Back to Journals » Infection and Drug Resistance » Volume 14
Molecular Characteristics of Rifampin-Sensitive and -Resistant Isolates and Characteristics of rpoB Gene Mutations in Methicillin-Resistant Staphylococcus aureus
Authors Guo Y, Wang B, Rao L, Wang X, Zhao H, Li M, Yu F
Received 2 September 2021
Accepted for publication 22 October 2021
Published 4 November 2021 Volume 2021:14 Pages 4591—4600
DOI https://doi.org/10.2147/IDR.S336200
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yinjuan Guo,1 Bingjie Wang,1 Lulin Rao,1 Xinyi Wang,1 Huilin Zhao,1 Meilan Li,2 Fangyou Yu1
1Department of Clinical Laboratory, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, Shanghai, 200433, People’s Republic of China; 2Respiratory Intensive Care Unit, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, Shanghai, 200433, People’s Republic of China
Correspondence: Meilan Li
Respiratory Intensive Care Unit, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, 3rd Floor, Building 2, No. 507 Zhengmin Road, Yangpu District, Shanghai, People’s Republic of China
Email [email protected]
Fangyou Yu
Department of Clinical Laboratory, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, 3rd Floor, Building 2, No. 507 Zhengmin Road, Yangpu District, Shanghai, People’s Republic of China
Tel +86 13575440803
Email [email protected]
Introduction: Methicillin-resistant Staphylococcus aureus (MRSA) infections have become a leading cause of severe infections in both healthcare and community settings. Mutations in the rpoB gene cause resistance to rifampin (RIFR), a critical antibiotic for the treatment of multidrug-resistant Staphylococcus aureus. The aim of this study was to detect the molecular characteristics of RIFR MRSA and analyze the rpoB gene mutations involved in RIF resistance.
Methods: A total of 49 RIFR MRSA and 38 RIFS MRSA isolates collected from seven cities in China were analyzed by multilocus sequence typing, staphylococcus chromosomal cassette mec (SCCmec) typing, spa typing, and rpoB gene mutations.
Results: ST239-III-t030 (35/49, 71.4%), the major clone in RIFR MRSA isolates; ST45-IV-t116 (16/38, 42.1%), the major clone in RIFS MRSA isolates with rpoB mutations. RIFR MRSA isolates were resistant to erythromycin, ciprofloxacin, tetracycline, gentamicin, and clindamycin. By contrast, RIFS MRSA isolates with rpoB mutation were more susceptible to ciprofloxacin, tetracycline, and gentamicin. Forty-three (87.8%) isolates present the mutational change H481N and L466S, conferring 128– 512 μg/mL RIF resistance. The four isolates with RIF MIC ≥ 1024 μg/mL had additional amino acid substitution: H481N, L466S, A473T (n=2); H481Y (n=2), associated with a high-level RIF resistance. Of 38 RIFS MRSA isolates, two mutations were observed, including H481N (n=37) and A477D (n=1).
Conclusion: In conclusion, the predominant RIFR MRSA clones in China were ST239-III-t030. Molecular characteristics, antibiotic-resistant profiles, and rpoB mutations between RIFR MRSA and RIFS MRSA were diverse. Antibiotics for treating patients with MRSA infections can be selected based on molecular characteristics.
Keywords: MRSA, rifampin, rpoB mutations, MLST, SCCmec, spa
Introduction
Staphylococcus aureus is a major human pathogen that causes a diversity of diseases ranging from relatively minor to invasive and systemic diseases with significant morbidity and mortality, which results in significant economic and societal costs.1 Since the first European isolate2 of methicillin-resistant Staphylococcus aureus (MRSA) was detected in the 1960s, MRSA infections have become a leading cause of bacterial infections in both healthcare and community settings and a global concern.3 The spread of different clones from different geographic regions has been reported.4 Sequence type (ST239) clone was the most important hospital-associated MRSA (HA-MRSA) around the world and disseminated in hospitals through Europe, North America, South America, and Asia.5 A previous study showed that MRSA ST239 and MRSA ST5 were also predominant in Chinese hospitals.6,7 However, ST228 was the predominant clone of RIFR MRSA isolates in Spain.8
MRSA was generated when methicillin-susceptible S. aureus (MSSA) acquires mecA gene encoding the penicillin-binding protein 2a (PBP2a) and acquired by horizontal transfer of a mobile genetic element designated staphylococcal cassette chromosome mec (SCCmec).9 To date, 13 SCCmec types have been identified among S. aureus in the world.5 Generally, HA-MRSA typically belongs to SCCmec I, II, and III, while CA-MRSA carries SCCmec IV or V.5 In addition, spa typing can be used for the investigation of both molecular evolution and hospital outbreaks.10
Most MRSA isolates are resistant to multiple antibiotics.5 Glycopeptides such as vancomycin are the primary treatment option for severe infections caused by MRSA and most strains of multidrug-resistant S. aureus.11 Because of poor tissue diffusion and moderate bactericidal activity, vancomycin is often combined with rifampin for deep-seated infections.12 However, the efficacy of vancomycin has declined with the emergence of vancomycin-intermediate S. aureus (VISA) and heterogeneous VISA.13 A number of studies have revealed a worrying link between certain rpoB mutations and decreased susceptibility not only to rifampin but also other last line anti-MRSA antibiotics such as beta-lactams, imipenem, vancomycin, or daptomycin in S. aureus.14–17 One study reported that 86% of all resistance to rifampin isolates in their global sample carried the mutations promoting cross-resistance to vancomycin and 52% to both vancomycin and daptomycin.18
Rifampin is a potent anti-staphylococcal agent and acts by interacting specifically with the β subunit of the bacterial RNA polymerase encoded by the rpoB gene.19 Rifampin is indicated in combination therapy for implant-associated S. aureus infections and to eradicate asymptomatic carriage of MRSA.20–22 However, the emergence and spread of rifampin-resistant MRSA during vancomycin–rifampin combination therapy in an intensive care unit has been reported.23 In China, the frequency of the RIF-R MRSA isolates decreased from 2017 to 2020 reported by the China Antimicrobial Surveillance Network (CHINET): 16.2% (986/6084) of all MRSA clinical isolates in 2017, 12.2% (894/7327) of all MRSA clinical isolates in 2018, 11.5% (834/7251) of all MRSA clinical isolates in 2019, and 8.2% (588/7170) of all MRSA clinical isolates in 2020 (http://www.chinets.com).
Resistance to rifampin occurs through mutation in the rpoB gene that codes for the Beta subunit of RNA polymerase which inactivates the drug. Resistance to rifampin in M. tuberculosis is largely associated with mutations within an 81 bp RIF resistance determining region (RRDR) in the rpoB gene. In S. aureus, rifampin resistance is associated with mutations in particular regions (cluster I and cluster II) of the gene rpoB (462 to 488 and 515 to 530).24,25 Not all rpoB mutations have the same phenotypic consequences.
In this study, we aim to investigate the molecular profile and antimicrobial resistance associated with RIFR and RIFS MRSA isolates and analyze mutations in rpoB gene related to rifampin resistance in MRSA and epidemiology.
Materials and Methods
Bacterial Strains
From 2011 to 2020, a total of 565 non-duplicate MRSA isolates were collected from the seven regions (Inner Mongolia, Wuhan, Chengdu, Guangzhou, Shanghai, Nanchang, Wenzhou) in China. Our team performed whole-genome sequencing on 565 isolates of MRSA, of which 49 (8.7%) isolates were resistant to rifampicin, and 38 isolates of the remaining RIF-sensitive MRSA had mutations in rpoB gene, and 84 isolates were randomly selected from RIF-sensitive MRSA without rpoB mutations.
The clinical isolates were identified as S. aureus using Matrix-Assisted Laser Desorption/Ionization Time of Flight (MOLDI-TOF) by VITEK Mass Spectrometry. Escherichia coli ATCCC8739 was used as a control strain for the identification of bacteria. MRSA was determined based on the minimal inhibitory concentrations (MICs) of oxacillin and cefoxitin and confirmed by detecting the presence of mecA gene. The proportions of MRSA isolates isolated from various specimens were as follows: 34.5% (30/87), sputum; 43.7% (38/87), pus; 34.5% (30/87), blood. This study was approved by the research ethics board at Shanghai Pulmonary Hospital.
Whole-Genome Sequencing
All of S. aureus isolates were sequenced using the HisSeq 2500 sequencing platform (Illumina Inc., San Diego, CA), with 150 base pair paired-end reads. The data generated from the Illumina platform were analyzed after quality control was performed. De novo assembly of the genomes of all S. aureus isolates was performed using Spades v3.1426 and annotated using Prokka v1.12.27
Molecular Typing
Molecular typing was performed using multi-locus sequence typing (MLST) as previously described. Staphylococcal cassette chromosome mec (SCCmec) type and spa type were performed using the web-based SCCmecFinder (https://cge.cbs.dtu.dk/services/SCCmecFinder/) and web-based spaFinder (https://cge.cbs.dtu.dk/services/spatyper/), respectively.
Antibiotic Susceptibility Testing
Antimicrobial susceptibility testing of 18 antimicrobial agents including ciprofloxacin (CIP), clindamycin (CLI), tetracycline (TET), erythromycin (ERY), quinupristin–dalfopristin (QD), ceftaroline (CPT), rifampin (RIF), sulfamethoxazole/trimethoprim (SXT), gentamicin (GEN), daptomycin (DAP), mupirocin (MOP), teicoplanin (TCL), linezolid (LN), fusidic acid (FA), vancomycin (VAN), dalbavancin (DAL), and cefoxitin (FOX) was determined in accordance with the protocols recommended by the Clinical and Laboratory Standards Institute (CLSI). Susceptibility testing of MRSA isolates was performed routinely by the disk diffusion method on Mueller–Hinton agar plates to the following antibiotics: CIP (5 μg), CLI (2 μg), TET (30 μg), ERY (15 μg), QD (15 μg), and CPT (30 μg). MICs of RIF, SXT, GEN, DAP, MOP, TCL, LN, FA, VAN, DAL, and FOX were determined in all strains by microdilution following CLSI recommendations. S. aureus ATCC 29213 and ATCC 25923 were used as quality controls per the CLSI breakpoints.
Data Analysis and Statistical Methods
The statistical analyses were accomplished using SPSS software (SPSS, Chicago, IL, USA). Comparisons were made between RIFR and RIFS MRSA isolates using the chi-square test. P-value with <0.05 was considered statistical significance. The MIC distribution result was analyzed with Prism 8.0 software (GraphPad, San Diego, CA). The detailed information of MRSA isolates resistance to RIF was listed in the Supplementary Table 1 (Molecular characteristics and drug sensitivity results of MRSA (n=49) isolates resistance to RIF), and MRSA isolates sensitivity to RIF was listed in the Supplementary Table 2 (Molecular characteristics and drug sensitivity results of MRSA (n=38) isolates sensitivity to RIF).
Results
Rifampin Resistance Levels and Associated rpoB Mutations
The majority (n=40, 81.6%) of the 49 RIFR MRSA isolates, showed RIF MICs of 256 μg/mL. The MIC values of RIF for remaining isolates were as followed: >1024 μg/mL, 3; 1024 μg/mL, 1; 512 μg/mL, 3; 128 μg/mL, 1; 8 μg/mL, 1. The mutations in the rifampin resistance-determining region of rpoB gene are shown in Tables 1 and 2. The MIC distributions for RIF in relation to mutations in rpoB are shown in Figure 1. Forty-three (87.8%) isolates present the mutational change H481N and L466S, conferring 128–512 μg/mL RIF resistance. The four isolates with MIC ≥1024 μg/mL had additional amino acid substitution: H481N, L466S, A473T (n=2); H481Y (n=2), associated with a high-level RIF resistance. Of 38 RIFS MRSA isolates, two mutations were observed, including H481N (n=37) and A477D (n=1).
 |
Table 1 Molecular Characteristics of Main Clones Among MRSA (n=49) Isolates Resistance to RIF. |
 |
Table 2 Molecular Characteristics of Main Clones Among MRSA (n=38) 8 isolates Sensitivity to RIF. |
 |
Figure 1 Distribution of the MIC of rifampin for 87 MRSA in relation to mutations in rpoB. |
SCCmec Typing, MLST, and spa Typing
The evolution of MRSA isolates was analyzed by MLST (Tables 1 and 2). There were five distinct CCs (CC8, CC59, CC45, CC5, and CC398) identified within the 49 RIFR MRSA isolates (Table 1). ST239 (CC8) was the most predominant ST (44/49, 89.8%) in RIFR MRSA isolates, and was distributed in five cities. By spa typing, ST239 included spa types t030, t459, t037, t233, and t2270 in RIFR MRSA isolates. The most predominant spa type in ST239 RIFR MRSA isolates was t030 (35/49, 71.4%), followed by t459 (5/49, 10.2%). In addition, three SCCmec types were found in RIFR MRSA isolates: III, IV, and V. The most common type was type III, which was present in 43 (87.8%) RIFR MRSA isolates.
However, 10 STs that could be clustered into 7 CCs (CC45, CC5, CC8, CC9, CC1, CC59, and CC121) were identified in 39 RIFS MRSA isolates with mutations in rpoB gene (Table 2). ST45 (CC45) was the most common ST (22/38, 57.9%) in RIFS MRSA isolates with mutations in rpoB gene, followed by ST5 (5/38, 13.2%), and ST239 (5/38, 13.2%). spa type t116 was the most common type (16/22, 72.7%) in ST45 RIFS MRSA isolates with mutations in rpoB gene. SCCmec type IV was the most predominant type, present in 63.2% (24/38) of the RIFS MRSA isolates and five provinces, being most prevalent in Guangzhou (15/38, 39.5%).
Antimicrobial Susceptibility Profiles
As shown in Tables 3 and 4, the results of antibiotic susceptibility testing showed that all the isolates were susceptible to DAP, TCL, LNZ, VAN, and DLA. Of 49 RIFR MRSA isolates, 69.4% (34/49) with resistance to three or more classes of antimicrobial agents tested were identified as multidrug-resistant isolates. Excluding intermediate resistance, 71.4% of the RIFR MRSA isolates were resistant to ERY and 69.4% to CLI. Similarly, 78.9% of the RIFS MRSA isolates were resistant to ERY and CLI. The resistance rates of the 49 RIFR MRSA isolates to TET (77.6%), CIP (89.8%), and GEN (83.7%) were relatively high. However, the resistance rates of 38 RIFS MRSA isolates to TET, CIP, and GEN were 31.6%, 31.6%, and 23.7%, respectively, which were significantly lower than that of RIFR MRSA isolates. The resistance rates to other antibiotics (FA, MOP, SXT, and CPT) were relatively low. Among 84 RIFS MRSA without rpoB mutations isolates, except CIP (57.1%) and SXT (0%), the drug resistance rate of other agents was similar to that of RIFS MRSA with rpoB mutations isolates.
 |
Table 3 The MIC Distribution of rpoB Gene Mutations in Rifampicin-Resistant and -Sensitive MRSA Isolates in China. |
 |
Table 4 The MIC Distribution of Rifampicin-Sensitive MRSA Isolates in China. |
Resistance Genes
As shown in Table 5, resistance genes (gyrA, erm (A), tet (M), and aac(6ʹ)-Ie/aph(2”)-Ia) of RIFR MRSA were significantly higher than those of RIFS MRSA with rpoB mutations isolates.
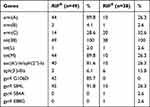 |
Table 5 Resistance Genes of Other Agents of rpoB Gene Mutations in Rifampicin-Resistant and -Sensitive MRSA Isolates. |
Discussion
MRSA is an increasing problem and HA-MRSA infections have been found worldwide. The growing number of antibiotic-resistant pathogens is increasingly threatening the efficacy of healthcare institutions worldwide. Antibiotic discovery needs to be re-energized, to rival the threat of the post-antibiotic era.28 Although a steady decrease in the prevalence of RIFR MRSA among Chinese hospitals within recent years has been already reported by the CHINET, and the relationship between RIF MICs and rpoB mutation of MRSA have been reported, there have been few reports, however, associating the decrease in the prevalence of RIFR MRSA with molecular characteristics.
ST239-III is the predominant clone among HA-MRSA strains in Asia, Middle East, Africa, New Zealand, and Australia.5 The major pandemic clones are usually related to specific geographical locations. The ST5-I/II clone in the USA, Canada, Mexico, and South America, ST36-II in Europe.5 Evidence suggests that the CC8-ST239 subgroup (ST239-III) lineage from South Korea, Hong Kong, Taiwan, and Vietnam and CC5(ST5-II) from South Korea and Sri Lanka have traveled from hospitals into the community.29 Belgium is the only location where ST239 has been detected in livestock so far.5 In China, ST239-III and ST5-II are both the major HA-MRSA clones.30 Similarly, 87.8% (43/49) RIFR MRSA ST239-III isolates were detected, while one ST5 MRSA isolate was detected in the present study. Li et al found ST239-t030 clone and ST239-t037 clone, which accounted for the large proportion of S. aureus, were on the wane and progressively replaced by ST59-t2460 in China.7 However, ST239-III-t030, the major clone in RIFR MRSA isolates, had a stronger survival advantage and could easily transmit in Chinese hospitals, which was in concordance with a previous study that reported that the MRSA isolates of the ST239-III-t030 clone were more resistant to RIF.30,31
Interestingly, ST45-IV-t116 MRSA was the predominant clone in RIFS MRSA isolates with rpoB mutation. CC45 is common in the United States (ST45-II) and Europe (ST45-IV/V).5 ST45-II is the hospital-associated clone and ST45-IV is community-associated clone.5 A previous study reported that a multicenter outbreak of ST45 MRSA containing deletions in the spa gene in New South Wales, Australia.32 Of 131 ST45 MRSA clinical isolates, 72 (54.9%) represented Australian Staphylococcal Sepsis Outcome Program bacteremia isolates.32 In the present study, 10 (10/22, 45.5%) isolates were isolated from blood. However, ST239 and ST5, the second predominant clones in RIFS MRSA isolates with rpoB mutation, were isolated from pus and sputum.
In general, RIFR MRSA isolates showed much higher resistance rates to all the tested antibiotics than RIFS MRSA. The antibiotic testing results of this research revealed that RIFR MRSA isolates were resistant to ERY, CIP, TET, GEN, and CLI. By contrast, RIFS MRSA isolates with rpoB mutation were more susceptible to CIP, TET, and GEN. The molecular characteristics of RIFR and RIFS MRSA with rpoB gene mutation were different, so the drug resistance profiles were also different.
Almost all MRSA isolates showed the mutational change H481N. It has previously been reported that the RpoB H481Y mutation can be associated with a remarkably persistent S. aureus infection.33 Forty-three (87.8%) isolates present the mutational change H481N and L466S, conferring 128–512 μg/mL RIF resistance. High-level rifampicin resistance could be attributable to double mutations within rpoB, as previously described.24 In addition, the single amino acid substitution H481Y also causes high-level resistance. In the present study, the two MRSA isolates with RIF MIC ≥ 1024 μg/mL had additional amino acid substitution: H481N, L466S, and A473T. Although H481N, L466S, and A473T have been described separately, they have not been detected in one clinical isolate. The two isolates with triple mutations, which belong to ST239-III-t037 clone, were from one region. Additionally, we also found two RIFR isolates revealing no mutations.
In conclusion, ST239-III-t030, the major clone in RIFR MRSA isolates; ST45-IV-t116, the major clone in RIFS MRSA isolates with rpoB mutations. RIFR MRSA isolates showed much higher resistance rates to all the tested antibiotics than RIFS MRSA. High-level rifampicin resistance was attributable to double mutations within rpoB.
Data Sharing Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
The protocols applied in this study were also approved by the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University School of Medicine Academy of Sciences, and informed consent was obtained from all patients whose specimens were used in scientific studies. This study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Science Foundation of China [81902122].
Disclosure
The authors have no financial or non-financial conflicts of interest for this work.
References
1. Gould IM. Costs of hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) and its control. Int J Antimicrob Agents. 2006;28:379–384. doi:10.1016/j.ijantimicag.2006.09.001
2. Gajdacs M, Urban E. Epidemiology and resistance trends of Staphylococcus aureus isolated from vaginal samples: a 10-year retrospective study in Hungary. Acta Dermatovenerol Alp Pannonica Adriat. 2019;28:143–147.
3. Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi:10.1056/NEJM199808203390806
4. Turner NA, Sharma-Kuinkel BK, Maskarinec SA, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17:203–218. doi:10.1038/s41579-018-0147-4
5. Lakhundi S, Zhang K. Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. 2018;31. doi:10.1128/CMR.00020-18.
6. Liu Y, Wang H, Du N, et al. Molecular evidence for spread of two major methicillin-resistant Staphylococcus aureus clones with a unique geographic distribution in Chinese hospitals. Antimicrob Agents Chemother. 2009;53:512–518. doi:10.1128/AAC.00804-08
7. Dai Y, Liu J, Guo W, et al. Decreasing methicillin-resistant Staphylococcus aureus (MRSA) infections is attributable to the disappearance of predominant MRSA ST239 clones, Shanghai, 2008–2017. Emerg Microbes Infect. 2019;8:471–478. doi:10.1080/22221751.2019.1595161
8. Mick V, Domínguez MA, Tubau F, et al. Molecular characterization of resistance to rifampicin in an emerging hospital-associated methicillin-resistant Staphylococcus aureus clone ST228, Spain. BMC Microbiol. 2010;10:68. doi:10.1186/1471-2180-10-68
9. Gajdacs M. The continuing threat of methicillin-resistant Staphylococcus aureus. Antibiotics (Basel). 2019;8. doi:10.3390/antibiotics8020052.
10. Koreen L, Ramaswamy SV, Graviss EA, et al. spa typing method for discriminating among Staphylococcus aureus isolates: implications for use of a single marker to detect genetic micro- and macrovariation. J Clin Microbiol. 2004;42:792–799. doi:10.1128/JCM.42.2.792-799.2004
11. Walsh C. Deconstructing vancomycin. Science. 1999;284:442–443. doi:10.1126/science.284.5413.442
12. Graziani AL, Lawson LA, Gibson GA, Steinberg MA, MacGregor RR. Vancomycin concentrations in infected and noninfected human bone. Antimicrob Agents Chemother. 1988;32:1320–1322. doi:10.1128/AAC.32.9.1320
13. Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2010;23:99–139. doi:10.1128/CMR.00042-09
14. Watanabe Y, Cui L, Katayama Y, Kozue K, Hiramatsu K. Impact of rpoB mutations on reduced vancomycin susceptibility in Staphylococcus aureus. J Clin Microbiol. 2011;49:2680–2684. doi:10.1128/JCM.02144-10
15. Cui L, Isii T, Fukuda M, et al. An rpoB mutation confers dual heteroresistance to daptomycin and vancomycin in Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:5222–5233. doi:10.1128/AAC.00437-10
16. Aiba Y, Katayama Y, Hishinuma T, et al. Mutation of RNA polymerase beta-subunit gene promotes heterogeneous-to-homogeneous conversion of beta-lactam resistance in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57:4861–4871. doi:10.1128/AAC.00720-13
17. Matsuo M, Hishinuma T, Katayama Y, et al. Mutation of RNA polymerase β subunit (rpoB) promotes hVISA-to-VISA phenotypic conversion of strain Mu3. Antimicrob Agents Chemother. 2011;55:4188–4195. doi:10.1128/AAC.00398-11
18. Guerillot R, Gonçalves da Silva A, Monk I, et al. Convergent evolution driven by rifampin exacerbates the global burden of drug-resistant Staphylococcus aureus. mSphere. 2018;3. doi:10.1128/mSphere.00550-17
19. Aboshkiwa M, Rowland G, Coleman G. Nucleotide sequence of the Staphylococcus aureus RNA polymerase rpoB gene and comparison of its predicted amino acid sequence with those of other bacteria. Biochim Biophys Acta. 1995;1262:73–78. doi:10.1016/0167-4781(95)00054-k
20. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537–1541. doi:10.1001/jama.279.19.1537
21. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–e25. doi:10.1093/cid/cis803
22. Senobar Tahaei SA, Stájer A, Barrak I, et al. Correlation between biofilm-formation and the antibiotic resistant phenotype in Staphylococcus aureus isolates: a laboratory-based study in Hungary and a review of the literature. Infect Drug Resist. 2021;14:1155–1168. doi:10.2147/IDR.S303992
23. Ju O, Woolley M, Gordon D. Emergence and spread of rifampicin-resistant, methicillin-resistant Staphylococcus aureus during vancomycin-rifampicin combination therapy in an intensive care unit. Eur J Clin Microbiol Infect Dis. 2006;25:61–62. doi:10.1007/s10096-005-0063-1
24. Aubry-Damon H, Soussy CJ, Courvalin P. Characterization of mutations in the rpoB gene that confer rifampin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:2590–2594. doi:10.1128/AAC.42.10.2590
25. Wichelhaus TA, Schafer V, Brade V, Boddinghaus B. Molecular characterization of rpoB mutations conferring cross-resistance to rifamycins on methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:2813–2816. doi:10.1128/AAC.43.11.2813
26. Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi:10.1089/cmb.2012.0021
27. Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi:10.1093/bioinformatics/btu153
28. Gajdacs M. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24:892. doi:10.3390/molecules24050892
29. Song JH, Hsueh PR, Chung DR, et al. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J Antimicrob Chemother. 2011;66:1061–1069. doi:10.1093/jac/dkr024
30. Cheng H, Yuan W, Zeng F, et al. Molecular and phenotypic evidence for the spread of three major methicillin-resistant Staphylococcus aureus clones associated with two characteristic antimicrobial resistance profiles in China. J Antimicrob Chemother. 2013;68:2453–2457. doi:10.1093/jac/dkt213
31. Chen H, Liu Y, Jiang X, Chen M, Wang H. Rapid change of methicillin-resistant Staphylococcus aureus clones in a Chinese tertiary care hospital over a 15-year period. Antimicrob Agents Chemother. 2010;54:1842–1847. doi:10.1128/AAC.01563-09
32. Beukers AG, Newton P, Hudson B, et al. A multicentre outbreak of ST45 MRSA containing deletions in the spa gene in New South Wales, Australia. J Antimicrob Chemother. 2020;75:1112–1116. doi:10.1093/jac/dkz560
33. Gao W, Cameron DR, Davies JK, et al. The rpoB H(4)(8)(1)Y rifampicin resistance mutation and an active stringent response reduce virulence and increase resistance to innate immune responses in Staphylococcus aureus. J Infect Dis. 2013;207:929–939. doi:10.1093/infdis/jis772
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
