Back to Journals » International Journal of Women's Health » Volume 15
Molecular Mechanisms Behind Vascular Mimicry as the Target for Improved Breast Cancer Management
Authors Wei Y, Jiao Z, Sun T , Lai Z, Wang X
Received 29 January 2023
Accepted for publication 20 June 2023
Published 12 July 2023 Volume 2023:15 Pages 1027—1038
DOI https://doi.org/10.2147/IJWH.S406327
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Everett Magann
Yali Wei,1,* Zheng Jiao,2,* Tianpei Sun,3,* Zhiwei Lai,4 Xiaochun Wang1
1Department of Breast Surgery, Affiliated Hospital of Hebei University, Baoding, Hebei Province, People’s Republic of China; 2Department of Neurosurgery, Youanmen Hospital, Beijing, People’s Republic of China; 3Clinical School of Medicine, Hebei University, Baoding, Hebei Province, People’s Republic of China; 4Department of Thoracic Surgery, Shanghai Sixth People’s Hospital Fujian Campus, Jinjiang, Fujian Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaochun Wang, Department of Breast Surgery, Affiliated Hospital of Hebei University, 212 Yuhua Dong Road, Baoding, Hebei Province, 071000, People’s Republic of China, Tel +86312-5983922, Email [email protected]
Introduction: Breast cancer has a high incidence and mortality rate in women due to metastasis and drug resistance which is associated with vasculogenic mimicry (VM). We purposed to explore VM formulation in breast cancer and mechanism of which is involved in EphA2/PIK3R1/CTNNB1 in the present study.
Methods: The expression of EphA2/PIK3R1/CTNNB1 and breast cancer patient prognosis was analyzed from TCGA data, both gene and protein expression as well as VM were measured in human breast cancer tissue samples collected in our study. The relationship between EphA2/PIK3R1/CTNNB1 and the formation of VM in breast cancer and its possible regulatory mechanism was explored.
Results: The results of the bioinformatics analysis based on TCGA showed that the expression of PIK3R1/ CTNNB1/ PECAM1 (CD31) in tumor tissues was significantly lower than that in normal tissues. EphA2 was positively correlated with PIK3R1, PIK3R1 with CTNNB1, and CTNNB1 with PECAM1 expression in breast cancer tissues. The results of detection in breast cancer and adjacent tissues indicated that the expression of EphA2/PIK3R1/CTNNB1 in cancer tissues was significantly lower than that in adjacent tissues. The expression of PIK3R1 was positively correlated with EphA2 and CTNNB1 expression, respectively, as well as EphA2 expression correlated with CTNNB1 expression positively. VM formation was significantly increased in breast cancer tissues compared with adjacent tissues.
Conclusion: Our results suggested that the formation of VM in breast cancer may be related to the EphA2/PIK3R1/CTNNB1 molecular signaling pathway.
Keywords: breast cancer, vasculogenic mimicry, EphA2/PIK3R1/CTNNB1 pathway, targeted prediction and prevention
Introduction
Breast cancer is the most common malignant tumor in female patients around the world with increasing incidence year by year, and it is also the main cause of most cancer-related deaths.1 Although systemic treatment has improved the cure rate of breast cancer, patients with metastatic or highly malignant breast cancer still have a poor prognosis and lower five-year survival rate.2 It is still needed to explore new and potential targets for breast cancer prediction and intervention presently.
Metastasis and drug resistance of breast cancer may be important reasons for its high mortality. At present, the effect of treatment for metastatic breast cancer remains to be improved and correlation treatment is still a serious problem, so in-depth study of the pathological mechanism of high malignant breast cancer has guiding significance for breast cancer treatment.
Tumor metastasis and invasion and tumor cell survival, growth method of the microenvironment is necessary. As the tumor progresses, when the tumor cells grow rapidly, the surrounding nutrients cannot meet the needs of the tumor cells, the tumor cells will appear a state of hypoxia. Vasculogenic mimicry (VM) is reported as one type of vascular supply modes for malignant tumors.3 VM is a new vascular supply mode, which is a microvascular channel composed of non-endothelial cells (tumor cells). Tumor cells secrete extracellular matrix components and self-shaping, thus enclosing the pipeline structure. Malignant tumor cells have the specificity to form vasculoid networks that provide adequate blood supply for tumor growth. Studies have confirmed the existence of VM in most tumor tissues with high malignant degree and easy metastasis, such as breast cancer, liver cancer, glioma, melanoma.4–6 Patients with malignant tumors in the presence of VM tend to have poor survival and poor prognosis. Since tumor microenvironment affects the occurrence and development of tumors, changes in tumor microenvironment, such as the initial hypoxic environment of solid tumors, are inseparable from VM.5 Hypoxic environment can increase the degree of malignancy of tumor cells, promote tumor metastasis, and promote the ability of tumor cells to form pipelines, thus promoting VM formation, affecting the poor prognosis of patients.7 VM has been recognized as a novel model of invasive tumor angiogenesis.
In recent years, a large number of studies have pointed out that some anti-angiogenesis clinical treatment effects are not effective enough, possibly due to VM activation. Researchers have proposed a variety of molecular mechanisms that regulate VM formation, particularly Erythropoietin-producing hepatocellular carcinoma-A2 (EphA2), phosphatidylinositol 3-kinase (PI3K), matrix metalloproteinases (MMPs), vascular endothelial growth factor receptor (VEGFR1), and hypoxia induction factor (HIF-1α) are involved in the formation of VM and are associated with tumor migration and invasion. Hypoxia, cancer stem cells (CSCs) and epithelial-mesenchymal transition (EMT) are important factors for VM formation and tumor metastasis.4 In particular, VE-Cadherin, EphA2, and nodal signaling pathways are involved in VM formation.7 CTNNB1 encodes cadherin-related protein β-catenin is stimulated and interacts with signal molecules, leading to transcription of genes related to cell proliferation.8 β-catenin plays a role in regulation of metabolism and energy homeostasis in breast cancer.9 β-catenin is involved in Wnt signaling which correlated with breast cancer progress.10 However, the role of CTNNB1 in VM of breast cancer and the mechanism of the VM has not been fully clarified to date.
In general, we hypothesized EphA2/PIK3R1/CTNNB1 might play a role in VM to correlate with prognosis of breast cancer patients. We purposed to explore VM formulation in breast cancer and mechanism of which involved in EphA2/PIK3R1/CTNNB1 in the present study. The expression of EphA2/PIK3R1/CTNNB1 and breast cancer patient prognosis was analyzed from TCGA data, both gene and protein expression as well as VM were measured in human breast cancer tissue samples collected in our study. The relationship between EphA2/PIK3R1/CTNNB1 and the formation of VM in breast cancer and its possible regulatory mechanism was investigated, in order to provide theoretical basis and data support for molecular targeted therapy of breast cancer.
Materials and Methods
Reagents and Antibodies
Antibodies of EphA2 (220092), CTNNB1 (R22820) and CD31 (347526) were purchased from ZENBIO (http://www.zen-bio.cn/, China). Antibody of PIK3R1 (Bsm-33219M) was applied by Bioss (http://www.bioss.com.cn/, China). All primers were synthesized and obtained from Invitrogen. Trizol reagent (R0016), SYBR Green Master Mix (D7262) and PAS kit (C0142S) were purchased from Beyotime (China).
Clinical Samples
Twenty patients with breast cancer including 11 luminal A, 4 luminal B, 3 HER2-positive and 2 triple-negative cancers confirmed by X-ray examination and pathological assessment in our hospital from December 2020 to May 2021 were included in the study. Inclusion criteria: (1) aged 18 to 45 premenopausal; (2) first time diagnosed without radiation or chemotherapy; (3) breast cancer in situ. Exclusion criteria: (1) history of hypertension and diabetes; (2) smoking history; (3) ovarian cancer and other complicated malignant tumors; (4) non-breast carcinoma in situ. This study was approved by the institutional research ethics committee of Hospital Affiliated to Hebei University (HDFY-LL-2021-240). All the patients involved in the present study have written informed consents in accordance with the Declaration of Helsinki.
Transcriptional Expression Analysis of Genes
Box plots of EphA2, PIK3R1, CTNNB1 and PECAM1 (CD31) expression breast tumors and normal tissues were obtained from Multiple Datasets of Breast invasive carcinoma (BRCA) of TCGA and Genotype-Tissue Expression (GTEx) databases8 in the Expression Analysis Module of GEPIA2 (http://gepia2.cancer-pku.cn).
Survival Prognosis Analysis
Survival prognosis analysis in breast cancer patients with EphA2/PIK3R1/CTNNB1/PECAM1 expression was performed using the Kaplan–Meier plotter (https://kmplot.com/analysis) and the data was from Multiple Datasets of Breast invasive carcinoma (BRCA) of TCGA based on Affymetrix microarray information. The prognostic value of EphA2/PIK3R1/CTNNB1/PECAM1 in breast cancer was evaluated by OS (overall survival).
Real-Time Quantitative PCR (qRT-PCR)
Real-time quantitative PCR was used to detect mRNA expression of EphA2, PIK3R1 and CTNNB1 in breast cancer tissues and corresponding para-carcinoma tissues. Briefly, total RNA was extracted using Trizol reagent according to the manufacturer’s instructions. Then, the RNA samples were reversely transcribed into cDNA in a reaction of 20 μL. Fluorescence quantitative PCR was performed on real-time quantitative PCR instrument (QuantStudio 6, ABI) with SYBR Green Master Mix (D7262), the reaction system was under a condition of pre-denaturation for 2min at 50°C, 10 min for 10 min, 40 cycles were conducted including 30s at 95°C and 30s at 60°C. The dissolution curve was drawn and the final data were quantified using 2−ΔΔCt method. The primers used in this study are listed in Table 1.
 |
Table 1 Primer Sequences for Real-Time PCR Used in the Study |
Immunohistochemistry (IHC)
Resected breast cancer tissues and corresponding para-carcinoma tissues from breast cancer patients who underwent surgical resection were fixed in 4% paraformaldehyde overnight. Then, the tissues were embedded with Paraffin, and cut into 4μm slices. After deparaffinization, dehydration, antigen retrieval and blocking endogenous peroxidase, the sections were incubated with primary antibodies against EphA2 (1:500, ZENBIO, 220092), CTNNB1 (1:500, ZENBIO, R22820) and PIK3R1 (1:500, Bioss, Bsm-33219M) at 4°C overnight. The sections were then incubated with HRP‐conjugated secondary antibodies conjugated with HRP followed by exposing to DAB to visualize signals of the antigen and counterstaining with hematoxylin. The sections were imaged under a microscope (BX43, OLYMPUS). Then, the stained slices were quantitatively analyzed by image-Pro Plus Image analysis system.
CD31-Periodic Acid Schiff (PAS) Double Staining for VM
Sections were stained for CD31 using primary antibody (1:500, ZENBIO, 347526) and HRP‐conjugated secondary antibody to visualize blood endothelial cells. Then, the sections were placed in PAS solution (0.5%) and incubated for 5 min followed by being stained using Schiff reagent for 20min. After being rinsed using distilled water, the sections were counterstained with hematoxylin. Photographs of sections were taken under a microscope and analyzed by image-Pro Plus Image analysis system.
Statistical Analysis
The results are expressed as the mean and standard error of the mean (mean ± SEM). Statistical calculations were performed with GraphPad Prism 8 (GraphPad Software, Inc., San Diego, USA). Student’s t-test was used to compare the two groups. Pearson correlation coefficient was used to analyze the correlation between studied genes in this research, P < 0.05 was considered statistically significant.
Results
Expression of EphA2/PIK3R1/CTNNB1/PECAM1 in Breast Cancer Tissues Compared with Normal Tissues and Correlation of the Expression with Survival of Patients with Breast Cancer Based on Bioinformatics Analysis
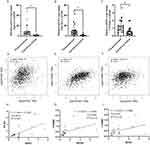
Gene expression of EphA2, PIK3R1, CTNNB1 and PECAM1 in breast cancer tissues (n=1097) compared with normal tissues (n=114) was obtained from data of TCGA analysis using GEPIA2. As shown in Figure 1, the expression of EphA2, PIK3R1, CTNNB1 and PECAM1 was down regulated in primary tumor compared with normal tissue.
 |
Figure 1 Expression level of the prognostic value in normal tissues and breast cancer samples from the TCGA Database. (A) EPHA2, (B) PIK3R1, (C) CTNNB1, (D) PECAM1. |
Survival of breast cancer patients with different expression levels of EphA2, PIK3R1, CTNNB1 and PECAM1 was then analyzed from data based on Kaplan–Meier Plotter database. All the breast cancer patients with high expression levels of EphA2 (n=2461), PIK3R1 (n=2462), CTNNB1 (n=2465) or PECAM1 (n=2465) had significantly longer survival than those with low expression levels of EphA2 (n=2468), PIK3R1 (n=2467), CTNNB1 (n=2464) or PECAM1 (n=2464) (P<0.05) (Figure 2).
 |
Figure 2 Survival curves comparing low and high expressions of EphA2/PIK3R1/CTNNB1/PECAM1 in breast cancer. (A) EPHA2, (B) PIK3R1, (C) CTNNB1, (D) PECAM1. All the survival analyses were conducted via Kaplan–Meier Plotter database (https://kmplot.com/analysis). |
mRNA Expression of EphA2, PIK3R1 and CTNNB1 in Breast Cancer Tissues and Corresponding Para-Carcinoma Tissues from Breast Cancer Patients Collected in Our Study and the Correlation of EphA2, PIK3R1 and CTNNB1 with Each Other
The mRNA expression of EphA2, PIK3R1 and CTNNB1 in 20 pairs of breast cancer and corresponding para-carcinoma tissues was measured. Results was showed in Figure 3A–C that the expression of EphA2, PIK3R1 and CTNNB1 was significantly downregulated compared with paracancerous (P<0.05) which was consistent with the bioinformatics analysis of data from TCGA. The correlation of EphA2, PIK3R1 and CTNNB1 with each other, especially PIK3R1 and CTNNB1 (R=0.32) expression was positively in cancerous tissues of breast, which was obtained from data of TCGA (Figure 3D–F). Moreover, positive correlation among EphA2, PIK3R1 and CTNNB1 expression as well as significantly positive correlation between EphA2 and PIK3R1 (R2=0.4257) was further found in the results measured in the collected cancer samples in our study (Figure 3G–I).
Protein Expression of EphA2, PIK3R1 and CTNNB1 in Breast Cancer Tissues and Corresponding Para-Carcinoma Tissues from Breast Cancer Patients Collected in Our Study
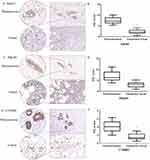
The protein expression of EphA2, PIK3R1 and CTNNB1 in breast cancer tissues and corresponding para-carcinoma tissues from breast cancer patients was detected using immunohistochemical staining. It was consistent with mRNA expression that the protein expression of EphA2, PIK3R1 and CTNNB1 was significantly increased in cancerous tissue compared with paracancerous tissue (P<0.05) (Figure 4).
Vascular Mimicry (VM) Denoted by PAS-CD31 Staining and Its Relation with PIK3R1 and CTNNB1 Expression in Breast Cancer Tissues and Corresponding Para-Carcinoma Tissues from Breast Cancer Patients Collected in Our Study
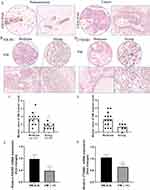
Considering PECAM1 (CD31) lower expression in primary tumor compared with normal tissue obtained from TCGA and involved in marking vascular mimicry which associated with tumor progression, vascular mimicry was detected in breast cancer tissues and corresponding para-carcinoma tissues from breast cancer patients collected in our study. As shown in Figure 5A, there are two lumen morphologies in breast cancer tissue, including endothelium-dependent vessels and VM. Tissues stained by PAS were purplish red and CD31 positive marks endothelial cells around the lumen are brown. VM formation (PAS positive and CD31 negative) was increased in breast cancer tissues, the presence of pale red blood cells could be observed in the lumen, and there was no bleeding around the lumen, cell necrosis and inflammatory cell infiltration. Paracancer tissues are mostly PAS-positive and CD31-positive blood vessels. VM formation in moderate-PIK3R1 (n=12) (Figure 5B and C) or CTNNB1 (n=14) (Figure 5D and E) expression (IHC score less than or equal to 2) tissues was more than that in Strong-PIK3R1 (n=8) or CTNNB1 expression (n=6) (IHC score more than 2). Furthermore, the mRNA expression of PIK3R1 and CTNNB1 in samples with more VM (VM more than or equal to 3) was significantly low compared with those with less VM (VM less than 3) (Figure 5F and G).
Discussion
Breast cancer is one of the most common cancers in the world.11 Prolongation of patient survival and improvement of life quality are the main goals of breast cancer treatment. Breast cancer biomarkers and subtypes, and genomics exploration affect future breast cancer diagnosis, treatment, and survival.12 VM of vascular channels independent of endothelial cells is involved in tumor invasion, metastasis, and drug resistance associated with poor prognosis of breast cancer.13 VM is the formation of PAS positive and vascular endothelial marker CD31 negative structures in tumor tissues.14 Erythropoietin is reported to produce EphA2, VE-cadherin, PI3K, MMPs, VEGFR1, HIF-1α and other molecular mechanisms are involved in the formation and progression of VM.4 In the present study, 114 cases of normal tissues and 1097 tumor cases were extracted from Multiple Datasets of Breast invasive carcinoma (BRCA) of TCGA. EphA2/PIK3R1/CTNNB1 expression and correlation between their expression and prognosis of breast cancer patients was analyzed, results of which showed that the expression of EphA2 in tumor tissues was significantly lower than that in normal tissues and positively correlated with the prognosis of patients. Moreover, the gene and protein expression of EphA2/PIK3R1/CTNNB1 were detected in the samples collected in our study and the correlation of EphA2, PIK3R1 and CTNNB1 with each other was analyzed, results of which were consistent with bioinformatics analysis of data from TCGA. However, it has been reported that the tyrosine kinase EphA2 belongs to the Eph receptor family and is produced in large quantities in tumor tissues, while the content is relatively low in most normal adult tissues so that EphA2 is a potential target for the treatment of malignant tumors.15 EphA2 is overexpressed in invasive breast cancer that includes the HER2+ subtype and associated with poor prognosis.16 The relationship between EphA2 and prognosis of breast cancer patients remains to be further explored.
Abnormalities of the PI3K/PTEN pathway are common events in cancer, and PIK3CA and PTEN are the most common mutated genes after TP53. PIK3R1 is the main regulatory subtype of PI3K. The results of our study showed that the expression of PIK3R1 in breast cancer tissues is significantly lower than that in normal tissues, which is consistent with the report that PIK3R1 is a tumor suppressor gene.8 Mutations in related signaling molecules are also important factors in cancer development. CTNNB1 participates in Wnt/β-catenin signaling pathway, which plays an important role in stem cell maintenance.17 CTNNB1 is up-regulated in endometrial circulating tumor cells and is a potential therapeutic target for endometrial cancer.18 However, the analysis results of bioinformatics analysis of data from TCGA indicated that the expression of CTNNB1 in Normal tissues was significantly higher than that in tumor tissues which was consistent with those expressions detected in the breast cancer samples collected in our study.
VM is composed of tumor cells, which mimic endothelial cells to surround the lumen and create endothelium-dependent vascular connectivity.19 More VM was found in breast cancer tissues compared with paracancerous tissue which was similar to previous study that a variety of highly invasive tumors were involved in VM.20 Angiogenesis is a fundamental process of tumorigenesis, growth, invasion and metastatic spread, as exosomal EPHA2 derived from highly metastatic breast cancer cells promotes angiogenesis.21 EPHA2 expression constitutes a potential prognostic factor for TNBC patients.22,23 PIK3R1 alterations or reduced mRNA expression tended to be associated with worse clinical outcomes in prostate cancer.24 Previous study demonstrated that the PI3K/MMPS/LN-5 γ2 signaling pathway mediates the formation of VM in invasive human gallbladder carcinoma which was analogous to our study that PIK3R1 expression in para-cancer tissues is significantly higher than that in breast cancer tissues, and PIK3R1 may be a tumor suppressor factor.25 Meanwhile, the expression of EphA2 and PIK3R1 was positively correlated in breast cancer tissues. In addition, more VM formations were found in breast cancer tissue with less PIK3R1 expression in our study.
The protein β-catenin encoded by CTNNB1 is a multifunctional relatively evolutionary conserved molecule that plays a key role in animal development and homeostasis. Imbalances in the structure and signal transduction properties of β-catenin often lead to disease and dysregulated growth associated with cancer and metastasis. In-depth study of β-catenin is helpful to elucidate the mechanism of cancer occurrence and malregulated growth.26 EMT was considered associating with tumor metastasis, and the expression of EMT-related protein e-cadherin was down-regulated with the down-regulation of TGFβ.27 Researchers have found that the occurrence of VM in tumors may be driven by TGFβ to jointly induce tumor cells to mesenchymal phenotypic transformation, with high expression rate ofof stem cell markers.20 The expression of CTNNB1 (β-catenin) in breast cancer tissues and adjacent tissues was lower than that in adjacent tissues. Preliminary analysis indicated that EphA2 and PIK3R1 were positively correlated with CTNNB1 in breast cancer tissues. According to previous studies,28,29 promising targets for predictive, preventive, and personalized medicine in breast cancer with the highest incidence among all cancers to benefit the patient and society remain deserved. Therefore, VM and its related molecular mechanism may be important for prognosis of patients with breast cancer. Moreover, VM and EphA2/PIK3R1/CTNNB1 expression in breast cancer including tumor subtypes in larger size of samples would be explored in our further study.
Conclusions and Recommendation
Results based on bioinformatics analysis and the detection results breast cancer and adjacent tissues showed that the expression of EphA2/PIK3R1/CTNNB1 in cancer tissues, which correlated with breast patient prognosis, was significantly lower than that in adjacent tissues and correlated with breast patient prognosis. The expression of PIK3R1 was positively correlated with EphA2 and CTNNB1 expression, respectively, as well as EphA2 expression correlated with CTNNB1 expression positively. VM formation was significantly increased in breast cancer tissues compared with adjacent tissues. The formation of VM in breast cancer may be related to the EphA2/PIK3R1/CTNNB1 molecular signaling pathway. VM as well as its related molecular signals of EphA2/PIK3R1/CTNNB1 may be the promising targets for breast cancer prediction and prevention.
Abbreviations
VM, vasculogenic mimicry; EphA2, erythropoietin-producing hepatocellular carcinoma-A2; PI3K, phosphatidylinositol 3-kinase; MMPs, matrix metalloproteinases; VEGFR1, vascular endothelial growth factor receptor; HIF-1α, hypoxia induction factor; EMT, epithelial-mesenchymal transition; CSCs, cancer stem cells; qRT-PCR, real-time quantitative PCR; IHC, Immunohistochemistry.
Data Sharing Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the institutional research ethics committee of Hospital Affiliated to Hebei University (HDFY-LL-2021-240). All the patients involved in the present study have written informed consents.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Liang Y, Zhang H, Song X, et al. Metastatic heterogeneity of breast cancer: molecular mechanism and potential therapeutic targets. Semin Cancer Biol. 2020;60:14–27. doi:10.1016/j.semcancer.2019.08.012
2. Zhang Y, Lv F, Yang Y, et al. Clinicopathological features and prognosis of metaplastic breast carcinoma: experience of a Major Chinese Cancer Center. PLoS One. 2015;10(6):e0131409. doi:10.1371/journal.pone.0131409
3. Zhang S, Zhang D, Sun B. Vasculogenic mimicry: current status and future prospects. Cancer Lett. 2007;254(2):157–164. doi:10.1016/j.canlet.2006.12.036
4. Zhang J, Qiao L, Liang N, et al. Vasculogenic mimicry and tumor metastasis. J buon. 2016;21(3):533–541.
5. Wei X, Chen Y, Jiang X, et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol Cancer. 2021;20(1):7. doi:10.1186/s12943-020-01288-1
6. Treps L, Faure S, Clere N. Vasculogenic mimicry, a complex and devious process favoring tumorigenesis - interest in making it a therapeutic target. Pharmacol Ther. 2021;223:107805. doi:10.1016/j.pharmthera.2021.107805
7. Guan YY, Luan X, Lu Q, et al. Natural products with antiangiogenic and antivasculogenic mimicry activity. Mini Rev Med Chem. 2016;16(16):1290–1302. doi:10.2174/1389557516666160211115507
8. Tanabe S, Aoyagi K, Yokozaki H, et al. Regulation of CTNNB1 signaling in gastric cancer and stem cells. World J Gastrointest Oncol. 2016;8(8):592–598. doi:10.4251/wjgo.v8.i8.592
9. Vergara D, Stanca E, Guerra F, et al. β-catenin knockdown affects mitochondrial biogenesis and lipid metabolism in breast cancer cells. Front Physiol. 2017;8:544. doi:10.3389/fphys.2017.00544
10. Xu X, Zhang M, Xu F, et al. Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities. Mol Cancer. 2020;19(1):165. doi:10.1186/s12943-020-01276-5
11. Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. doi:10.1016/S0140-6736(16)31891-8
12. Odle TG. Precision medicine in breast cancer. Radiol Technol. 2017;88(4):401m–421m.
13. Andonegui-Elguera MA, Alfaro-Mora Y, Cáceres-Gutiérrez R, et al. An overview of vasculogenic mimicry in breast cancer. Front Oncol. 2020;10:220. doi:10.3389/fonc.2020.00220
14. Lin PP. Aneuploid Circulating Tumor-Derived Endothelial Cell (CTEC): a novel versatile player in tumor neovascularization and cancer metastasis. Cells. 2020;9(6):1539. doi:10.3390/cells9061539
15. Xiao T, Xiao Y, Wang W, et al. Targeting EphA2 in cancer. J Hematol Oncol. 2020;13(1):114. doi:10.1186/s13045-020-00944-9
16. Liang Z, Wang X, Dong K, et al. Expression pattern and prognostic value of EPHA/EFNA in breast cancer by bioinformatics analysis: revealing its importance in chemotherapy. Biomed Res Int. 2021;2021:5575704. doi:10.1155/2021/5575704
17. Yang Z, Zhao T, Liu H, et al. Ginsenoside Rh2 inhibits hepatocellular carcinoma through β-catenin and autophagy. Sci Rep. 2016;6:19383. doi:10.1038/srep19383
18. Alonso-Alconada L, Muinelo-Romay L, Madissoo K, et al. Molecular profiling of circulating tumor cells links plasticity to the metastatic process in endometrial cancer. Mol Cancer. 2014;13:223. doi:10.1186/1476-4598-13-223
19. Sun H, Nan Y, Siqi C, et al. Cancer stem-like cells directly participate in vasculogenic mimicry channels in triple-negative breast cancer. Cancer Biol Med. 2019;16(2):299–311. doi:10.20892/j.issn.2095-3941.2018.0209
20. Morales-Guadarrama G, García-Becerra R, Méndez-Pérez EA, et al. Vasculogenic mimicry in breast cancer: clinical relevance and drivers. Cells. 2021;10(7):1758. doi:10.3390/cells10071758
21. Han B, Zhang H, Tian R, et al. Exosomal EPHA2 derived from highly metastatic breast cancer cells promotes angiogenesis by activating the AMPK signaling pathway through Ephrin A1-EPHA2 forward signaling. Theranostics. 2022;12(9):4127–4146. doi:10.7150/thno.72404
22. Nikas I, Giaginis C, Petrouska K, et al. EPHA2, EPHA4, and EPHA7 expression in triple-negative breast cancer. Diagnostics (Basel). 2022;12(2):366.
23. Zhang X. The expression profile and prognostic values of EPHA family members in breast cancer. Front Oncol. 2021;11:619949. doi:10.3389/fonc.2021.619949
24. Chakraborty G, Nandakumar S, Hirani R, et al. The impact of PIK3R1 mutations and insulin-PI3K-glycolytic pathway regulation in prostate cancer. Clin Cancer Res. 2022;28:3603–3617. doi:10.1158/1078-0432.CCR-21-4272
25. Liu Y, Wang D, Li Z, et al. Pan-cancer analysis on the role of PIK3R1 and PIK3R2 in human tumors. Sci Rep. 2022;12(1):5924. doi:10.1038/s41598-022-09889-0
26. Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31(12):2714–2736. doi:10.1038/emboj.2012.150
27. Kusunose M, Hashimoto N, Kimura M, et al. Direct regulation of transforming growth factor beta-induced epithelial-mesenchymal transition by the protein phosphatase activity of unphosphorylated PTEN in lung cancer cells. Cancer Sci. 2015;106(12):1693–1704. doi:10.1111/cas.12831
28. Zhan X, Li J, Guo Y, et al. Mass spectrometry analysis of human tear fluid biomarkers specific for ocular and systemic diseases in the context of 3P medicine. Epma j. 2021;12(4):449–475. doi:10.1007/s13167-021-00265-y
29. Mazurakova A, Koklesova L, Samec M, et al. Anti-breast cancer effects of phytochemicals: primary, secondary, and tertiary care. Epma J. 2022;13(2):315–334. doi:10.1007/s13167-022-00277-2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.