The fundamental abnormality leading to impaired vergence in PD leads to four compensatory strategies, as depicted in Figure 5. One strategy incorporates pure “slow” vergence gaze shift, called “pure slow” (Figure 5A). The second strategy includes saccades, called “pure fast” (Figure 5B). The third and fourth strategies combine slow and fast, either slow followed by fast or vice versa (Figure 5C and D). Unlike disparity-driven vergence, blur-driven vergence had only two strategies: fast followed by slow (Figure 5E) or pure fast (Figure 5F).
 |
Figure 5 Different gaze-shifting strategies to compensate for disparity- and blur-driven vergence deficits in PD patients. The vergence position is plotted on y-axis while corresponding time is plotted on x-axis. Grey line depicts desired target position. Panels A-D depict strategies in binocular viewing: (A) pure slow, (B) pure fast, (C) slow fast, (D) fast slow. Panels E and F depict strategies in monocular viewing: (E) fast slow, (F) pure fast. Readers are referred to Gupta et al 202110 for detailed patient demographics. Adapted with permission from Wolters Kluwer Health, Inc.: Gupta P, Beylergil S, Murray J et al. Effects of Parkinson Disease on Blur-Driven and Disparity-Driven Vergence Eye Movements. J Neuroophthalmol. 2021;41(4):442–451. Available from: https://journals.lww.com/jneuro-ophthalmology/Fulltext/2021/12000/Effects_of_Parkinson_Disease_on_Blur_Driven_and.4.aspx.10 |
Figure 6A, B, E and F summarizes the prevalence of four strategies for disparity-driven vergence in PD patients and healthy controls. As expected, pure slow movements are most common in healthy controls, followed by a slow-fast and pure fast. On the contrary, vergence movements were absent or preceded by fast saccades in most instances when PD patients attempted disparity-driven vergence; slow-fast or pure-fast were also seen. Pure slow strategy, the majority in healthy control, were much less frequent in PD (Figure 6A, B, E and F). Monocular viewing condition, depicting blur-driven component, has prominent fast-slow movements followed by a pure fast. The distribution was comparable in healthy controls and PD patients (Figure 6C, D, G and H).
 |
Figure 6 Gaze-shift strategies utilized by healthy controls and PD patients. For healthy controls, (A and B) depict strategies in binocular viewing during (A) convergence and (B) divergence, while (C and D) depict strategies in monocular viewing during (C) convergence and (D) divergence. For PD patients, (E and F) depict strategies in binocular viewing during (E) convergence and (F) divergence, while (G and H) depict strategies in monocular viewing during (G) convergence and (H) divergence. Readers are referred to Gupta et al 202110 for detailed patient demographics. Adapted with permission from Wolters Kluwer Health, Inc.: Gupta P, Beylergil S, Murray J et al. Effects of Parkinson Disease on Blur-Driven and Disparity-Driven Vergence Eye Movements. J Neuroophthalmol. 2021;41(4):442–451. https://journals.lww.com/jneuro-ophthalmology/Fulltext/2021/12000/Effects_of_Parkinson_Disease_on_Blur_Driven_and.4.aspx.10 |
PD not only affects the ability to make vergence eye movements, but it also impacts the latency with which vergence is made. In addition to increased vergence latency, the velocity is also decreased.9–11 It is also shown that decreasing the vergence velocity gain correlates with the propensity for generating compensatory fast, saccadic eye movements.10,11 These findings are consistent with previous studies in non-human primates, which described separate brain areas controlling divergence and convergence and noted the significant role that the midbrain supra oculomotor area plays in controlling vergence movements.49,50 The mesencephalic reticular formation, involved in mediating vergence velocity, is complemented by a separate group of convergence burst cells located in the dorsal mesencephalic region rostral to the superior colliculus.51 It is possible that a more robust neural network is in place for mediating the convergence eye movements, enabling them to compensate for motor insufficiency seen in PD.
Saccades
PD affects saccades made to novel visual targets (ie visually-guided saccades), but even more so, the saccades to the remembered locations (ie memory-guided saccades). Impaired visually-guided saccades may affect patients’ ability to read seamlessly and precisely scan their visual environment. PD patients also have a greater anti-saccadic (ie inhibition of reflexive saccades) error rate compared to healthy individuals.4,52 Impairment of visually-guided saccades and anti-saccades is present early in PD, and both have been associated with cognitive dysfunction in PD.4,53,54 The study of saccadic eye movements in PD offers a prototype to examine the physiology of complex motor and perceptual phenomena. Such studies often elucidate etiologies of motor abnormalities in physiologically simpler circuits of visually guided saccades. By delineating such pathophysiology, we can better understand complex motor and perceptual phenomenologies of the movement disorders in PD.
The superior colliculus is the critical structure for visuomotor coordination; its malfunction leads to abnormal saccade scaling, as seen in PD.55,56 Visually guided saccades in PD are hypometric in the setting of increased inhibition of the superior colliculus through the abnormal basal ganglia output via SNr; this pathway also affects pre-attentional visual processing and saccade execution.28,29,57,58 There is a reduced fMRI activation of the frontal eye fields in PD patients in the preparatory phase of the visually-guided saccade.59,60 It was suggested that the underlying physiology might involve the superior colliculus, which sends corollary information to the frontal ocular motor and occipital visual regions at the onset of eye movement.61,62 It was also suggested that interruption or malprogramming of saccade size and curvature, as seen in PD and progressive supranuclear palsy (though to a smaller extent), could be partially due to an abnormality affecting the brainstem burst generators.
The example of a visually-guided saccade from a PD patient and a healthy control is illustrated in Figure 7. The saccadic eye movement in the healthy control is uninterrupted, as evident from a single peak in the saccade velocity (Figure 7A and B). The trajectory of the shown saccade from the healthy control is straight (Figure 7C). Unlike the healthy control, the PD patient has an interruption in the ongoing saccade (Figure 7D). The interruption is prominent in the vertical axis and is clearly shown in the plotted velocity trace (Figure 7E). The saccade is not only interrupted, but it also has a curved trajectory (Figure 7F). The saccade depicted in Figure 7D-F is a vertical saccade. The interruptions are also seen in horizontal saccade (Figure 7G and H), but their curvature irregularity is less pronounced (Figure 7I).
 |
Figure 7 Examples of visually guided saccades from healthy subject (A-C) and PD patient (G-I). The black line depicts the right eye, and the grey trace depicts the left eye. In the first row of subplots, the eye position is plotted on the y-axis while the x-axis depicts the corresponding time in seconds. (A) Illustrates a normal visually-guided vertical saccade from a healthy subject. (D and G) Depict examples of visually-guided (D) vertical and (G) horizontal saccades from the same PD subject. Red arrows depict interruption in ongoing saccades. The middle row of subplots depicts eye velocity; eye velocity is plotted on the y-axis while the x-axis illustrates the corresponding time. (B) Depicts the eye velocity of a normal visually-guided saccade recorded from a healthy subject, while (E and H) depict vertical and horizontal eye velocity respectively from a PD patient. Red arrows illustrate the interruptions in a saccade when the eye velocity was zero (H) or when the eye moved at slower velocity in the opposite direction (E). The bottom row of the subplots depict trajectories of horizontal and vertical saccades; the green dot is start point, and the red dot is the stop point. (C) Depicts normal saccade from the healthy subject, while panel F and I depict (F) vertical and (I) horizontal saccades in a PD patient. Arrows in (F) highlight curvature in the saccade trajectory. Adapted from Prog Brain Res. 249, Shaikh AG, Ghasia FF. Saccades in Parkinson’s disease: Hypometric, slow, and maladaptive. 81–94, Copyright 2019, with permission from Elsevier.63 |
Three types of abnormalities in visually-guided saccades in PD subjects—frequent interruption of the ongoing saccade, irregular saccade trajectory resulting in longer time to complete the movement, and slowing of segmented saccade velocity—can be explained by maladaptation in burst generation and colliculus network. Timely saccade initiation requires transient cessation of the tonic GABAergic inhibition from the SNr neurons on the superior colliculus.33,64–66 Removal of such inhibition by injecting muscimol in the SNr causes saccadic intrusions and contralateral spontaneous saccades.28,29 In contrast, the electrical stimulation of SNr causes decreased latency and hypometria of visually-guided saccades.57 PD is expected to disinhibit SNr, prematurely interrupting the ongoing saccades by imposing phasic inhibition to the superior colliculus. This theory, however, predicts equal impairment in horizontal and vertical saccades and saccade velocity always reaching zero during the break (ie complete cessation of the eye movements during saccades). However, data in PD patients are inconsistent with such prediction (Figure 7), hence an alternative explanation is needed.63
The abnormal saccades in PD may be due to pathology in the burst generators. The saccade signals from the cerebral cortex follow two pathways: the nucleus reticularis tegmenti pontis (NRTP) of the pontine reticular formation, and the superior colliculus.67 The projections of the NRTP go to the oculomotor vermis (OMV), located in lobules 5–7 of the cerebellum.67 OMV projects to the caudal fastigial nucleus (the fastigial oculomotor region, FOR) via GABAergic inhibition. The FOR projects to the omnidirectional pause neurons (OPN) in the nucleus raphe interpositus of the midline pons, and inhibitory and excitatory burst neurons (IBNs and EBNs, respectively).67 The choke in external inhibition triggers an abrupt peak in firing, known as the post-inhibitory rebound (PIR), necessary for high saccade velocity.68–73 The abrupt cessation of the OPN activity and spike in the burst neuron firing is correlated with saccade velocity and acceleration.68,69,72,73 The caveat of this theory is that the OPNs equally affect horizontal and vertical saccades via its influence on NRTP; therefore, an OPN abnormality alone does not account for the asymmetric involvement of horizontal and vertical saccades. However, burst neurons for horizontal and vertical saccades are located in different anatomical regions; hence impaired excitation of vertical and horizontal saccades generators can result in differences in abnormal matrices of horizontal and vertical saccades in PD.
If stimulated during the saccade, the superior colliculus fixation zone can cause directional changes in the ongoing eye movements.74–76 The redirected saccade path depends upon the coordinates of the stimulated site in the collicular fixation zone.76 Idiosyncrasy and irregularity in the saccade trajectory in PD may be explained by premature activation of superior colliculus fixation zones.63 It is possible that PD first affects EBNs and IBNs for vertical saccades, and a degenerative lesion of IBNs causes an asynchronous, irregular discharge pattern during an ongoing vertical saccade.63 The such discharge pattern of IBNs during an ongoing vertical saccade is inferred as a command to stop the eye movement, hence the abrupt interruption of the ongoing saccade. It is then forwarded into a feedback loop to the superior colliculus, leading to superior colliculus fixation zone stimulation. Such unwanted activation of the superior colliculus leads to redirected saccades in an arbitrary direction. Such predictions underlying the pathophysiology of abnormal visually-guided saccades are supported by the histology literature in PD, which suggests the involvement of the mesencephalic reticular formation in the area for vertical saccades.77–79 In other words, it was predicted that the irregularities and slowing, prominently seen in the vertical saccades in PD, could be due to impaired function of EBNs and IBNs leading to maladaptive feedback causing the premature activation of the superior colliculus.63
Summary
Abnormal eye movements in PD include abnormal fixational saccades, strabismus, impaired convergence, and impaired visually-guided saccades. Abnormal fixational saccades in PD are thought to be modulated by the basal ganglia, as a result of increased inhibition of the superior colliculus by SNr. With subthalamic DBS, the characteristics of the fixational eye movements are reset to a different baseline, corroborating the basal ganglia’s role in gaze fixation. Strabismus in PD suggests a critical role of dopamine in the vergence pathway, further supported by the improvement of convergence with levodopa and the DBS. Furthermore, these binocular abnormalities correlate with disease severity and may possess a utility as a disease progression marker. PD patients demonstrate more impairment in disparity-driven convergence compared to healthy individuals, with differing adaptive strategies for convergence (fast saccades in PD, slow movements in healthy individuals).
Furthermore, PD patients demonstrate increased latency and the decreased velocity of vergence eye movements. Vergence is largely controlled by the supraoculomotor area in the midbrain, involving the mesencephalic reticular formation and burst cells. The pathophysiology of eye deviation due to DBS differs from that, causing strabismus and vergence insufficiency in PD. Clinically, monitoring for eye deviation in postoperative period following DBS implantation is prudent. Visually-guided saccades are frequently interrupted, irregular in trajectory, and often slow in PD. Visually-guided saccades are mediated through the superior colliculus; increased inhibition through SNr, decreased activation of frontal eye fields, and abnormalities in brainstem burst generators contribute to saccadic abnormalities in PD. Altogether, eye movement abnormalities in PD not only offer insight into the pathophysiology of the disease and the networks involved, it also may prove to be a viable marker for disease monitoring in the future.
Acknowledgments
Grants from the US Department of Veterans Affairs SPiRE I21-RX003878-01 (Shaikh), The American Academy of Neurology (Shaikh), American Parkinson’s Disease Association George C Cotzias Memorial Fellowship (Shaikh), Dystonia Medical Research Foundation Research (Shaikh), and philanthropic funds to the Department of Neurology at University Hospitals (The Allan Woll Fund). Shaikh also has Penni and Steven Weinberg Chair in Brain Health.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Parkinson’s Foundation. Statistics; 2023. Available from: https://www.parkinson.org/understanding-parkinsons/statistics.
2. Almer Z, Klein KS, Marsh L, Gerstenhaber M, Repka MX. Ocular Motor And Sensory Function In Parkinson’s disease. Ophthalmology. 2012;119(1):178–182. doi:10.1016/j.ophtha.2011.06.040
3. Armstrong RA. Oculo-visual dysfunction in Parkinson’s disease. J Parkinson’s Dis. 2015;5(4):715–726. doi:10.3233/jpd-150686
4. Antoniades CA, Demeyere N, Kennard C, Humphreys GW, Hu MT. Antisaccades and executive dysfunction in early drug-naive Parkinson’s disease: the discovery study. Mov Disord. 2015;30(6):843–847. doi:10.1002/mds.26134
5. Lepore FE. Parkinson’s Disease and Diplopia. Neuro-Ophthalmology. 2006;30(2–3):37–40. doi:10.1080/01658100600742838
6. Urwyler P, Nef T, Killen A, et al. Visual complaints and visual hallucinations in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(3):318–322. doi:10.1016/j.parkreldis.2013.12.009
7. Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19(8):871–884. doi:10.1002/mds.20115
8. Davidsdottir S, Wagenaar R, Young D, Cronin-Golomb A. Impact of optic flow perception and egocentric coordinates on veering in Parkinson’s disease. Brain. 2008;131(Pt 11):2882–2893. doi:10.1093/brain/awn237
9. Hanuska J, Bonnet C, Rusz J, et al. Fast vergence eye movements are disrupted in Parkinson’s disease: a video-oculography study. Parkinsonism Relat Disord. 2015;21(7):797–799. doi:10.1016/j.parkreldis.2015.04.014
10. Gupta P, Beylergil S, Murray J, et al. Effects of Parkinson disease on blur-driven and disparity-driven vergence eye movements. J Neuroophthalmol. 2021;41(4):442–451. doi:10.1097/WNO.0000000000001422
11. Gupta P, Beylergil S, Murray J, Kilbane C, Ghasia FF, Shaikh AG. Computational models to delineate 3D gaze-shift strategies in Parkinson’s disease. J Neural Eng. 2021;18(4):346.
12. Beylergil SB, Murray J, Noecker AM, et al. Temporal patterns of spontaneous fixational eye movements: the influence of basal ganglia. J Neuroophthalmol. 2022;42(1):45–55. doi:10.1097/WNO.0000000000001452
13. Beylergil SB, Murray J, Noecker AM, et al. Effects of subthalamic deep brain stimulation on fixational eye movements in Parkinson’s disease. J Comput Neurosci. 2021;49(3):345–356. doi:10.1007/s10827-020-00773-2
14. Gitchel GT, Wetzel PA, Baron MS. Pervasive ocular tremor in patients with Parkinson disease. Arch Neurol. 2012;69(8):1011–1017. doi:10.1001/archneurol.2012.70
15. Kaski D, Bronstein AM. Ocular tremor in Parkinson’s disease: discussion, debate, and controversy. Front Neurol. 2017;8:134. doi:10.3389/fneur.2017.00134
16. Saifee TA, Kaski D, Buckwell D, Bronstein AM. Tremor of the eyes in Parkinson’s disease: merely a measure of the head movement. Parkinsonism Relat Disord. 2014;20(12):1447–1448. doi:10.1016/j.parkreldis.2014.07.009
17. Kaski D, Saifee TA, Buckwell D, Bronstein AM. Ocular tremor in Parkinson’s disease is due to head oscillation. Mov Disord. 2013;28(4):534–537. doi:10.1002/mds.25342
18. Kaski D, Saifee TA, Buckwell D, Bronstein AM. Reply to letter-eye oscillations in Parkinson’s disease relate to the vestibulo-ocular reflex. Mov Disord. 2013;28(6):845–846. doi:10.1002/mds.25465
19. Troncoso XG, Macknik SL, Martinez-Conde S. Microsaccades counteract perceptual filling-in. J Vis. 2008;8(14):15 1–9. doi:10.1167/8.14.15
20. Martinez-Conde S. Fixational eye movements in normal and pathological vision. Prog Brain Res. 2006;154:151–176. doi:10.1016/S0079-6123(06)54008-7
21. Martinez-Conde S, Macknik SL, Troncoso XG, Dyar TA. Microsaccades counteract visual fading during fixation. Neuron. 2006;49(2):297–305. doi:10.1016/j.neuron.2005.11.033
22. Abadi RV, Gowen E. Characteristics of saccadic intrusions. Vision Res. 2004;44(23):2675–2690. doi:10.1016/j.visres.2004.05.009
23. Zhou H, Wang X, Ma D, et al. The differential diagnostic value of a battery of oculomotor evaluation in Parkinson’s disease and multiple system atrophy. Brain Behav. 2021;11(7):e02184. doi:10.1002/brb3.2184
24. Shaikh AG, Xu-Wilson M, Grill S, Zee DS. ‘Staircase’ square-wave jerks in early Parkinson’s disease. Br J Ophthalmol. 2011;95(5):705–709. doi:10.1136/bjo.2010.179630
25. Otero-Millan J, Schneider R, Leigh RJ, Macknik SL, Martinez-Conde S. Saccades during attempted fixation in parkinsonian disorders and recessive ataxia: from microsaccades to square-wave jerks. PLoS One. 2013;8(3):e58535. doi:10.1371/journal.pone.0058535
26. Averbuch-Heller L, Stahl JS, Hlavin ML, Leigh RJ. Square-wave jerks induced by pallidotomy in parkinsonian patients. Neurology. 1999;52(1):185–188. doi:10.1212/wnl.52.1.185
27. Fisk JD, Goodale MA, Burkhart G, Barnett HJ. Progressive supranuclear palsy: the relationship between ocular motor dysfunction and psychological test performance. Neurology. 1982;32(7):698–705. doi:10.1212/wnl.32.7.698
28. Wurtz RH, Hikosaka O. Role of the basal ganglia in the initiation of saccadic eye movements. Prog Brain Res. 1986;64:175–190. doi:10.1016/S0079-6123(08)63412-3
29. Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol. 1985;53(1):266–291.
30. Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983;49(5):1268–1284. doi:10.1152/jn.1983.49.5.1268
31. Hikosaka O, Wurtz RH. Effects on eye movements of a GABA agonist and antagonist injected into monkey superior colliculus. Brain Res. 1983;272(2):368–372. doi:10.1016/0006-8993(83)90586-3
32. Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol. 1983;49(5):1230–1253. doi:10.1152/jn.1983.49.5.1230
33. Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol. 1983;49:1285–1301.
34. Hikosaka O, Wurtz RH. Saccadic eye movements following injection of lidocaine into the superior colliculus. Exp Brain Res. 1986;61(3):531–539. doi:10.1007/BF00237578
35. Otero-Millan J, Serra A, Leigh RJ, Troncoso XG, Macknik SL, Martinez-Conde S. Distinctive features of saccadic intrusions and microsaccades in progressive supranuclear palsy. J Neurosci. 2011;31(12):4379–4387. doi:10.1523/JNEUROSCI.2600-10.2011
36. Otero-Millan J, Macknik SL, Serra A, Leigh RJ, Martinez-Conde S. Triggering mechanisms in microsaccade and saccade generation: a novel proposal. Ann N Y Acad Sci. 2011;1233:107–116. doi:10.1111/j.1749-6632.2011.06177.x
37. Racette BA, Gokden M, Tychsen L, Perlmutter JS. Convergence insufficiency in idiopathic Parkinson’s disease responsive to levodopa. Strabismus. 1999;7(3):169–174. doi:10.1076/stra.7.3.169.636
38. Shields DC, Gorgulho A, Behnke E, Malkasian D, DeSalles AA. Contralateral conjugate eye deviation during deep brain stimulation of the subthalamic nucleus. J Neurosurg. 2007;107(1):37–42. doi:10.3171/jns-07/07/0037
39. Krack P, Fraix V, Mendes A, Benabid AL, Pollak P. Postoperative management of subthalamic nucleus stimulation for Parkinson’s disease. Mov Disord. 2002;17(Suppl 3):S188–97.
40. Wang Y, Liu ZY, Dou WC, Ma WB, Wang RZ, Guo Y. Application of Preoperative CT/MRI Image Fusion in Target Positioning for Deep Brain Stimulation. Chine Med Sci j. 2016;31(3):161–167.
41. Ortiz-Perez S, Sanchez-Dalmau B, Molina J, Adan A, Candela S, Rumia J. Ocular tilt reaction as a delayed complication of deep brain stimulation for Parkinson disease. J Neuroophthalmol. 2009;29(4):286–288. doi:10.1097/WNO.0b013e3181b2822d
42. Fincham EF, Walton J. The reciprocal actions of accommodation and convergence. J Physiol. 1957;137(3):488–508. doi:10.1113/jphysiol.1957.sp005829
43. Krishnan VV, Phillips S, Stark L. Frequency analysis of accommodation, accommodative vergence and disparity vergence. Vision Res. 1973;13(8):1545–1554. doi:10.1016/0042-6989(73)90013-8
44. Krishnan VV, Stark L. A heuristic model for the human vergence eye movement system. IEEE Trans Biomed Eng. 1977;24(1):44–49. doi:10.1109/TBME.1977.326207
45. Rashbass C, Westheimer G. Independence of conjugate and disjunctive eye movements. J Physiol. 1961;159:361–364. doi:10.1113/jphysiol.1961.sp006813
46. Hung GK, Semmlow JL. Static behavior of accommodation and vergence: computer simulation of an interactive dual-feedback system. IEEE Trans Biomed Eng. 1980;27(8):439–447. doi:10.1109/TBME.1980.326752
47. Amphetamine WG. Barbiturates, and accommodation-convergence. Arch Ophthalmol. 1963;70:830–836. doi:10.1001/archopht.1963.00960050832019
48. Repka MX, Claro MC, Loupe DN, Reich SG. Ocular motility in Parkinson’s disease. J Pediatr Ophthalmol Strabismus. 1996;33(3):144–147.
49. Das VE. Cells in the supraoculomotor area in monkeys with strabismus show activity related to the strabismus angle. Ann N Y Acad Sci. 2011;1233:85–90. doi:10.1111/j.1749-6632.2011.06146.x
50. Das VE. Responses of cells in the midbrain near-response area in monkeys with strabismus. Invest Ophthalmol Vis Sci. 2012;53(7):3858–3864. doi:10.1167/iovs.11-9145
51. Mays LE, Porter JD, Gamlin PD, Tello CA. Neural control of vergence eye movements: neurons encoding vergence velocity. J Neurophysiol. 1986;56(4):1007–1021.
52. Rivaud-Pechoux S, Vidailhet M, Brandel JP, Gaymard B. Mixing pro- and antisaccades in patients with parkinsonian syndromes. Brain. 2007;130(Pt 1):256–264. doi:10.1093/brain/awl315
53. Stuart S, Lawson RA, Yarnall AJ, et al. Pro-Saccades Predict Cognitive Decline in Parkinson’s Disease: ICICLE-PD. Mov Disord. 2019;34(11):1690–1698. doi:10.1002/mds.27813
54. Hanuska J, Rusz J, Bezdicek O, et al. Eye movements in idiopathic rapid eye movement sleep behaviour disorder: high antisaccade error rate reflects prefrontal cortex dysfunction. J Sleep Res. 2019;28(5):e12742. doi:10.1111/jsr.12742
55. Terao Y, Fukuda H, Yugeta A, et al. Initiation and inhibitory control of saccades with the progression of Parkinson’s disease - changes in three major drives converging on the superior colliculus. Neuropsychologia. 2011;49(7):1794–1806. doi:10.1016/j.neuropsychologia.2011.03.002
56. Terao Y, Fukuda H, Ugawa Y, Hikosaka O. New perspectives on the pathophysiology of Parkinson’s disease as assessed by saccade performance: a clinical review. Clin Neurophysiol. 2013;124(8):1491–1506. doi:10.1016/j.clinph.2013.01.021
57. Basso MA, Liu P. Context-dependent effects of substantia nigra stimulation on eye movements. J Neurophysiol. 2007;97(6):4129–4142. doi:10.1152/jn.00094.2007
58. Lieb K, Brucker S, Bach M, Els T, Lucking CH, Greenlee MW. Impairment in preattentive visual processing in patients with Parkinson’s disease. Brain. 1999;122(Pt 2):303–313.
59. Rieger JW, Kim A, Argyelan M, et al. Cortical functional anatomy of voluntary saccades in Parkinson disease. Clin EEG Neurosci. 2008;39(4):169–174. doi:10.1177/155005940803900404
60. Javaid MA, Weeden J, Flom P, Avitable M, Glazman S, Bodis-Wollner I. Perisaccadic gamma modulation in Parkinson disease patients and healthy subjects. Clin EEG Neurosci. 2010;41(2):94–101. doi:10.1177/155005941004100209
61. Sommer MA, Wurtz RH. Visual perception and corollary discharge. Perception. 2008;37(3):408–418. doi:10.1068/p5873
62. Javaid MA, Amassian V, Glazman S, Fesharaki A, Stefanov D, Bodis-Wollner I. Cortical control of voluntary saccades in Parkinson’s disease and pre-emptive perception. Parkinsonism Relat Disord. 2012;18 Suppl 1:S100–3. doi:10.1016/S1353-8020(11)70032-3
63. Shaikh AG, Ghasia FF. Saccades in Parkinson’s disease: hypometric, slow, and maladaptive. Prog Brain Res. 2019;249:81–94. doi:10.1016/bs.pbr.2019.05.001
64. Fisher RS, Buchwald NA, Hull CD, Levine MS. The GABAergic striatonigral neurons of the cat: demonstration by double peroxidase labeling. Brain Res. 1986;398(1):148–156.
65. Francois C, Percheron G, Yelnik J. Localization of nigrostriatal, nigrothalamic and nigrotectal neurons in ventricular coordinates in macaques. Neuroscience. 1984;13(1):61–76.
66. Handel A, Glimcher PW. Quantitative analysis of substantia nigra pars reticulata activity during a visually guided saccade task. J Neurophysiol. 1999;82(6):3458–3475.
67. Leigh RJ, Zee DS. The Neurology of Eye Movements. Oxford University Press; 2015.
68. Enderle JD, Engelken EJ. Simulation of oculomotor post-inhibitory rebound burst firing using a Hodgkin-Huxley model of a neuron. Biomed Sci Instrum. 1995;31:53–58.
69. Miura K, Optican LM. Membrane channel properties of premotor excitatory burst neurons may underlie saccade slowing after lesions of omnipause neurons. J Comput Neurosci. 2006;20(1):25–41.
70. Optican L. Saccadic burst cell membrane dysfunction is responsible for saccadic oscillations. J Neuroophthalmol. 2008;28(4):329–336.
71. Shaikh AG, Miura K, Optican LM, Ramat S, Leigh RJ, Zee DS. A new familial disease of saccadic oscillations and limb tremor provides clues to mechanisms of common tremor disorders. Brain. 2007;130(Pt 11):3020–3031. doi:10.1093/brain/awm240
72. Shaikh AG, Wong AL, Optican LM, Miura K, Solomon D, Zee DS. Sustained eye closure slows saccades. Vision Res. 2010;50(17):1665–1675. doi:10.1016/j.visres.2010.05.019
73. Shaikh AG, Zee DS, Optican LM, Miura K, Ramat S, Leigh RJ. The effects of ion channel blockers validate the conductance-based model of saccadic oscillations. Ann N Y Acad Sci. 2011;1233:58–63. doi:10.1111/j.1749-6632.2011.06130.x
74. Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol. 1993;70(2):559–575.
75. Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J Neurophysiol. 1993;70(2):576–589.
76. Gandhi NJ, Keller EL. Comparison of saccades perturbed by stimulation of the rostral superior colliculus, the caudal superior colliculus, and the omnipause neuron region. J Neurophysiol. 1999;82(6):3236–3253.
77. Halliday GM, Del Tredici K, Braak H. Critical appraisal of brain pathology staging related to presymptomatic and symptomatic cases of sporadic Parkinson’s disease. J Neural Transm Suppl. 2006;99–103.
78. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211.
79. Halliday GM, Li YW, Blumbergs PC, et al. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol. 1990;27(4):373–385. doi:10.1002/ana.410270405
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
