Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Molluscum Contagiosum in HIV Patient Treated with 20% Topical Glycolic Acid After Resistance with Topical Tretinoin
Authors Achdiat PA , Andiani S , Hindritiani R , Gondokaryono SP , Nuzuliyah G , Usman HA , Maharani RH
Received 5 June 2023
Accepted for publication 21 September 2023
Published 29 September 2023 Volume 2023:16 Pages 2749—2755
DOI https://doi.org/10.2147/CCID.S423304
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Pati Aji Achdiat,1 Syafira Andiani,1 Reti Hindritiani,1 Srie Prihianti Gondokaryono,1 Gempita Nuzuliyah,1 Hermin Aminah Usman,2 Retno Hesty Maharani1
1Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin General Hospital, Bandung, West Java, Indonesia; 2Department of Anatomical Pathology, Faculty of Medicine, Universitas Padjadjaran – Dr. Hasan Sadikin General Hospital, Bandung, Indonesia
Correspondence: Pati Aji Achdiat, Email [email protected]
Abstract: Molluscum contagiosum (MC) is a benign papular skin infection caused by Molluscum contagiosum virus (MCV). Over the past 30 years, the incidence of MK has continued to increased association with sexually transmitted infections and human immunodeficiency virus (HIV) infection. The incidence of MC in HIV patients is quite high at 5– 8%. Until now there is no standard therapy used for the treatment of MC in patients with HIV. In HIV patients, anti retro viral therapy (ARV) is the main therapy with several other additional therapies such as cantaridin, chemical peeling agents such as glycolic acid (20– 70%) and trichloroacetic acid (20– 100%), cryosurgery, electrosurgery, incision, lactic acid, laser surgery, podophyllin, retinoic acid, and urea. There have been no studies regarding the administration of topical 20% glycolic acid in MC patients. We report a case of MC in an HIV patient who was treated with 20% topical glycolic acid after failing treatment with topical tretinoin. The diagnosis was made clinically, cytologically, and histopathologically, a white mass was found on compression of the lesion and Henderson-Paterson bodies. The lesions on the face, arms, and legs were given glycolic acid lotion 20% which was applied once a day at night. The lesions started to show responses to the treatment at week 6th as some of the MC papules became hyperpigmented macules. The side effects of therapy that appeared were itching and hyperpigmentation. Topical 20% glycolic acid can be used for MC therapy with minimal side effects, easy to apply and safe.
Keywords: molluscum contagiosum, molluscum contagiosum virus, HIV patient, glycolic acid
Introduction
Molluscum contagiosum (MC) is a skin infection in the form of benign popular lesions caused by Molluscum contagiosum virus (MCV).1 It is more common in children and young adults.2–4 In Indonesia, the prevalence of molluscum contagiosum is high, at 40.4% of other skin diseases. The incidence of molluscum contagiosum in HIV patients is quite high at 5–8%, and in patients with CD4+ <100 the incidence of molluscum contagiosum is reported at 33%.5–7 Molluscum contagiosum has a typical clinical picture of a white, pink, or skin-colored, pearly, dome-shaped protrusion with central umbilication, measuring 1 mm to 1 cm in size, so that the diagnosis can be made clinically.1,8 Unusual clinical manifestations in HIV patients include atypical, disseminated, facial MC, or can be large with a diameter of more than 1 cm, referred to as giant MC.9,10 Management of MC in HIV patients is very difficult.11 There are several aspects that must be considered in choosing therapy, namely the patient’s physical and psychological endurance to receive therapy, especially if the number of lesions is large, the choice of the patient or the patient’s parents, cost, availability of therapeutic materials or tools, and ease of application. Curettage has been used as the gold standard therapy for MC.12 Tretinoin is one of the chemicals that can be used for molluscum therapy. There are a number of other destructive therapy modalities such as topical glycolic acid (GA) 20–70%.13
Here we report one case MC in an HIV patient who was treated with 20% topical GA after failing therapy with 0.05% topical tretinoin.
Clinical Case
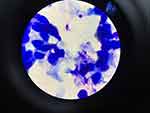
A 26-year-old homosexual male presented with a chief complaint of skin-colored pustules on the face, right arm, and right leg that were neither itchy nor painful and had not improved after eight weeks of treatment with vitacid® cream. In the anamnesis, it was found that since two years before treatment, the patient experienced excessive weight loss and diarrhea for one month and was diagnosed with HIV accompanied by the onset of skin-colored papules on the face with size of a pin on the right cheek that did not feel itchy or painful. Two months later the facial papules increased in number on the left cheek, right arm and right leg. The patient did not treat the skin disorder. Three months before he came to RSHS, complaints of papules getting bigger and more numerous to the eyelids, so the patient sought treatment at Cicendo Eye Hospital and they excised the papules on the eyelids. The patient was then referred to Dr. Hasan Sadikin General Hospital for further treatment of papules on the face, arms and legs. The patient was said to have a viral infection and was treated with vitacid® for two months but there was no improvement and the complaints of papules became erythema with the size of corn kernels. There was a history of repeated homosexual-anogenital exposures with the patient being the receptive partner. The patient has first sexual intercourse at the age of 18 years with a male casual friend, unprotected, anogenital, and orogenital. He has had sex with approximately 20 men in her lifetime. The last sexual intercourse with a male acquaintance six months ago was anogenital and orogenital, without using a condom. On physical examination, vital signs were within normal limits. The generalized status was found to be underweight. On the face, right arm, and right leg there were multiple lesions, discrete, round shape, size 0.2×0.2x0.1 cm to 0.8×0.3x0.2 cm, firm borders, arising from the skin surface, dry, in the form of skin-colored papules with central umbilication (Figure 1). The results of cytological examination of Tzanck smears with Giemsa staining of white masses taken from skin lesions on the face and neck found round-shaped hyperbasophilic masses (Henderson-Paterson bodies) (Figure 2). Histopathologic examination of the lesion on the right cheek revealed hyperplastic, endophytic, lobularly arranged, keratinized spongy epithelium with nuclei within normal limits (Figure 3). The cytoplasm appeared to form molluscum bodies in the form of eosinophilic inclusion bodies. In the center of the lesion, there was ostia formation containing keratinous debris. The dermis in the form of fibrocollagenous connective tissue is pressed into the lesion forming septa between the lobules. CD4+ test result was 26 cells/uL. Skin disorders showed improvement after 54 months of therapy with post inflammatory hyperpigmentation (Figure 4).
 |
Figure 1 Clinical manifestation of MC lesion on face, right arm, and right leg before therapy. |
 |
Figure 3 Histopathologic examination of the lesion on the right cheek revealed hyperplastic, endophytic, lobularly arranged, keratinized spongy epithelium with nuclei within normal limits. |
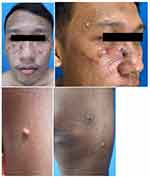 |
Figure 4 Clinical manifestation of MC lesion on face, right arm, and right leg before therapy, after 54th days of therapy. |
Discussion
Molluscum contagiosum (MC) is a self-limiting disease caused by infection with molluscum contagiosum virus (MCV) which is the only genus of molluscipoxvirus in the poxviridae family.1,9 MCV has 4 major subtypes namely MCV-1, MCV-2, MCV-3, and MCV-4 with MCV-1 being the most common cause of MC covering 75–96% of cases.14,15 MC cases in adolescents and adults are more often caused by MCV-2 because they experience transmission through sexual intercourse. In patients with HIV, 60% of MC cases are caused by MCV-2.8 MCV infection can also occur in adults who are sexually active or in immunosuppressed conditions, especially patients with HIV.16 The prevalence of MC co-infection with HIV or AIDS is around 5–18%.1,6 The World Health Organization (WHO) divides HIV into 4 clinical stages based on comorbidities found in history and physical examination. Supportive examinations such as CD4+ values can also be used to classify the degree of immunodeficiency of HIV patients which can be distinguished based on the number of CD4+ T cells, namely the acute phase, stage 1–2 with CD4+ counts >200 cells/uL (early chronic phase), stage 3–4 with CD4+ counts <200 cells/uL (advanced chronic phase), and <100 cells/uL in stage 4 (severe immunosuppression). Each of these stages can cause different mucocutaneous clinical manifestations, MC is usually found in patients with severe immunosuppression (stage 4) and CD4+ T cell count <100 cells/uL.10 The incubation period of the virus ranges from one week to six months, with an average time of 2–3 months.1,12 Transmission of MC can occur through direct skin-to-skin contact through sexual and non-sexual routes.12 In adults, MC with genital lesions is generally transmitted through sexual intercourse, so it is necessary to screening for sexually transmitted infections.14,17 The spread of MC through non-sexual routes can occur through the shared use of contaminated clothes, towels, and fomites.18 Living in the same house with an infected person, poor hygiene, overcrowded environment, having a history of atopic dermatitis, the use of topical steroids, immunosuppression such as in malignancy, and HIV are risk factors for the incidence of MC.17,19 Transmission of MCV to other locations is obtained through inoculation of the virus on the skin after trauma or micro-abrasion of the lesion. MC virus can enter the host epithelial cells by endocytosis or cell fusion and will replicate in the stratum spinosum of the epidermis. The virus will release the capsid and viral DNA transcription occurs to produce infective virions. Cells infected with the virus will increase the expression of epidermal growth factor receptors resulting in increased cell mitosis. The virus replicates in the cytoplasm of the cell producing cytoplasmic inclusion bodies (Henderson-Paterson bodies). The clinical picture of MC is a skin-colored papule, pearly or pink, dome-shaped, has a smooth surface, spongy consistency, with umbilication in the central part, contains a white mass containing molluscum bodies and can be seen when removed with pressure.20,21 Within a few weeks the size of MC can reach 3–5 mm in diameter, sometimes it can reach 1.5 cm in diameter, especially in immunosuppressed patients.21,22 The number of papules is usually less than 20 or can even reach 100 pieces or more.22 In HIV patients, the majority of MC lesions are found on the face and neck.6 In addition, although rare, MC lesions in HIV patients can appear in other locations, such as the soles of the feet, areolas, fingers, or eyelids.23 In this case report patient, MC lesions were in the form of skin-colored papules with umbilication in the center, white masses were found when pressing on the lesions, and most of the lesions were in the facial area in accordance with the majority of MC predilection in patients with HIV. The diagnosis of MC can be made based on the typical characteristics of the lesion.14 On suppression of the lesion, a grayish white mass is found.9 The discovery of molluscum bodies (Henderson-Paterson bodies) on cytological examination of Tzanck smear with Giemsa staining is pathognomonic in MC.20,24 On histopathologic examination MC shows hypertrophy or hyperplasia of the epidermis surrounding lobules containing keratinized debris and molluscum bodies.20 The diagnosis can be made through histopathological examination of the tissue using methenamine silver staining and the characteristic yeast cells divided by the septum as small cells (2–4 μm in diameter).25 In this case report patient, the diagnosis of MC was made based on the typical clinical picture, supported by the discovery of round masses (Henderson-Paterson bodies) on direct cytological examination with Giemsa staining and on histopathological examination. The duration of onset to disappearance of lesions varies with most occurrences self-limiting within 6–9 months in immunocompetent individuals. Some cases of lesions may persist for more than 3 or 4 years.4 In HIV patients, MC may not undergo spontaneous resolution without treatment.26 In this case report patient, the lesions appeared since two years before seeking treatment at RSHS and the complaints were still apparent when the patient sought treatment at RSHS when the patient came to RSHS. In general, there are 4 therapeutic approaches for MC, destructive therapy, chemical agents, immunomodulators, and antivirals.3 Destructive therapy works by damaging the intracytoplasmic sac containing viral particles and triggering an immunological response that will clear the infection.1 Some destructive therapies that can be performed are cryosurgery, curettage, tape stripping, and laser.3,9 In HIV patients, optimal management to restore immune system function with antiretroviral therapy is the main treatment of choice with several other adjunctive therapies.23 There are a number of treatment modalities available for HIV patients with MC including cantaridine, chemical peeling agents such as glycolic acid (20–70%) and trichloroacetic acid (20–100%), cryosurgery, electrosurgery, incision, lactic acid, laser surgery, podophylline, retinoic acid, urea,13 and intravenous or topical cidofovir.27 Treatment that destroys lesions such as curettage is not ideal as it may increase the risk of infection and disease transmission. In patients with HIV, MC lesions often do not undergo spontaneous resolution and may expand.13,14 Tretinoin is a retinol (vitamin A) derivative that can be used for MC therapy and is available in various concentrations. Tretinoin concentration of 0.05% can be an appropriate choice in MC therapy. The mechanism of tretinoin to break the viral lipid protein by controlling the growth and differentiation of keratinocytes, affecting the body’s immune response by producing cytokines, and inducing inflammation. The patient in this case report was treated with tretinoin 0.05% at night for 8 weeks, but there was no improvement in the lesions. Glycolic acid (GA) is one of the alpha hydroxy acids (AHA) belonging to the family of carboxylic acids derived from sugarcane.28 At low concentrations, AHA causes a decrease in corneocyte adhesion. At higher concentrations they promote epidermolysis. Glycolic acid 20%-70% belongs to superficial peeling agents and has anti-inflammatory, keratolytic, and antioxidant effects. Corneocyte are the main target of GA by increasing breakdown and reducing cohesiveness, leading to desquamation.29 In 2009, Fabbrocini classified GA as very superficial peeling agents (30–50% GA, applied for 1–2 minutes); superficial peeling agents (50–70% GA, applied for 2–5 minutes); and medium-depth peeling agents (70% GA, applied for 3–15 minutes).20 Glycolic acid is available in various concentrations. Concentrations of less than 20% can be used by patients themselves as home peels.30 In this case report, the patient was treated with 20% GA lotion applied every night to the MC papules lesion on the face, right arm and right leg. At first application, the 20% GA lotion was applied for 5 minutes, then rinsed off with water. The application time was increased according to the patient’s endurance. Therapeutic response in the form of lesion rupture and some of the MC papules lesion has become hyperpigmented macules starting from the 2nd to 6th week of observation after regular use for 8 hours. Post-inflammatory hyperpigmentation was found in the lesion areas that had resolved.
Conclusion
Overall, topical 20% glycolic acid can be used for MK therapy with minimal side effects, easy to apply and safe.
Ethic Statement
The publications of images were included in the patient’s consent for publication of the case. Institutional approval has been obtained to publish the case details.
Consent Statement
The authors certify that they have obtained all appropriate patient consent forms. The patient signed a consent form for the publication of the case details and images.
Acknowledgments
The authors would like to thank the staff of the Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Douglas JM. Molluscum contagiosum. In: Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, editors. Sexually Transmitted Diseases.
2. Gur I. The epidemiology of molluscum contagiosum in HIV-seropositive patients: a unique entity or insignificant finding? Int J STD AIDS. 2008;19(8):503–506. doi:10.1258/ijsa.2008.008186
3. Pérez-Blázquez E, Villafruela I, Madero S. Eyelid molluscum contagiosum in patients with human immunodeficiency virus infection. Orbit. 1999;18(2):75–81. doi:10.1076/orbi.18.2.75.2712
4. Wouden JC, Sande R, Kruithof EJ, Sollie A, Suijlekom-Smit LW, Koning S. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2017;5(5):CD004767. doi:10.1002/14651858.CD004767.pub4
5. Nguyen HP, Franz E, Stiegel KR, Hsu S, Tyring SK. Treatment of molluscum contagiosum in adult, pediatric, and immunodeficient populations. J Cutan Med Surg. 2014;18(5):299–306. doi:10.2310/7750.2013.13133
6. Schwartz JJ, Myskowski PL. Molluscum contagiosum in patients with human immunodeficiency virus infection: a review of twenty-seven patients. J Am Acad Dermatol. 1992;27(4):583–588. doi:10.1016/0190-9622(92)70226-6
7. Bhatia AC. Molluscum Contagiosum. Medscape; 2020. Available from: https://emedicine.medscape.com/article/910570-overview?form=fpf#a3.
8. Haddock ES, Friedlander SF. Poxvirus infection. In: Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, editors. Fitzpatrick Dermatology.
9. Chen X, Anstey AV, Bugert J. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013;13(10):877–888. doi:10.1016/S1473-3099(13)70109-9
10. Filo-Rogulska M, Pindycka-Piaszczyńska M, Januszewski K, Jarząb J. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol I Alergol. 2013;30(1):56–58. doi:10.5114/pdia.2013.33380
11. Kaufman WS, Ahn CS, Huang WW. Molluscum contagiosum in immunocompromised patients: AIDS presenting as molluscum contagiosum in a patient with psoriasis on biologic therapy. Cutis. 2018;101(2):136–140.
12. Vardhan P, Goel S, Goyal G, Kumar N. Solitary giant molluscum contagiosum presenting as lid tumor in an immunocompetent child. Indian J Ophthalmol. 2010;58(3):236. doi:10.4103/0301-4738.62652
13. Sadick N, Sorhaindo L. A comparative split-face study of cryosurgery and trichloroacetic acid 100% peels in the treatment of HIV-associated disseminated facial molluscum contagiosum. Cutis. 2009;83(6):299–302.
14. Fernando I, Pritchard J, Edwards SK, Grover D. UK national guideline for the management of genital molluscum in adults, 2014 clinical effectiveness group, British association for sexual health and HIV. Int J STD AIDS. 2015;26(10):687–695. doi:10.1177/0956462414554435
15. Silverberg NB. Pediatric molluscum contagiosum. Pediatric Drugs. 2003;5(8):505–511. doi:10.2165/00148581-200305080-00001
16. Qureshi A, Zeb M, Jalal-ud-din M, et al. Comparison of efficacy of 10% potassium hydroxide solution versus cryotherapy in treatment of molluscum contagiosum. J Ayub Med Coll Abbottabad. 2016;28(2):382–385.
17. Mohan RP, Verma S, Singh AK, Singh U. Molluscum contagiosum: report of one case with overview. BMJ Case Rep. 2013;2013:bcr2013008744. doi:10.1136/bcr-2013-008744
18. Leung AK, Barankin B, Hon KL. Molluscum contagiosum: an update. Recent Pat Inflamm Allergy Drug Discov. 2017;11(1):22–31. doi:10.2174/1872213X11666170518114456
19. Robinson MR, Udell IJ, Garber PF, Perry HD, Streeten BW. Molluscum contagiosum of the eyelids in patients with acquired immune deficiency syndrome. Ophthalmology. 1992;99(11):1745–1747. doi:10.1016/S0161-6420(92)31737-3
20. Piggott C, Friedlander SF, Wynnis T. Poxvirus infection. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffel DJ, Wolff K, editors. Fitzpatrick’s Dermatology in General Medicine.
21. Maluki A, Kadhum QJ. Treatment of molluscum contagiosum by potassium hydroxide solution 20% with and without pricking and by pricking alone: a comparative study with review of literature. Int J Dermatol Clin Res. 2015;1(2):031–041. doi:10.17352/2455-8605.000011
22. Brown ST, Nalley MD, James FB, et al. Molluscum contagiosum. Sex Transm Dis. 1981;8(3):227–234. doi:10.1097/00007435-198107000-00012
23. Beutler BD, Cohen PR. Molluscum contagiosum of the eyelid: case report in a man receiving methotrexate and literature review of molluscum contagiosum in patients who are immunosuppressed secondary to methotrexate or HIV infection. Dermatol Online J. 2016;22(3). doi:10.5070/D3223030363
24. Ahmad N, Khan S, Jetley S. Molluscum contagiosum: rapid cytological diagnosis using Tzanck smear in a case with unusual presentation. Natl J Med. 2017;6(2):C17–19.
25. Hay RJ. Deep fungal infection. In: Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, editors. Fitzpatrick Dermatology.
26. Al-Sudany NK, Abdulkareem DR. A comparative study of topical 10% KOH solution and topical 25% podophyllin solution as home-base treatments of molluscum contagiosum. J Dermatol dermatosurg. 2016;20:107–114.
27. De Clercq E. Cidofovir for the treatment of molluscum contagiosum virus. Viruses. 2022;14(11):2484. doi:10.3390/v14112484
28. O’Connor AA, Lowe PM, Shumack S, Lim AC. Chemical peels: a review of current practice. Australas J Dermatol. 2018;59(3):171–181. doi:10.1111/ajd.12715
29. Sharad J. Glycolic acid peel therapy–a current review. Clin Cosmet Investig Dermatol. 2013;6:281. doi:10.2147/CCID.S34029
30. Erbağcı Z, Akçalı C. Biweekly serial glycolic acid peels vs. long‐term daily use of topical low‐strength glycolic acid in the treatment of atrophic acne scars. Int J Dermatol. 2000;39(10):789–794. doi:10.1046/j.1365-4362.2000.00076.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.