Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Monitoring and Management of Respiratory Function in Pompe Disease: Current Perspectives
Authors El Haddad L, Khan M , Soufny R, Mummy D , Driehuys B, Mansour W , Kishnani PS, ElMallah MK
Received 13 May 2023
Accepted for publication 14 August 2023
Published 1 September 2023 Volume 2023:19 Pages 713—729
DOI https://doi.org/10.2147/TCRM.S362871
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Léa El Haddad,1 Mainur Khan,1 Rania Soufny,1 David Mummy,2 Bastiaan Driehuys,2 Wissam Mansour,3 Priya S Kishnani,4 Mai K ElMallah1
1Division of Pulmonary and Sleep Medicine, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA; 2Department of Radiology, Duke University Medical Center, Durham, NC, USA; 3Division of Pulmonary and Sleep Medicine, Department of Medicine, Duke University Medical Center, Durham, NC, USA; 4Division of Medical Genetics, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA
Correspondence: Mai K ElMallah, Division of Pulmonary and Sleep Medicine, Department of Pediatrics, Duke University Medical Center, Box 2644, Durham, NC, 27710, USA, Tel +1-919-684-3577, Email [email protected]
Abstract: Pompe disease (PD) is a neuromuscular disorder caused by a deficiency of acid alpha-glucosidase (GAA) – a lysosomal enzyme responsible for hydrolyzing glycogen. GAA deficiency leads to accumulation of glycogen in lysosomes, causing cellular disruption. The severity of PD is directly related to the extent of GAA deficiency – if no or minimal GAA is produced, symptoms are severe and manifest in infancy, known as infantile onset PD (IOPD). If left untreated, infants with IOPD experience muscle hypotonia and cardio-respiratory failure leading to significant morbidity and mortality in the first year of life. In contrast, late-onset PD (LOPD) patients have more GAA activity and present later in life, but also have significant respiratory function decline. Despite FDA-approved enzyme replacement therapy, respiratory insufficiency remains a major cause of morbidity and mortality, emphasizing the importance of early detection and management of respiratory complications. These complications include impaired cough and airway clearance, respiratory muscle weakness, sleep-related breathing issues, and pulmonary infections. This review aims to provide an overview of the respiratory pathology, monitoring, and management of PD patients. In addition, we discuss the impact of novel approaches and therapies on respiratory function in PD.
Keywords: Pompe disease, respiratory complications, respiratory monitoring, respiratory therapy
Introduction
Pompe Disease
Pompe disease (PD), or glycogen storage disease type II, is an autosomal recessive disorder caused by a deficiency of acid alpha-glucosidase (GAA) - an enzyme responsible for hydrolyzing lysosomal glycogen.1 PD is a neuromuscular disorder characterized by systemic lysosomal glycogen accumulation causing diffuse cellular disruption in muscles and motor neurons. PD encompasses a broad spectrum of disease severity ranging from severe infantile-onset to late-onset Pompe disease (IOPD and LOPD, respectively). The classification of PD is determined by the age at diagnosis, amount of the residual GAA enzyme activity, and cardiac involvement.2,3 In untreated infants with IOPD, symptoms can manifest at birth or in the first few weeks of life. These symptoms include muscle hypotonia, cardiomegaly, and respiratory decline, ultimately leading to cardiorespiratory failure and death within the first 2 years of life.2 On the other hand, LOPD can present at various ages, ranging from early childhood to adulthood. A key distinguishing feature of LOPD is the absence of cardiomyopathy in the first year of life.2
To date, the only FDA-approved treatment for PD is enzyme replacement therapy (ERT) with human recombinant GAA (rhGAA). rhGAA breaks down the excess glycogen in the lysosomes and improves muscle function. A meta-analysis performed on the efficacy of ERT in patients with LOPD revealed a significantly improved walking distance on the six-minute walk test.4 However, the respiratory function and muscle strength in these patients remained unchanged.4 In contrast, other studies have shown stable respiratory function and improved motor function with ERT in LOPD patients.5 Similarly, increased ventilator-free survival is seen in IOPD patients.6 Despite improved clinical outcomes in patients treated with ERT, the clinical response to ERT varies considerably between patients due to many factors including age, stage of treatment, dose of ERT, the Cross-Reactive Immunological Material (CRIM) status, and muscle fiber type.7,8 CRIM-positive (CRIM+) patients produce some residual but nonfunctional GAA enzyme and better tolerate ERT. They typically have low neutralizing antibody titers against rhGAA, making the treatment effective. On the other hand, CRIM negative (CRIM-) patients are unable to produce any GAA protein and generally do not tolerate ERT because of a significant immune response against rhGAA resulting in respiratory decline, ventilator dependence, and early death despite continuation of ERT treatment.7 Thus, the latter led to the investigation of immunomodulation regimens to induce immune tolerance.9,10 A combination of immune tolerance induction (ITI) protocol and ERT demonstrated clinical success by suppressing the immune response to the exogenous GAA protein and preventing the occurrence of high antibody titers. This combination of ITI and ERT is now the standard of care for CRIM- PD patients.9,10
In addition to establishing care with ERT and ITI regimens, the clinical evaluation of patients with PD is managed by a multidisciplinary team including geneticists, neurologists, cardiologists, pulmonologists, nutritionists, physical therapists, and speech therapists, working to optimize care for these patients.
Respiratory Involvement
PD results in respiratory complications that can occur in both IOPD and LOPD. These respiratory complications contribute to respiratory failure and are attributed to common various factors such as diaphragm muscle weakness, macroglossia and upper airway dysfunction.11–13 Diaphragm muscle weakness can be evident even before significant limb muscle weakness, meaning that pulmonary function can be compromised in patients with relatively minor mobility issues.14 In addition, macroglossia may also be present before the onset of limb muscle weakness.15 This upper airway pathology in both IOPD and LOPD patients can lead to dysphagia and feeding difficulties due to buccal muscle pathology, as well as motor neuron and nerve involvement in controlling the upper airways, increasing the risk of aspiration pneumonia.16 Quantitative assessment of lingual strength was evaluated in LOPD patients, ranging from mild to severe tongue weakness. Patients with dysarthria had greater lingual weakness than those without. In the 37% of participants with moderate or severe lingual weakness, dysarthria and/or dysphagia were also present.17 This early respiratory weakness highlights the need for regular respiratory monitoring in PD.
Extensive neuropathology was seen in both autopsy cases of IOPD patients and postmortem tissues from the central and peripheral nervous system of PD mouse model, specifically anterior cervical spinal cord and motor neurons,18,19 as well as in neurons throughout the ventral horn, including motor neurons of an adult LOPD patient.20 Although ventilatory failure is common in IOPD patients, many questions are still unanswered regarding the respiratory pathology and lung function data are almost completely lacking. This can be explained by the requirement for respiratory support during early stages of life and the accompanying significant orofacial weakness, making it challenging to accurately measure lung function parameters. Moreover, tracheo-bronchomalacia21 in IOPD patients or airway collapse in adults LOPD patients have been identified and described in patients with declining of their respiratory function despite ERT or diaphragm pacing.13,22
Overall, these respiratory deficits in both infantile and late onset PD lead to difficulty breathing, a reduced ability to cough and clear secretions from the airways, recurrent respiratory infections, and potentially life-threatening respiratory failure.2,23,24
Respiratory complications also cause sleep-disordered breathing as the respiratory motor units become more affected by PD.25 Diaphragm muscle weakness can lead to nocturnal hypoventilation, resulting in sleep disruption, morning headache, and excessive daytime sleepiness.25 Nocturnal hypoventilation disrupts sleep resulting in daytime somnolence, headaches, and impaired intellectual function.23 The mechanical disadvantage of the supine position along with a weak diaphragm, upper airway obstruction, and REM-related atonia26 further compromises ventilation in PD patients. Consequently, patients with PD experience reduced sleep quality and diminished health-related quality of life.23 In the absence of treatment, ventilatory failure continues to be a significant cause of morbidity and mortality as PD progresses. Regular assessment of respiratory function and early intervention can help mitigate the impact of respiratory complications, improve sleep quality, and enhance overall quality of life for individuals with PD.
Objective
This review aims to describe the respiratory complications associated with PD and provide healthcare providers of patients with PD with information on standard and novel approaches for monitoring the respiratory function (Figure 1). In addition, this review will highlight emerging therapeutic perspectives for managing respiratory complications in patients with PD (Figure 2).
Methodology
A PubMed search was carried out using the following search terms ((“Pompe disease” [MeSH]) AND (“Respiratory function” [MeSH] OR “breathing” [Title/Abstract] OR “pulmonary” [Title/Abstract] OR “Sleep apnea” [Title/Abstract] OR “monitoring” [Title/Abstract] OR “management” [Title/Abstract] AND English [Language]. The literature search was performed without time constraints to ensure that our review manuscript engulfs all the old, existing, and new perspectives on monitoring and management of the respiratory function of patients with PD in a clinical setting.
Clinical Respiratory Manifestation in Patients with PD
Respiratory Symptoms in Infantile-Onset Pompe Disease (IOPD) Patients
IOPD manifests within the first year of life, with early multiorgan involvement occurring around the median age of two months. Clinical symptoms include cardiomegaly, muscle weakness, respiratory distress, feeding difficulties, and failure to thrive, necessitating early medical evaluation.27,28 Prior to the advent of ERT, IOPD patients who exhibited symptoms before 12 months of age often required ventilator support by the age of 5.9 months, and death would typically follow shortly after, at a median age of 8.7 months.27,29 The introduction of ERT has significantly improved the survival and ventilator-free survival rates of IOPD patients.30 However, there is a distinction between patients who are cross-reactive immunologic material (CRIM) positive (CRIM+) and those who are CRIM negative (CRIM-). CRIM- patients poorly tolerate ERT (when given without immunomodulation) due to the induction of an immune response against the foreign protein, leading to death or the need for invasive ventilation by around 27.1 months of age.7 CRIM+ patients, who generally produce some amount of the deficient enzyme (GAA), exhibit a less robust immune response compared to CRIM- patients. Nonetheless, a subset of CRIM+ IOPD patients may still develop high titers of anti-rhGAA antibodies, resulting in overall and ventilator-free survival rates similar to those of CRIM- patients8 Currently, the standard approach for managing patients with CRIM- IOPD involves a combination of ERT and immune tolerance induction (ITI) therapy (described in more detail below). This combined ERT and ITI has dramatically improved the clinical outcomes in CRIM- patients.10
As PD progresses and respiratory muscle weakness worsens, patients develop an impaired cough and compromised airway clearance which significantly increases the risk of recurrent respiratory tract infections.21,28,31 An epidemiologic study by Marsden et al, revealed that respiratory distress was the most significant physical finding in 72% of patients with PD, with 46% of patients presenting with respiratory infections.32 In addition to respiratory infections, dysphagia and feeding difficulties also contribute to the heightened risk of aspiration pneumonia and life-threatening respiratory complications in PD patients.2,16 The compromised function of the muscles involved in swallowing and controlling the upper airways leads to difficulty in swallowing and an increased likelihood of food or liquid entering the lungs, resulting in aspiration pneumonia. These complications further exacerbate respiratory challenges in PD patients.
Respiratory Symptoms in Late-Onset Pompe Disease (LOPD) Patients
Patients with LOPD exhibit a broader range of disease presentation compared to patients with IOPD, with symptoms manifesting from infancy and early childhood to adulthood. LOPD is primarily characterized by muscle weakness and typically does not result in cardiomyopathy.2 The most reported pathology in LOPD patients include diaphragm, paraspinal, and proximal lower limb muscle weakness.33 Respiratory complications in LOPD arise from muscle weakness, leading to a restricted ability of the chest to expand during respiration, resulting in restrictive lung disease. This is characterized by a reduction in VC and FVC.2,34,35 Consequently, individuals with LOPD may experience dyspnea (shortness of breath) during exertion or at rest, dyspnea upon immersion in water, orthopnea (breathing difficulty while lying flat), and reduced exercise capacity.36 Prior to the initiation of ERT, FVC typically declines by an annual rate of 1.0% to 4.6%.2,35 However, early treatment with ERT in LOPD patients can prevent or attenuate the decline in respiratory function rate.37 Stabilization of pulmonary function tests (PFTs) to baseline levels can occur following initiation of ERT. The extent of stabilization depends on the disease’s initial status at the time of treatment initiation, with better clinical outcomes observed when treatment is initiated earlier.38
Lingual and pharyngeal muscle weakness is another manifestation of LOPD. This muscle weakness results in sleep-disordered breathing and obstructive sleep apnea (OSA).39 Sleep-disordered breathing is further exacerbated by diaphragm weakness, resulting in nocturnal hypoventilation.40 Treatment to cover both OSA and nocturnal hypoventilation is non-invasive positive pressure ventilation (NIPPV) via a face or nasal mask. Before initiation of NIPPV, a study of overnight polysomnography in five LOPD patients showed increased apnea/hypopnea index (AHI) ranging from 7 to 28 events per hour with significant desaturation events.41 In another study, the introduction of NIV led to significant improvement of ventilation and oxygenation without deterioration of sleep outcomes.42 In addition, LOPD patients with weakened respiratory muscles experience impaired cough and poor airway clearance, leading to an elevated risk of aspiration pneumonia and respiratory tract infections.24 In summary, disease progression in LOPD patients is slower compared to patients with IOPD, and respiratory dysfunction is the major cause of death in untreated patients.
Monitoring of the Respiratory Function in Patients with PD
Clinical Evaluation of Respiratory Function
Although PD is characterized by a decline of both respiratory and locomotor function over time, the correlation between both is weak, with some patients presenting with respiratory pathology prior to significant locomotor involvement. Thus, respiratory function evaluation should be done independently and not according to the degree of limb muscle weakness.41 The respiratory status of patients with PD should be evaluated by physicians at diagnosis, annually for stable patients, or every 6 months if respiratory function begins to decline. A screening questionnaire can be used which focuses on the history. Specifically, questions about the extent and strength of cough, presence of shortness of breath or wheezing, energy level, morning headaches, sleep disturbance, and daytime tiredness.43,44 The clinical history of the patients should include a history of respiratory infections, previous hospitalizations for serious respiratory complications, immunization status, and use of supplemental oxygen or noninvasive respiratory support.2 Monitoring of respiratory function in patients with PD is imperative, including respiratory signs and symptoms, measurement of pulmonary function tests (PFTs) in the upright and supine position, pulse oximetry, end-tidal carbon dioxide, thoracic gas volumes, maximal inspiratory and expiratory pressures, evaluation of tongue strength, and swallow function, and polysomnography studies for sleep disorders.2,45 A novel assessment of pulmonary function, ventilation, and diffusion will also be discussed. Clinical recommendations for respiratory function monitoring are summarized in Figure 1.
Pulmonary Function Testing (PFTs)
Spirometry Tests
Given the risk for progressive respiratory muscle weakness and restrictive lung disease, respiratory function testing is an essential part of the management of PD. Total lung capacity and motor function deterioration over time occurs as the disease progresses.35 Although we are not really able to test in specifically any muscle in isolation, some respiratory function testing can help differentiate, to a certain extent, between inspiratory and expiratory muscle impairment, and investigating lung function in the upright and supine positions helps determine the extent of diaphragmatic involvement, as a major inspiratory muscle.46 Since the decline in respiratory function can precede the decline in locomotor function, it is important to perform annual evaluations of PFTs including the forced vital capacity (FVC).2,41 FVC measures the maximal amount of forced exhaled air from the lungs after taking a deep breath.47 It is correlated with respiratory status, patient-reported outcomes,48 and depends on activation of both inspiratory and expiratory muscles.49 However, the characteristic abnormality of diaphragmatic weakness is a low supine FVC with a normal upright FVC detected on spirometry.50 A postural FVC decline by more than 10% suggests diaphragm weakness.49–51 Thus, a normal supine FVC makes the presence of clinically significant diaphragmatic weakness unlikely.49 In case of sleep disordered breathing (SDB), an FVC of <60% predicted along with a decrease in the supine value of FVC by greater than 10% should raise suspicion for sleep-related hypoventilation.2
Manometry Tests
In addition, manometry testing such as maximal inspiratory and expiratory pressure and sniff nasal inspiratory pressure (MIP, MEP, and SNIP, respectively) can predict respiratory muscle impairment and frequently used for testing respiratory muscle strength.2,52,53 Both SNIP and MIP, if less than 60 cmH2O, are easy, noninvasive complementary techniques that assess inspiratory muscle strength.50 However, these metrics are effort dependent and are impacted by poor technique and effort. Cough strength is affected by both inspiratory and expiratory muscle weakness, and can be assessed by the peak cough flow (PCF) using a non-invasive portable device.54 In adults with PD, PCF values higher than 270 L/min are considered normal, values between 160–270 L/min are associated with an increased risk of an ineffective cough and PCF values below ~160 L/min indicate a poor cough and an inability to effectively clear the airways.49 The latter is associated with increased risk of respiratory problems, indicating the need of incorporating pulmonary hygiene in patients’ daily routine.54
Assessment of Diaphragm and Intercostal Muscle Strength
Trans-Diaphragmatic Pressure Measurement
As mentioned above, assessing inspiratory muscle weakness using traditional tests such as upright and supine FVC and MIP is dependent on patient effort. To address this limitation, a study explored non-volitional assessments of diaphragm strength by measuring trans-diaphragmatic pressure (PDi) and dynamic muscle compliance using esophageal and gastric balloons.50 By maximal diaphragm stimulation, this technique allows assessment of the patient’s voluntary effort. If no additional PDi is observed, then the contraction is maximal. A study involving phrenic nerve conduction of 13 LOPD patients revealed significantly lower PDi recordings compared to healthy controls, indicating diaphragm weakness in LOPD patients.11 Another study included 30 LOPD patients for diaphragmatic muscle evaluation. Results showed that PDi correlated with PFTs and manometry studies but that the drop in FVC did not correlate strongly with PDi. These investigators hypothesized that this discrepancy with supine FVC values could be attributed to expiratory muscle pathology and dysfunction.55
Electrophysiology
Phrenic nerve conduction and motor-evoked potentials of the diaphragm have also been used to study diaphragm strength in LOPD. In order to investigate neural contributions to diaphragm dysfunction, 30 LOPD underwent respiratory muscle strength testing by combining spirometry and phrenic nerve conductions studies.11 Posterior cervical magnetic stimulation of the phrenic nerve was done and results showed no significant difference in the latency and amplitude of motor-evoked potentials of the diaphragm following phrenic nerve stimulation.11 These findings could not confirm the phrenic nerve involvement in LOPD patients, but it should be emphasized that diaphragmatic twitch pressures could also be influenced by not only the conduction impairment along the motor nerve, but also alterations in the neuromuscular junction. Additional research is required to identify any potential neurogenic involvement in diaphragm weakness among LOPD patients similar to the phrenic nerve and neuromuscular junction pathology that has been observed in the PD mouse model.19
Imaging
The radiologic assessment of respiratory accessory muscles and diaphragm pillars using T1-weighted magnetic resonance imaging (MRI) has the potential to predict respiratory insufficiency. Specifically, this technique identifies fat replacement in paraspinal, abdominal, and thoracic muscles and these findings correlate with low FVC.56 Furthermore, intercostal and abdominal muscle weakness is noted by whole-body MRI imaging data,15 which emphasizes the importance of assessing respiratory muscle weakness. MRI imaging is capable of capturing myopathic changes, and dynamic MRI techniques may provide insights into the functional involvement of the respiratory muscles. However, it is important to note that further validation is required to establish the accuracy and reliability of these methods. Noninvasive simple and reproducible ultrasound and tissue Doppler imaging of the diaphragm also provide an ability to monitor and assess vital diaphragm function in PD patients.11,57–59 Classically, the drop of VC from upright to supine position is an indirect measure of diaphragm weakness, however, diaphragm weakness characterized by a paradoxical motion during a sniff test, has been observed utilizing M-mode ultrasound and tissue Doppler imaging techniques in a patient diagnosed with LOPD.57 Furthermore, 17 LOPD patients were included in a study to evaluate the correlation between diaphragm thickness and mobility using ultrasonography and respiratory function using spirometry.58 Diaphragm excursion, thickness at functional residual capacity and total lung capacity, and thickness fraction were decreased and significantly correlated with the routine PFTs and other respiratory tests.58 These findings provide confirmation that diaphragm ultrasonography serves as a valuable, safe, and alternative technique for assessing clinical, functional, and neurophysiological aspects of respiratory muscle weakness in patients with PD.
Gas and Acid-Base Status
As PD progresses, muscle weakness leads to gas exchange abnormalities. Hypoventilation caused by progressive respiratory muscle weakness causes CO2 retention (elevated arterial pressure of CO2 (PaCO2>45mmHg), or >55 mmHg for ≥10 minutes during sleep60) and hypoxemia (lower arterial pressure of O2 or SaO2 <90%).2 Hypoventilation is initially REM sleep-related, but over time, it occurs throughout sleep and then progresses to become diurnal. Thus, as the PD progresses, patients will exhibit symptoms suggestive of nocturnal hypoventilation, oxygen desaturation, and daytime hypercapnia such as headache and daytime fatigue.61 Thus, gas exchange analysis should be evaluated in every patient, at each visit, by pulse oximetry and capnography, to assess respiratory failure. If normal, invasive arterial blood gas is usually not necessary.2,62
Upper and Lower Airway (Proximal Airway) Evaluation
Respiratory insufficiency in patients with PD has also been attributed to the inability to keep the airway patency due to macroglossia in IOPD patients, and tongue atrophy and weakness in LOPD patients. Thus, the detection of tongue weakness is a part of the clinical diagnosis, physical examination, and follow-up in these patients. On physical examination, macroglossia can be revealed by a protruding tongue with the presence of dental marks on it. While these signs are relatively straightforward to identify, their interpretation and assessment can vary depending on the evaluator, so the awareness depends on the evaluator, but not the clinical sign itself. Clinical and radiological identification of macroglossia and upper airway weakness can lead to early diagnosis and faster decisions for the treatment plan. Whole-body MRI with facial sections have been used in studies to identify tongue fatty infiltration and atrophy in adult patients with LOPD.12 These studies highlight the importance of tongue pathology in LOPD and the need to monitor patients for upper airway weakness. Ultrasound may also be used to assess the tongue’s overall appearance, echo intensity, and thickness. Statistically significant decreases in quantitative tongue strength and sonographic muscle thickness were detected in 10 LOPD patients naïve to ERT treatment.17,63 Moreover, excessive glycogen buildup in the buccal area and the airways leads to airway anatomic collapsibility, including narrowed nasal tract, compromised oral cavity, oropharynx, tracheal, and bronchial lumens.13,22,64 We do not recommend the routine use of MRI and ultrasound imaging to assess the upper airways, but recommend the clinical assessment of tongue weakness and dysphagia in these patients.
Within the lower airways, proximal airway pathology is present in PD patients22 and was also noted in the PD mouse model. Specifically, PD results in trachea-bronchomalacia, airway smooth muscle dysfunction, and impaired smooth muscle contractility.65 In a recent study, fifteen CRIM+ patients with IOPD (median age of 21mo), treated with biweekly ERT, were evaluated for airway abnormalities.21 Clinically, patients developed facial muscle weakness, speech disorders, and recurrent otitis media. On FB, all patients had a narrowed nasal tract and oral cavity, compromised oropharynx with posterior displacement of the soft palate and uvula, and increased rigidity of the pharyngeal wall and tongue base, along with the reduced motion of the bilateral vocal cords.21 In another study, LOPD patients, treated biweekly with ERT, were also evaluated for airway abnormalities using FB.64 All patients had facial muscle weakness, narrowed nasal tracts, and a compromised oropharynx, characterized by posterior displacement of the soft palate, uvula, and collapse of the pharyngeal wall. Mild tracheomalacia and bronchomalacia were also noted. One patient in this study also experienced a dramatic deterioration of FVC.64 Even with early treatment with ERT in IOPD,21 or LOPD patients,64 airway abnormalities persist. Therefore, If there is a concern regarding malacia (abnormal softening) of the airways, flexible bronchoscopy can be instrumental in assessing and evaluating the condition.
Polysomnography
Polysomnography should be performed at baseline and then again when the FVC declines. With a significant drop in supine FVC, the ETCO2 is increased and symptoms of early morning headaches, daytime somnolence, and low energy will occur.2,25 OSA and hypoventilation occur in both infantile and late-onset PD patients.25,34 In a retrospective study of 38 IOPD patients treated with ERT, polysomnogram monitoring was analyzed and showed an increased risk of airway obstruction during sleep, and two-thirds of the cohort had sleep apnea and/or hypoventilation.25 In another study of 27 LOPD patients, polysomnography was compared to daytime upright and supine respiratory measures, including inspiratory vital capacity (IVC), peak inspiratory pressure, and FVC.34 Ventilatory restriction and diaphragm muscle weakness were noted in 17 of the 27 patients, whereas sleep-disordered breathing (SDB) was seen in 13 of the 27 patients. SDB included OSA, REM sleep hypopnea with REM sleep hypoventilation and continuous sleep stage–independent hypoventilation. A decrease in supine IVC was correlated with total sleep time spent with SaO2<90% and PCO2>50mmHg, which indicates that diaphragm weakness results in SDB.34
Regarding diagnostic sleep studies, overnight transcutaneous capnometry has been shown to be superior to pulse oximetry for detection of nocturnal hypoventilation in patients with neuromuscular disorders and is indispensable for baseline evaluation of PD patients with suspected sleep related hypoventilation.66 In a study that included patients with slowly progressive myopathies (including PD), the authors investigated whether daytime tests of respiratory muscle function and diaphragm ultrasound predict hypercapnia during sleep. Nocturnal hypercapnia is defined as transcutaneous CO2 tension ptcCO2 ≥ 50 mmHg for ≥30 min or an overnight increase in the ptcCO2 of ≥10 mmHg.67 As a result, a reduction in FVC and MIP reliably predict nocturnal hypercapnia when specific thresholds are applied (<60% of predicted for FVC and <120% of lower limit of normal for MIP). In contrast, a clinically reliable method for excluding nocturnal hypercapnia involves assessing diaphragm excursion velocity using ultrasound during a voluntary sniff maneuver, especially when it exceeds 8.0 cm/s.67 These three measures can help identify patients at risk for sleep-related hypoventilation and guide clinical decisions regarding the need for further assessments such as sleep studies and overnight capnometry.
Novel Assessment of Pulmonary Function Using XeMRI
Hyperpolarized (HP) 129Xenon (Xe) magnetic resonance imaging (MRI) is a promising modality for a 3-dimensional assessment of ventilation and gas exchange. This method is rapid (requiring only a single 10-s breath hold), well-tolerated, and non-invasive. Inhaled xenon distributes through the pulmonary branches and into the alveoli, where it freely diffuses into the interstitial membrane tissues and capillary red blood cells (RBCs). The distribution of 129Xe within each of these three compartments (gas, membrane, and RBCs) can be imaged separately by taking advantage of the distinct frequency signature of 129Xe within them. XeMRI has been of particular interest in pediatric patients, where traditional imaging techniques can be challenging due to patient size, motion artifacts, and the need to minimize radiation exposure. Imaging of gas-phase xenon in the airspaces alone has proven useful for assessing ventilatory obstruction arising from cystic fibrosis,68 bronchopulmonary dysplasia,69 asthma70 and stem cell transplantation.71 Extensions of XeMRI that utilize its absorption into the membrane and RBC compartments provide further region-specific quantification of interstitial membrane thickness and RBC transfer efficiency.72 These capabilities have been used to evaluate abnormalities in membrane and capillary conductances associated with interstitial lung diseases and pulmonary hypertension.73 This gas exchange imaging technique has recently been applied to pediatric lung disease.74
We used 129Xe MRI to assess ventilation and gas exchange function in three subjects with PD. Two subjects had IOPD (subjects 209 and 210) and one had LOPD (subject 211), and all were on ERT at Duke Children’s Hospital. The study was approved by the Duke University Health System Institutional Review Board. Representative panels of 129Xe ventilation, membrane uptake, and RBC transfer are shown for an age-matched healthy young control subject as well as the three PD patients (Figure 3). Ventilation imaging and membrane uptake were normal in all three subjects. RBC transfer was higher than normal in subjects 209 and 210, while small defects could be seen at the bases and apices of the lung in subject 211. None of the subjects exhibited significantly increased membrane uptake (as seen in those with interstitial lung disease), nor the substantial defects in RBC transfer typically seen across all cardiopulmonary diseases.73 Secondary markers derived from 129Xe spectroscopy such as the RBC shift and cardiogenic RBC signal oscillations were also generally in the normal range for these subjects.75 However, subject 209 exhibited moderately enhanced oscillation amplitudes which have been associated with left heart failure.73
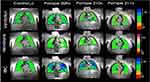 |
Figure 3 Comparative Panels of 129Xe Ventilation, Membrane Uptake, and Red Blood Cell (RBC) Transfer in Control and Pompe Disease Patients. |
While XeMRI may not be widely accepted as a clinical standard and may not be readily accessible, it is still worth considering its implications for studying air diffusion pathology, that might be responsible of respiratory complications despite enzyme replacement therapy in patients with PD.
Management of Respiratory Complication
The Impact of Enzyme Replacement Therapy (ERT) on Respiratory Function
Enzyme replacement therapy (ERT) with alglucosidase alfa - recombinant human acid alpha-glucosidase (rhGAA) - was approved for the treatment of PD in 2006. A pivotal clinical trial in IOPD patients demonstrated increased ventilator-free survival in the first 18 months at a cumulative dose of 20 mg/kg every other week.76 Another clinical study compared the effect of the latter to a dose of 40mg/kg weekly in IOPD and early LOPD (within the first 5 years of age).77 Higher rhGAA doses improved tongue strength and pulmonary function measures. Lingual strength increased by 28%, and the median FVC %predicted increased from 67% to 73% in the upright position and from 53% to 68.5% in the supine position. No increased need for ventilator support was needed.77 In a retrospective chart review of PFTs of CRIM+ IOPD patients treated with ERT, subjects were divided into 2 groups, a younger cohort (5–9 years old) and an older cohort (10–18 years).78 In the younger cohorts, a decline in the mean FVC %predicted from 70.3% to 64.9% occurred from the upright to the supine position. Similar findings were noted for the older cohort (upright FVC 61.5% to supine FVC 52.5% predicted). Stability in FVC over time was seen in half of the patients and MIP and MEP did not decline with age. Increasing the dose of ERT to 40mg/kg/week resulted in further stability in FVC. This study concluded that ERT was able to maintain respiratory function in a subgroup of patients, while before ERT, patients were unable to maintain ventilator-free breathing during the first years of life.78 Thus, treatment with rhGAA at a higher dose of 40 mg/kg weekly was generally well tolerated and led to improved ventilator‐free survival in children with classic CRIM+ IOPD.6,76
Similarly, LOPD patients treated with ERT over five years exhibited a stable FVC in the upright position.5 Withdrawal of ERT in patients with PD led to a reduced level of respiratory function, and resuming the treatment partially recovers it.79 In addition, ERT reduces ventilator dependency in LOPD patients and the need for hospitalization due to respiratory exacerbations,80 and the addition of ERT to ventilatory support at home showed improvements in lung function and gas exchange.81
Avalglucosidase alfa and Cipaglucosidase alfa are next-generation recombinant human GAA enzyme replacement therapies that that have shown promising results in increasing glycogen clearance and improving clinical outcomes in patients with LOPD. These new therapies have demonstrated improvements in respiratory function, ambulation, and functional endurance.82,83 In a study involving two groups of LOPD patients (a treatment-naïve group and a switch group consisting of patients previously treated with alglucosidase alfa for at least 9 months), both groups were treated with 20 mg/kg of avalglucosidase alfa every other week. The upright FVC %predicted remained stable in both treatment groups, with slope estimates of −0.473/year (−1.188, 0.242) in the treatment-naïve group and −0.648/year (−1.061, −0.236) in the switch group.84 Another study, known as the COMET study compared the efficacy of avalglucosidase alfa to the current FDA-approved treatment - alglucosidase alfa - and showed an improvement in FVC %predicted in the avalglucosidase alfa treated group.82 In the PROPEL study, LOPD naïve patients were assigned to receive either cipaglucosidase alfa (20 mg/kg) plus oral miglustat or alglucosidase alfa (20 mg/kg) plus oral placebo once every two weeks. Miglustat is administered to enhance the delivery of cipaglucosidase alfa to tissues. The LOPD patients treated with cipaglucosidase alfa showed increased stability in FVC overall compared to the group treated with alglucosidase alfa.83 These studies suggest that the next-generation enzyme replacement therapies, avalglucosidase alfa and cipaglucosidase alfa, hold promise in improving respiratory function and other clinical parameters in LOPD patients. Continued research and clinical trials are needed to further evaluate the efficacy and long-term benefits of these therapies in the management of LOPD.
Immune Tolerance Induction (ITI) Regimens with ERT
The efficacy of ERT is diminished for patients who mount an immune response against rhGAA.8 In a CRIM- cohort of IOPD patients treated with ERT as monotherapy, the median age of ventilator dependence was 13.8 months, and the median age of death was 32.5 months.85 When compared to CRIM+ patients, CRIM- patients clearly show attenuated response to ERT in all outcomes including reduced survival and ventilator-free survival.7 Hence, developing ITI protocols to prevent the emergence of high sustained antibody titers was essential and changed the course of the disease for CRIM- patients. Induction of immune tolerance was first reported through intravenous administration of immunoglobulin, anti-CD-20 monoclonal antibody, and methotrexate9 with successful respiratory clinical outcomes.10 Immunomodulation using a bortezomib-based regimen in addition to ERT demonstrated clinical improvement in terms of reduction of ventilator requirements in both CRIM- and CRIM+ IOPD patients, in the setting of an entrenched immune response.86
Gene Therapy for Respiratory Insufficiency
Gene therapy using adeno-associated virus (AAV) to deliver a functional GAA gene is ideal for a monogenetic disease such as PD.87 The first clinical trial investigating AAV-GAA gene therapy in PD was initiated in ventilator-dependent PD patients.88 The study consisted of five patients from 2–18 years of age. Two dose levels (1.0x1012 and 5.0x1012vg) of the rAAV1-CMV-GAA vector were studied. The vector was injected intramuscularly (IM) into the diaphragm and patients also underwent inspiratory muscle strength training. The primary outcome of the study was to test the safety of gene therapy delivery into the diaphragm muscle of PD patients. The secondary outcome was to assess the impact of inspiratory muscle strength training (IMST) in conjunction with AAV gene therapy on respiratory function.88 Overall, intradiaphragm injections of AAV-rhGAA resulted in gains in unassisted tidal volume and spontaneous ventilatory endurance. No appreciable changes were noted in maximal voluntary ventilation and MIP.89 Levels of circulating antibodies against the vector were low, stating that rAAV1-CMV-GAA vector injection was safe and led to improvement in spontaneous ventilatory endurance.
Another clinical study investigated the efficacy and safety of systemic gene therapy using a liver vector, the AAV8-LSPhGAA.90 This study involved patients with LOPD who received the study vector at a low dose of 1.6 × 10^12 viral genome per kilogram (vg/kg) of body weight. The vector was administered through controlled intravenous infusion three days after receiving biweekly ERT. ERT was discontinued at week 26 once quantifiable serum GAA levels were detected and no decline in motor and pulmonary function was observed.90 The study’s endpoints included motor and pulmonary performance 52 weeks following vector administration. At week 52, upright FVC showed no significant difference from baseline, indicating stability in respiratory function. Similarly, the supine FVC also remained stable. Furthermore, pulmonary function remained stable following the withdrawal of ERT.90 While it is true that systemic gene delivery may not be perceived as immediately crucial for general respiratory management, utilizing gene therapy in general holds immense potential in treating the respiratory pathology in PD. Additional work is needed to advance our understanding and move this forward.
Non-Invasive and Invasive Ventilatory Support
Untreated PD often leads to the progression of respiratory dysfunction and the need for non-invasive positive pressure ventilation (NIPPV).91 Effective management of chronic respiratory failure and respiratory muscle weakness relies on long-term ventilatory support which can be provided by NIPPV or more invasive tracheostomy and mechanical ventilation (IMV). Positive pressure ventilation significantly reduces the work of breathing.92 NIPPV and IMV expand the lungs and help prevent complications such as atelectasis, ventilation-perfusion mismatch, and respiratory infections.36 A systemic literature review determined if PFTs can predict the threshold for ventilator use. The PFTs used included MIP, MEP, upright and supine slow VC, and FVC. Patients included in the study were either treated or untreated with ERT.93 No statistical significance was noted between the patients untreated and treated with ERT when comparing assisted ventilation status (none, day, night) and PFTs parameters. However, PFTs were useful in predicting the need for both nocturnal and daytime ventilator support. Specifically, nocturnal and daytime ventilatory support demonstrated a stronger correlation with MIP values below 60 cmH2O (5.9 kPa) than with FVC or VC below 50% predicted. This study found that 31% LOPD patients using nocturnal NIPPV had VC > 50% predicted, whereas only 8% had MIP values greater than 60 cmH2O.93
The timing for initiating NIPPV varies based on factors such as the patient’s age, functional status, and rate of disease progression. Clinical indications for NIPPV include early morning headaches and daytime somnolence with a declining FVC from upright to supine of >10%, an FVC < 50% of predicted, SNIP/MIP <60 cmH2O, hypercapnia, or evidence of sleep-related hypoventilation.94 Notable progression of respiratory muscle weakness leads to hypoventilation, while macroglossia and upper airway weakness exacerbates respiratory compromise by causing OSA. Bi-level positive airway pressure (BiPAP) is the preferred treatment when nocturnal or diurnal hypoventilation is present. In addition to bi-level pressure support, NIPPV devices may incorporate a backup rate and adaptive pressure support with an assured volume. The selection of a specific mode depends on the degree of respiratory muscle involvement, rate of disease progression, and patient comfort. NIPPV treatment should be individualized to achieve ventilation goals (prevention of worsening of hypoventilation during sleep (less than 5% of nocturnal oximetry time with an SpO2<90%), improvement in sleep quality, relief of nocturnal dyspnea, and providing respiratory muscle rest95) and promote adherence. Regular evaluation of symptoms, device digital downloads, oximetry, and capnography are recommended to determine and maintain optimal settings.
In general, in any neuromuscular disorders, an earlier introduction of NIPPV yields a more favorable respiratory outcome.96 When NIPPV is initiated, patients have significant improvements in sleep, fatigue, headaches, reduction of daytime somnolence, and survival.97 In PD, NIPPV improves nocturnal gas exchange (oxygen and carbon dioxide) and alleviates sleep-related symptoms.42 In cases where patients fail to respond or unable to tolerate NIPPV, IMV via tracheostomy may be considered. IMV can also be considered for patients who require prolonged daytime use of NIPPV.36 However, alternative modes of non-invasive respiratory support, such as mouth-piece ventilation, are also an option for those who require more support.
Respiratory Muscle Strength Training and Cough Assistance
Intensive respiratory muscle training (RMT) offers potential benefits for both IOPD and LOPD patients to delay the progressive respiratory muscle weakness. RMT involves tailored and individualized muscle exercises that use calibrated pressure-threshold resistance during inspiration and expiration.98–100 Inspiratory muscle strength and respiratory muscle endurance training has shown significant improvements in muscle endurance, strength, and overall respiratory function.101 LOPD patients who underwent frequent RMT have increased MIP, MEP, and overall respiratory strength, with a particular improvement in inspiratory muscles. Thereby RMT stabilizes and decelerates the decline of the diaphragm strength.102 Importantly, these improvements have been observed to persist even after a 3-month period following RMT completion.98,99,103,104
In addition, effective clearance of airway secretions is crucial for mitigating respiratory compromise and reducing the risk of pulmonary infections in patients with respiratory muscle weakness and an impaired cough. PFTs such as PCF and MEP serve as predictive measures for the need for cough assistance. A PCF < 270L/min during a stable state should prompt conversations about ordering a cough assist device, specifically an insufflator/exsufflator, as this value correlates with a critical PCF value of <160 L/min during an exacerbation. Likewise, a MEP < 60 cm H2O in patients with a history of impaired airway clearance is another indication to initiate therapy.36 Manually assisted coughing in combination with lung volume recruitment techniques, such as air stacking using a bag valve mask or ventilator device, can be employed to promote lung inflation and enhance expiratory airflow in cooperative patients. More commonly, insufflation/exsufflation devices are ordered to improve PCF and airway clearance in patients with a weak cough, and high-frequency chest wall oscillation are often used promote mucociliary clearance and may help to propel secretions forward from the periphery to the central airways. In advanced cases, suctioning is a necessary aid to airway clearance.36
Management of Acute Respiratory Failure (ARF)
Acute respiratory failure (ARF) can arise from various factors, including infection, aspiration, and inadequate ventilation. These factors are exacerbated when respiratory muscle strength is weak and airway clearance is impaired. Therefore, a primary objective in managing respiratory failure is the prevention or prompt treatment of these complications. Early antibiotic treatment, in case of infection, and adequate immunization administration are important to prevent further pulmonary complications and help improve respiratory outcomes and quality of life.36
Despite adequate therapy, ARF can still develop. It is crucial to recognize that any condition that leads to ARF is potentially life-threatening. ARF can arise from severe respiratory infections, post-surgical complications, or worsening progression of respiratory muscle weakness. Management of ARF should be done in an intensive care unit setting.36 In the case of severe pneumonia, it is essential to initiate broad-spectrum antibiotics promptly once the diagnosis is established. When ARF occurs, the introduction of NIPPV as soon as possible leads to better respiratory outcomes and improved overall survival rates. When NIPPV fails to provide adequate support, is not tolerated, or is contraindicated, IMV is indicated.36 Following the resolution of ARF, physicians should initiate physical and respiratory rehabilitation as early as possible to prevent additional complications. Long-term prophylactic measures such as regular airway clearance, cough assistance, and immunizations should be reinforced.2 ERT should not be paused at any time during or after the resolution of ARF.36
Additional Management During Anesthesia
The presence of cardiopulmonary complications of PD poses significant challenges in the management of PD patients during anesthesia.105 Whenever feasible, local anesthesia is preferred. Regional anesthesia techniques such as femoral nerve and caudal epidural blockade have shown beneficial outcomes in IOPD patients undergoing procedures such as open muscle biopsy or the insertion of indwelling vascular access devices.106 However, cases where general anesthesia is mandatory, ensuring its safe administration is imperative. The primary goal during anesthesia in patients with cardiac involvement is to maximize coronary perfusion pressure and minimize arrhythmia, heart failure risk, and abrupt changes in diastolic blood pressure.107,108 Cardiac arrhythmias and cardiopulmonary arrest have been reported in IOPD patients under anesthesia following the use of propofol or high concentrations of sevoflurane.108 In addition, there are reports of intraoperative cardiac arrest during halothane anesthesia in patients with IOPD.109 Therefore, it is advised to consider using alternative agents such as ketamine for anesthesia induction in order to minimize these risks. It is also important to be aware of the potential occurrence of malignant hyperthermia in PD patient.110 To mitigate the risk of cardiorespiratory failure during anesthesia, it is recommended to avoid volatile anesthetics and muscle relaxants.111 Finally, meticulous monitoring of vital signs and close observation for any signs of cardiac or respiratory and cardiac distress is crucial. An experienced anesthesia team familiar with the unique considerations of PD patients should be involved in the perioperative management to ensure the safest possible outcomes.105
Conclusion
Respiratory insufficiency is a prominent and significant feature of PD, impacting the life, morbidity, and mortality of affected individuals. As a result, optimal care becomes crucial, necessitating the involvement of a multidisciplinary team well-versed in PD and experienced in managing anticipated respiratory complications. A comprehensive care plan should be established, encompassing diligent monitoring of respiratory muscle weakness and addressing sleep-disordered breathing. The goal of respiratory function monitoring is two-fold: to delay the onset of symptoms and to prevent potentially life-threatening complications (Figure 1). To achieve these goals, early initiation of treatment is vital. ERT, physical therapy, and respiratory interventions such as NIPPV play pivotal roles in altering the natural course of the disease (Figure 2). Thus, managing respiratory complications in patients with PD requires a comprehensive and multidisciplinary approach. Regular pulmonary function testing, ERT, non-invasive ventilation, airway clearance techniques, vaccinations, and regular follow-up are vital components of effective respiratory management. By implementing these practical recommendations, healthcare providers can improve the respiratory health and overall well-being of individuals living with PD.
Abbreviations
PD, Pompe Disease; IOPD, Infantile-Onset Pompe disease; LOPD, Late-Onset Pompe disease; ERT, Enzyme Replacement Therapy; ITI, Immune Tolerance Induction; CRIM, Cross-Reactive Immunological Material; CRIM+, CRIM positive; CRIM-, CRIM negative; FVC, Forced Vital Capacity; FEV, Forced Expiratory Volume; AHI, Apnea/hypopnea index; PFTs, Pulmonary Function Tests; MIP, Maximal Inspiratory Pressure; MEP, Maximal Expiratory Pressure; SNIP, Sniff Nasal Inspiratory Pressure; PCF, Peak Cough Flow; PDi, trans-diaphragmatic pressure; MRI, Magnetic Resonance Imaging; FB, Flexible Bronchoscopy; OSA, Obstructive Sleep Apnea; NIPPV, Non-Invasive Positive Pressure Ventilation; IMV, Invasive Mechanical Ventilation; SDB, Sleep Disordered Breathing; RMT, Respiratory Muscle Training; ARF, Acute respiratory failure.
Disclosure
Dr. Mai ElMallah is funded by NIH NICHD R01HD099486-01. Dr David Mummy reports personal fees from Polarean Inc, outside the submitted and he also provided occasional scientific consultant for Polarean. Polarean had no role in the submitted work. Dr Bastiaan Driehuys reports personal fees from Polarean Imaging as the founder and board member of the company. In addition, Dr Bastiaan Driehuys has a patent US8911709 with royalties paid to Polarean Imaging. Prof. Dr. Priya S Kishnani reports grants, personal fees from and a member of the Pompe and Gaucher Disease Registry Advisory Board for Sanofi Genzyme, Amicus Therapeutics; personal fees from and equity with Maze Therapeutics; personal fees from Bayer; held equity in Asklepios Biopharmaceuticals, and may receive milestone payments related to that equity in the future from Asklepios Biopharmaceutical, Inc. (AskBio). In addition, Priya S. Kishnani is a member of the Advisory Board for Baebies. The authors declare no other conflicts of interest in this work.
References
1. Kohler L, Puertollano R, Raben N. Pompe Disease: from Basic Science to Therapy. Neurotherapeutics. 2018;15(4):928–942. doi:10.1007/s13311-018-0655-y
2. Kishnani PS, Steiner RD, Bali D, et al. Pompe disease diagnosis and management guideline. Genet Med. 2006;8(5):267–288. doi:10.1097/01.gim.0000218152.87434.f3
3. Kroos M, Hoogeveen-Westerveld M, van der Ploeg A, et al. The genotype-phenotype correlation in Pompe disease. Am J Med Genet C Semin Med Genet. 2012;160c(1):59–68. doi:10.1002/ajmg.c.31318
4. Sarah B, Giovanna B, Emanuela K, et al. Clinical efficacy of the enzyme replacement therapy in patients with late-onset Pompe disease: a systematic review and a meta-analysis. J Neurol. 2022;269(2):733–741.
5. Stockton DW, Kishnani P, van der Ploeg A, et al. Respiratory function during enzyme replacement therapy in late-onset Pompe disease: longitudinal course, prognostic factors, and the impact of time from diagnosis to treatment start. J Neurol. 2020;267(10):3038–3053.
6. van Gelder C, Plug I, Kroos M, et al. A higher dose of enzyme therapy in patients with classic infantile Pompe disease seems to improve ventilator-free survival and motor function. BMC Musculoskelet Disord. 2013;14(Suppl 2):19. doi:10.1186/1471-2474-14-S2-P19
7. Kishnani PS, Goldenberg PC, DeArmey SL, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab. 2010;99(1):26–33.
8. Banugaria SG, Prater SN, Ng YK, et al. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet Med. 2011;13(8):729–736.
9. Mendelsohn NJ, Messinger YH, Rosenberg AS, et al. Elimination of antibodies to recombinant enzyme in Pompe’s disease. N Engl J Med. 2009;360(2):194–195.
10. Messinger YH, Mendelsohn NJ, Rhead W, et al. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet Med. 2012;14(1):135–142. doi:10.1038/gim.2011.4
11. Spiesshoefer J, Henke C, Kabitz HJ, et al. The nature of respiratory muscle weakness in patients with late-onset Pompe disease. Neuromuscul Disord. 2019;29(8):618–627. doi:10.1016/j.nmd.2019.06.011
12. Dupé C, Lefeuvre C, Solé G, et al. Macroglossia: a potentially severe complication of late-onset Pompe disease. Eur J Neurol. 2022;29(7):2121–2128. doi:10.1111/ene.15330
13. Brenn BR, Theroux MT, Shah SA, et al. Critical airway stenosis in an adolescent male with pompe disease and thoracic lordosis: a case report. A Case Rep. 2017;9(7):199–203.
14. Burghaus L, Liu W, Neuen-Jacob E, et al. Glykogenose Typ II (M. Pompe). Selektiver Befall der Atemmuskulatur --Eine seltene Erstmanifestation [Glycogenesis Type II (M. Pompe). Selective failure of the respiratory musculature--a rare first symptom]. Nervenarzt. 2006;77(2):181–2, 185–6. German. doi:10.1007/s00115-005-2005-7
15. Carlier RY, Laforet P, Wary C, et al. Whole-body muscle MRI in 20 patients suffering from late onset Pompe disease: involvement patterns. Neuromuscul Disord. 2011;21(11):791–799. doi:10.1016/j.nmd.2011.06.748
16. Jones HN, Muller CW, Lin M, et al. Oropharyngeal dysphagia in infants and children with infantile Pompe disease. Dysphagia. 2010;25(4):277–283. doi:10.1007/s00455-009-9252-x
17. Jones HN, Crisp KD, Asrani P, et al. Quantitative assessment of lingual strength in late-onset Pompe disease. Muscle Nerve. 2015;51(5):731–735. doi:10.1002/mus.24523
18. Gambetti P, DiMauro S, Baker L. Nervous system in Pompe’s disease. Ultrastructure and biochemistry. J Neuropathol Exp Neurol. 1971;30(3):412–430. doi:10.1097/00005072-197107000-00008
19. Falk DJ, Todd AG, Lee S, et al. Peripheral nerve and neuromuscular junction pathology in Pompe disease. Hum Mol Genet. 2015;24(3):625–636. doi:10.1093/hmg/ddu476
20. Byrne BJ, Fuller DD, Smith BK, et al. Pompe disease gene therapy: neural manifestations require consideration of CNS directed therapy. Ann Transl Med. 2019;7(13):290. doi:10.21037/atm.2019.05.56
21. Yang C-F, Niu D-M, Tai S-K, et al. Airway abnormalities in very early treated infantile-onset Pompe disease: a large-scale survey by flexible bronchoscopy. Am J Med Genet A. 2020;182(4):721–729. doi:10.1002/ajmg.a.61481
22. Yang CF, Niu D-M, Jeng M-J, et al. Late-onset Pompe disease with left-sided bronchomalacia. Respir Care. 2015;60(2):e26–9. doi:10.4187/respcare.03419
23. Boentert M, Karabul N, Wenninger S, et al. Sleep-related symptoms and sleep-disordered breathing in adult Pompe disease. Eur J Neurol. 2015;22(2):369–76, e27. doi:10.1111/ene.12582
24. Berger KI, Chan Y, Rom WN, et al. Progression from respiratory dysfunction to failure in late-onset Pompe disease. Neuromuscul Disord. 2016;26(8):481–489.
25. Kansagra S, Austin S, DeArmey S, et al. Polysomnographic findings in infantile Pompe disease. Am J Med Genet A. 2013;161a(12):3196–3200. doi:10.1002/ajmg.a.36227
26. Rowley JA, Zahn BR, Babcock MA, et al. The effect of rapid eye movement (REM) sleep on upper airway mechanics in normal human subjects. J Physiol. 1998;510(Pt 3):963–976. doi:10.1111/j.1469-7793.1998.00963.x
27. Kishnani PS, Hwu WL, Mandel H, et al. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr. 2006;148(5):671–676. doi:10.1016/j.jpeds.2005.11.033
28. Bay LB, Denzler I, Durand C¸ et al. Infantile-onset Pompe disease: diagnosis and management. Arch Argent Pediatr. 2019;117(4):271–278. doi:10.5546/aap.2019.eng.271
29. van den Hout HM, Hop W, van Diggelen OP, et al. The natural course of infantile Pompe’s disease: 20 original cases compared with 133 cases from the literature. Pediatrics. 2003;112(2):332–340. doi:10.1542/peds.112.2.332
30. Moravej H, Karamizadeh Z, Paran M. The outcome of infantile onset pompe disease in South of Iran. Iran J Pediatr. 2016;26(1):e4473. doi:10.5812/ijp.4473
31. Owens P, Wong M, Bhattacharya K, et al. Infantile-onset Pompe disease: a case series highlighting early clinical features, spectrum of disease severity and treatment response. J Paediatr Child Health. 2018;54(11):1255–1261.
32. Marsden D. Infantile onset Pompe disease: a report of physician narratives from an epidemiologic study. Genet Med. 2005;7(2):147–150.
33. van der Beek NA, de Vries JM, Hagemans ML, et al. Clinical features and predictors for disease natural progression in adults with Pompe disease: a nationwide prospective observational study. Orphanet J Rare Dis. 2012;7:88.
34. Mellies U, Ragette R, Schwake C, et al. Sleep-disordered breathing and respiratory failure in acid maltase deficiency. Neurology. 2001;57(7):1290–1295.
35. Sixel BS, Silva LD, Cavalcanti NC, et al. Respiratory manifestations in late-onset Pompe disease: a case series conducted in Brazil. J Bras Pneumol. 2017;43(1):54–59.
36. Boentert M, Prigent H, Várdi K, et al. Practical recommendations for diagnosis and management of respiratory muscle weakness in late-onset Pompe disease. Int J Mol Sci. 2016;17:10.
37. Chien YH, Lee NC, Huang HJ, et al. Later-onset Pompe disease: early detection and early treatment initiation enabled by newborn screening. J Pediatr. 2011;158(6):1023–1027.e1.
38. Schoser B, Stewart A, Kanters S, et al. Survival and long-term outcomes in late-onset Pompe disease following alglucosidase alfa treatment: a systematic review and meta-analysis. J Neurol. 2017;264(4):621–630.
39. Margolis ML, Howlett P, Goldberg R, et al. Obstructive sleep apnea syndrome in acid maltase deficiency. Chest. 1994;105(3):947–949.
40. Böing S, Randerath WJ. Chronic hypoventilation syndromes and sleep-related hypoventilation. J Thorac Dis. 2015;7(8):1273–1285.
41. Pellegrini N, Laforet P, Orlikowski D, et al. Respiratory insufficiency and limb muscle weakness in adults with Pompe’s disease. Eur Respir J. 2005;26(6):1024–1031.
42. Boentert M, Dräger B, Glatz C, et al. Sleep-Disordered Breathing and Effects of Noninvasive Ventilation in Patients with Late-Onset Pompe Disease. J Clin Sleep Med. 2016;12(12):1623–1632.
43. Veneruso G, Azzari C, Calandi C, Franchini V, Sabatini C, Vierucci A. Valutazione della funzionalità respiratoria in pazienti affetti da distrofia muscolare di Duchenne [Evaluation of the respiratory function in patients with Duchenne’s muscular dystrophy]. Pediatr Med Chir. 1987;9(1):15–19. Italian.
44. Pennati F, LoMauro A, D’Angelo MG, Aliverti A. Non-invasive respiratory assessment in Duchenne muscular dystrophy: from clinical research to outcome measures. Life. 2021;11(9):1.
45. Cupler EJ, Berger KI, Leshner RT, et al. Consensus treatment recommendations for late-onset Pompe disease. Muscle Nerve. 2012;45(3):319–333.
46. Katz S, Arish N, Rokach A, Zaltzman Y, Marcus EL. The effect of body position on pulmonary function: a systematic review. BMC Pulm Med. 2018;18(1):159.
47. MacKintosh EW, Chen ML, Benditt JO. Lifetime care of Duchenne muscular dystrophy. Sleep Med Clin. 2020;15(4):485–495.
48. Berger KI, Kanters S, Jansen JP, et al. Forced vital capacity and cross-domain late-onset Pompe disease outcomes: an individual patient-level data meta-analysis. J Neurol. 2019;266(9):2312–2321.
49. Laveneziana P, Albuquerque A, Aliverti A, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J. 2019;53(6):1.
50. Polkey MI, Green M, Moxham J. Measurement of respiratory muscle strength. Thorax. 1995;50(11):1131–1135.
51. American Thoracic Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518–624.
52. Evans JA, Whitelaw WA. The assessment of maximal respiratory mouth pressures in adults. Respir Care. 2009;54(10):1348–1359.
53. Uldry C, Fitting JW. Maximal values of sniff nasal inspiratory pressure in healthy subjects. Thorax. 1995;50(4):371–375.
54. Pitts T, Bordelon R, Huff A, Byrne BJ, Smith BK. Cough effectiveness and pulmonary hygiene practices in patients with pompe disease. Lung. 2019;197(1):1–8.
55. Prigent H, et al. Supine volume drop and diaphragmatic function in adults with Pompe disease. Eur Respir J. 2012;39(6):1545–1546.
56. Reyes-Leiva D, Alonso-Pérez J, Mayos M, et al. Correlation between respiratory accessory muscles and diaphragm pillars MRI and pulmonary function test in late-onset pompe disease patients. Front Neurol. 2021;12:621257.
57. Meng P, Ogna A, Fayssoil A. M mode ultrasound and tissue Doppler imaging to assess diaphragm feature in late onset pompe disease. Neurol Int. 2020;12(3):55–58.
58. Ruggeri P, Lo Monaco L, Musumeci O, et al. Ultrasound assessment of diaphragm function in patients with late-onset Pompe disease. Neurol Sci. 2020;41(8):2175–2184.
59. Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve. 2013;47(3):319–329.
60. D’Cruz RF, Murphy PB, Kaltsakas G. Sleep disordered breathing and chronic obstructive pulmonary disease: a narrative review on classification, pathophysiology and clinical outcomes. J Thorac Dis. 2020;12(Suppl 2):S202–s216.
61. Shah NM, Sharma L, Ganeshamoorthy S, Kaltsakas G. Respiratory failure and sleep-disordered breathing in late-onset Pompe disease: a narrative review. J Thorac Dis. 2020;12(Suppl 2):S235–s247.
62. Rosenow EC, Engel AG. Acid maltase deficiency in adults presenting as respiratory failure. Am J Med. 1978;64(3):485–491.
63. Jones HN, Hobson-Webb LD, Kuchibhatla M, et al. Tongue weakness and atrophy differentiates late-onset Pompe disease from other forms of acquired/hereditary myopathy. Mol Genet Metab. 2021;133(3):261–268.
64. Wang TH, Soong WJ, Niu DM, et al. Airway abnormalities and pulmonary complications in long-term treated late-onset Pompe disease: diagnostic and interventional by flexible bronchoscopy. Pediatr Pulmonol. 2022;57(1):185–192.
65. Keeler AM, Liu D, Zieger M, et al. Airway smooth muscle dysfunction in Pompe (Gaa(-/-)) mice. Am J Physiol Lung Cell Mol Physiol. 2017;312(6):L873–l881.
66. Spiesshoefer J, Runte M, Heidbreder A, et al. Sleep-disordered breathing and effects of non-invasive ventilation on objective sleep and nocturnal respiration in patients with myotonic dystrophy type I. Neuromuscul Disord. 2019;29(4):302–309.
67. Spiesshoefer J, Lutter R, Kabitz HJ, et al. Respiratory muscle function tests and diaphragm ultrasound predict nocturnal hypoventilation in slowly progressive myopathies. Front Neurol. 2021;12:731865.
68. Smith LJ, Horsley A, Bray J, et al. The assessment of short and long term changes in lung function in CF using (129)Xe MRI. Eur Respir J. 2020; 2020:1.
69. Higano NS, Ruoss JL, Woods JC. Modern pulmonary imaging of bronchopulmonary dysplasia. J Perinatol. 2021;41(4):707–717.
70. Lin NY, Roach DJ, Willmering MM, et al. (129)Xe MRI as a measure of clinical disease severity for pediatric asthma. J Allergy Clin Immunol. 2021;147(6):2146–2153.e1.
71. Walkup LL, Myers K, El-Bietar J, et al. 129Xe MRI detects ventilation deficits in pediatric stem-cell transplant patients unable to perform spirometry. Eur Respir J. 2019;2019:1.
72. Wang Z, Robertson SH, Wang J, et al. Quantitative analysis of hyperpolarized (129) Xe gas transfer MRI. Med Phys. 2017;44(6):2415–2428.
73. Wang Z, Bier EA, Swaminathan A, et al. Diverse cardiopulmonary diseases are associated with distinct xenon magnetic resonance imaging signatures. Eur Respir J. 2019;54(6):1.
74. Willmering MM, Walkup LL, Niedbalski PJ, et al. Pediatric (129) Xe Gas-Transfer MRI-feasibility and applicability. J Magn Reson Imaging. 2022;56(4):1207–1219.
75. Bier EA, Wang Z, Chalian H, et al. Noninvasive diagnosis of pulmonary hypertension with hyperpolarized 129Xe magnetic resonance imaging and spectroscopy. ERJ Open Res. 2022;2022:00035–2022.
76. Amalfitano A, Bengur AR, Morse RP, et al. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a Phase I/II clinical trial. Genet Med. 2001;3(2):132–138.
77. Khan AA, Case LE, Herbert M, et al. Higher dosing of alglucosidase alfa improves outcomes in children with Pompe disease: a clinical study and review of the literature. Genet Med. 2020;22(5):898–907.
78. ElMallah MK, Desai AK, Nading EB, et al. Pulmonary outcome measures in long-term survivors of infantile Pompe disease on enzyme replacement therapy: a case series. Pediatr Pulmonol. 2020;55(3):674–681.
79. Hundsberger T, Rösler KM, Findling O. Cessation and resuming of alglucosidase alfa in Pompe disease: a retrospective analysis. J Neurol. 2014;261(9):1684–1690.
80. Vianello A, Semplicini C, Paladini L, et al. Enzyme replacement therapy improves respiratory outcomes in patients with late-onset type II glycogenosis and high ventilator dependency. Lung. 2013;191(5):537–544.
81. Sayeed N, Sharma P, Abdelhalim M, Mukherjee R. Effect of enzyme replacement therapy (ERT) added to Home Mechanical Ventilation (HMV) in adult Pompe disease. Respirol Case Rep. 2015;3(4):159–161.
82. Diaz-Manera J, Kishnani PS, Kushlaf H, et al. Safety and efficacy of avalglucosidase alfa versus alglucosidase alfa in patients with late-onset Pompe disease (COMET): a Phase 3, randomised, multicentre trial. Lancet Neurol. 2021;20(12):1012–1026.
83. Schoser B, Roberts M, Byrne BJ, et al. Safety and efficacy of cipaglucosidase alfa plus miglustat versus alglucosidase alfa plus placebo in late-onset Pompe disease (PROPEL): an international, randomised, double-blind, parallel-group, phase 3 trial. Lancet Neurol. 2021;20(12):1027–1037.
84. Dimachkie MM, et al. Long-term safety and efficacy of avalglucosidase alfa in patients with late-onset pompe disease. Neurology. 2022;99(5):e536–48.
85. Berrier KL, Barohn RJ, Byrne B. CRIM-negative infantile Pompe disease: characterization of immune responses in patients treated with ERT monotherapy. Genet Med. 2015;17(11):912–918.
86. Banugaria SG, Prater SN, McGann JK, et al. Bortezomib in the rapid reduction of high sustained antibody titers in disorders treated with therapeutic protein: lessons learned from Pompe disease. Genet Med. 2013;15(2):123–131.
87. Roger AL, Sethi R, Huston ML, et al. What’s new and what’s next for gene therapy in Pompe disease? Expert Opin Biol Ther. 2022;22(9):1117–1135.
88. Byrne PI, Smith B, Mah C, et al. Phase I/II trial of diaphragm delivery of recombinant adeno-associated virus acid alpha-glucosidase (rAAaV1-CMV-GAA) gene vector in patients with Pompe disease. Hum Gene Ther Clin Dev. 2014;25(3):134–163.
89. Smith BK, Collins SW, Conlon TJ, et al. Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Hum Gene Ther. 2013;24(6):630–640.
90. Smith EC, Hopkins S, Case LE, et al. Phase I study of liver depot gene therapy in late-onset Pompe disease. Mol Ther. 2023;2023:1.
91. Hagemans ML, Winkel LP, Hop WC, et al. Disease severity in children and adults with Pompe disease related to age and disease duration. Neurology. 2005;64(12):2139–2141.
92. Silva PL, Rocco PRM. The basics of respiratory mechanics: ventilator-derived parameters. Ann Transl Med. 2018;6(19):376.
93. Johnson EM, Roberts M, Mozaffar T, et al. Pulmonary function tests (maximum inspiratory pressure, maximum expiratory pressure, vital capacity, forced vital capacity) predict ventilator use in late-onset Pompe disease. Neuromuscul Disord. 2016;26(2):136–145.
94. Khan A, Frazer-Green L, Amin R, et al. Respiratory management of patients with neuromuscular weakness: an American college of chest physicians clinical practice guideline and expert panel report. Chest. 2023;2023:1.
95. NPPV Titration Task Force of the American Academy of Sleep Medicine. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med. 2010;6(5):491–509.
96. Morelot-Panzini C, Bruneteau G, Gonzalez-Bermejo J. NIV in amyotrophic lateral sclerosis: the ‘when’ and ‘how’ of the matter. Respirology. 2019;24(6):521–530.
97. Mellies U, Dohna-Schwake C, Voit T. Respiratory function assessment and intervention in neuromuscular disorders. Curr Opin Neurol. 2005;18(5):543–547.
98. Jones HN, Crisp KD, Moss T, et al. Effects of respiratory muscle training (RMT) in children with infantile-onset Pompe disease and respiratory muscle weakness. J Pediatr Rehabil Med. 2014;7(3):255–265.
99. Jones HN, Crisp KD, Robey RR, et al. Respiratory muscle training (RMT) in late-onset Pompe disease (LOPD): effects of training and detraining. Mol Genet Metab. 2016;117(2):120–128.
100. Jones HN, Kuchibhatla M, Crisp KD, et al. Respiratory muscle training in late-onset Pompe disease: results of a sham-controlled clinical trial. Neuromuscul Disord. 2020;30(11):904–914. doi:10.1016/j.nmd.2020.09.023
101. Illi SK, Held U, Frank I, et al. Effect of respiratory muscle training on exercise performance in healthy individuals: a systematic review and meta-analysis. Sports Med. 2012;42(8):707–724. doi:10.1007/BF03262290
102. Wenninger S, Greckl E, Babačić H, et al. Safety and efficacy of short- and long-term inspiratory muscle training in late-onset Pompe disease (LOPD): a pilot study. J Neurol. 2019;266(1):133–147. doi:10.1007/s00415-018-9112-4
103. Jones HN, Moss T, Edwards L, et al. Increased inspiratory and expiratory muscle strength following respiratory muscle strength training (RMST) in two patients with late-onset Pompe disease. Mol Genet Metab. 2011;104(3):417–420. doi:10.1016/j.ymgme.2011.05.006
104. Jevnikar M, Metka K, Fabiana C, et al. Respiratory muscle training with enzyme replacement therapy improves muscle strength in late - onset Pompe disease. Mol Genet Metab Rep. 2015;5:67–71. doi:10.1016/j.ymgmr.2015.09.007
105. Ing RJ, Ryan Cook D, Bengur RA, et al. Anaesthetic management of infants with glycogen storage disease type II: a physiological approach. Paediatr Anaesth. 2004;14(6):514–519. doi:10.1111/j.1460-9592.2004.01242.x
106. Walker RW, Briggs G, Bruce J, et al. Regional anesthetic techniques are an alternative to general anesthesia for infants with Pompe’s disease. Paediatr Anaesth. 2007;17(7):697–702. doi:10.1111/j.1460-9592.2007.02196.x
107. Al Atassi A, Al Zughaibi N, Naeim A, et al. Anesthesia management in an infant with glycogen storage disease type II (Pompe Disease). Middle East J Anaesthesiol. 2015;23(3):343–346.
108. Wang LY, Ross AK, Li JS, et al. Cardiac arrhythmias following anesthesia induction in infantile-onset Pompe disease: a case series. Paediatr Anaesth. 2007;17(8):738–748. doi:10.1111/j.1460-9592.2007.02215.x
109. McFarlane HJ, Soni N. Pompe’s disease and anaesthesia. Anaesthesia. 1986;41(12):1219–1224. doi:10.1111/j.1365-2044.1986.tb13007.x
110. Edelstein G, Hirshman CA. Hyperthermia and ketoacidosis during anesthesia in a child with glycogen-storage disease. Anesthesiology. 1980;52(1):90–92. doi:10.1097/00000542-198001000-00025
111. Kotani N, Hashimoto H, Hirota K, et al. Prolonged respiratory depression after anesthesia for parathyroidectomy in a patient with juvenile type of acid maltase deficiency. J Clin Anesth. 1996;8(7):620. doi:10.1016/S0952-8180(96)00092-X
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


