Back to Journals » Infection and Drug Resistance » Volume 14
Molecular Characteristics of Escherichia coli Causing Bloodstream Infections During 2010–2015 in a Tertiary Hospital, Shanghai, China
Authors Li D, Li P, Yu X, Zhang X, Guo Q, Xu X, Wang M , Wang M
Received 10 February 2021
Accepted for publication 14 April 2021
Published 3 June 2021 Volume 2021:14 Pages 2079—2086
DOI https://doi.org/10.2147/IDR.S305281
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Dan Li, 1, 2,* Pei Li, 1, 2,* Xiaoyan Yu, 3,* Xuefei Zhang, 1, 2 Qinglan Guo, 1, 2 Xiaogang Xu, 1, 2 Minggui Wang, 1, 2 Minghua Wang 1, 2
1Institute of Antibiotics, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; 2Key Laboratory of Clinical Pharmacology of Antibiotics, Ministry of Health, Shanghai, People’s Republic of China; 3Department of Critical Care Medicine, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Minghua Wang
Institute of Antibiotics, Huashan Hospital, Fudan University, No. 12 Middle Wulumuqi Road, Shanghai, 200040, People’s Republic of China
Tel +86 021 52888193
Email [email protected]
Background: The bloodstream infections (BSI) caused by Escherichia coli pose a serious threat to human health. To explore molecular characteristics of E. coli causing BSI, we collected E. coli isolates causing BSI in Huashan Hospital, Shanghai, China during 2010– 2015.
Methods: In all E. coli isolates causing BSI collected from this study, polymerase chain reaction (PCR) was used to detect ESBLs and carbapenemase genes, and minimum inhibitory concentrations (MICs) were determined with agar dilution method. Outer membrane proteins were examined by SDS-PAGE in carbapenem-resistant strains. The genetic background of blaKPC gene was investigated by combining next-generation sequencing with a PCR mapping approach. Conjugation and transformation experiments were performed to verify the mobilization of blaKPC. The transcription levels of the blaKPC gene were measured by RT-PCR.
Results: During 2010– 2015, a total of 207 E. coli BSI strains were isolated. The positive rates of β-lactamase resistant genes were 0.48% (blaKPC), 57% (blaTEM), 23.67% (blaCTX-M-1), 18.84% (blaCTX-M-9), and 1.93% (blaSHV). High rates of blaTEM, blaCTX-M-1, and blaCTX-M-9 were consistent with the poor activity of third-generation cephalosporins and aztreonam in vitro, except for carbapenem and β-lactamase inhibitor combinations. Low susceptibility rates were observed for piperacillin (25.1%) in contrast to the increased susceptibility when combined with β-lactamase inhibitors, namely piperacillin-tazobactam (90.8%). Only one KPC-producing E. coli strain was detected. Despite the combination of OmpC loss, the low expression level of KPC may be responsible for its lower resistance to carbapenems compared to E. coli DH5α (pKP12-100).
Conclusion: E. coli strains isolated from BSI were still highly susceptible to carbapenems and β-lactamase inhibitor combinations, and blaCTX-M was the dominant genotype of ESBLs. The low expression of blaKPC may be the reason for the low resistance to carbapenems.
Keywords: Escherichia coli, bloodstream infections, resistance mechanism, ESBLs
Introduction
Escherichia coli, a gram-negative, motile, facultative anaerobic, rod-shaped bacterium, is one of the most common hospital-acquired pathogens which could cause urinary tract infections, abdominal infections, bloodstream infections (BSI), etc.1 Bacteremia represents a major cause of death with large increases in incidence and mortality.2 E. coli is a leading cause of bloodstream infection, it ranks first as a cause of community-acquired episodes and second as a cause of hospital-acquired BSI in different world regions.3 In addition, the incidence of E. coli BSI is increasing with associated high morbidity and mortality.4 In a study from England, all-cause mortality rate in individuals with E. coli bacteremia was 18.2%.5 And in China, one study showed that in 45 episodes of E. coli bacteremia, the 30-day all-cause mortality was 22.2%.6
β-lactams are commonly used in the treatment of BSI caused by E. coli. β-lactamase production remains the most important contributing factor to β-lactam resistance.7 Extended-spectrum β lactamases (ESBLs), one group of β lactamases, have the ability to hydrolyze and cause resistance to various types of the β-lactam antibiotics, including the third-generation cephalosporins and monobactams except the cephamycins and carbapenems.8,9 The most common ESBLs belong to three groups: TEM, SHV, and CTX-M types.8 The CTX-M β lactamases, now exceeding 50 different types, can be divided into five groups based on their amino acid identities: CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9, and CTX-M-25.10 Since their first description, class A extended-spectrum β-lactamases (ESBLs) producing E. coli continue to thwart our best clinical efforts. ESBLs-producing E. coli remains an important reason for therapy failure with cephalosporins and have serious consequences for infection control.7
Based on data from a multicenter randomized controlled trial, carbapenem is recommended as first-line treatment for infections outside of the urinary tract caused by ESBLs-producing E. coli.11 In E. coli, carbapenem resistance is typically caused by two main mechanisms: production of carbapenemases and β-lactamase activity combined with structural mutations.12 When combined with the mutation of outer membrane proteins or drug efflux pumps, ESBLs and AmpC are capable of conferring carbapenem resistance.12 According to the Ambler classification method, carbapenemases are members of the molecular class A, B, and D beta-lactamases. Class A and D enzymes have a serine-based hydrolytic mechanism, while class B enzymes are metallo-beta-lactamases that contain zinc in the active site.13,14 Of these, the KPC carbapenemases are the most prevalent, found mostly on plasmids in Klebsiella pneumoniae.15
Given the increasing importance and the fact that BSI caused by ESBLs-producing E. coli are an increasing therapeutic challenge, we investigated the molecular characteristics and antimicrobial susceptibility profiles of BSI caused by E. coli during 2010–2015 in Huashan Hospital, Shanghai, China.
Materials and Methods
Sources of Strains
A total of 207 non-duplicate E. coli isolates were collected from blood cultures of the inpatients of Huashan Hospital, Fudan University from 2010 to 2015. E. coli was identified using the Vitek 2 system. E. coli A49, selected from 207 strains mentioned previously, was used as the positive reference for outer membrane proteins with complete OmpC, OmpF, and OmpA. Plasmid pKP12-100 (Supplementary Data 1) was extracted from KPC-producing K. pneumoniae KP100-12 isolated from Huashan Hospital, not belonging to the 207 strains mentioned previously. This plasmid was used for transformation.
MIC Determination
In 207 E. coli strains in this study (Supplementary Data 2), minimum inhibitory concentrations (MICs) of cefotaxime, cefepime, ceftazidime, cefoxitin, ampicillin, aztreonam, piperacillin, piperacillin-tazobactam, meropenem, imipenem, ertapenem, fosfomycin, ciprofloxacin, amikacin, and gentamicin were determined with agar dilution method according to Clinical and Laboratory Standards Institute (CLSI) guidelines. Escherichia coli ATCC25922 and Pseudomonas aeruginosa ATCC27853 were used as routine controls for agents mentioned previously. The criterion of the susceptibility of fosfomycin was based on EUCAST (European Committee on Antimicrobial Susceptibility Testing) (Resistance standard: MIC ≥ 32μg/mL). We investigated the effect of efflux pump inhibitors cyanide 3-chlorophenylhydrazone (CCCP) on the carbapenems’ susceptibility in the carbapenem-resistant E. coli. The concentration of CCCP was 25μg/mL.
Detection of Resistance Genes
Polymerase chain reaction (PCR) was used to detect β-lactmase such as blaTEM, blaCTX-M-1, blaCTX-M-9, blaCTX-M-2, blaCTX-M-8, blaCTX-M-25, blaSHV and the carbapenemases genes such as blaKPC, blaNDM, blaIMP, blaSPM, blaAIM, blaVIM, blaOXA, blaGIM, blaBIC, blaSIM, blaDIM.16 And in our laboratory, we possessed isolates which were used as positive controls for the following genes: blaKPC, blaNDM, blaIMP, blaOXA, blaCTX-M-1, blaCTX-M-9, blaSHV and blaTEM. Amplification was carried out as follows: initial denaturation at 94°C for 5 min; 30 cycles of 94°C for 30s, 60°C for 30s and 72°C for 1 min; and a final elongation step at 72°C for 7 min. Primers were listed in Supplementary Data 3.
Analysis of Outer Membrane Proteins (OMPs) of KPC-Producing Strain
Briefly, the suspension was sonicated on ice for about 10 minutes (60 cycles for 5 seconds with 5-second intervals). The cell extracts were centrifuged at 15,600 g 4°C for 60 minutes, then we removed the supernatant and added 200 μL 1×PBS, 25 μL10% Sarcosyl to resolve the protein. We repeated the procedure and suspended the OMP with 80 μL 1×PBS. Porins were loaded onto 15% SDS-polyacrylamide gel. After a 150-min electrophoresis of 80 V, the membrane was stained with 0.1% Coomassie brilliant blue (Beyotime, China).17
Conjugation and Transformation Experiments
Conjugation and transformation experiments were performed to verify the transferability of blaKPC. Plasmid pKP12-100 was extracted from a blaKPC-positive isolate through phenol-chloroform method and then transformed into the recipient strain E. coli DH5α. E. coli J53, an azide-resistant strain was used for conjugation experiments.18 Agar plate containing ampicillin (50μg/mL) was used to screen for transformants. Conjugation strain was selected on LB agar plates supplemented with 50μg/mL ampicillin and 150μg/mL sodium azide. PCR with primers Kpc-RT (listed in Table 1) and sequencing were used to verify transformants and conjugation strain.
 |
Table 1 Primers Presented Below Were Used for RT-qPCR of blaKPC Gene in This Study |
Genetic Environment of blaKPC Positive Strain
DNA was extracted from EC-A59 (TIANamp Bacteria DNA Kit) and next-generation sequencing was performed (Supplementary Data 4). Flanking sequences of blaKPC were extracted from the contig harboring blaKPC and analyzed by Blastn. The genetic background of blaKPC gene in E. coli A59 obtained from this study was investigated by combining next-generation sequencing with a PCR mapping approach with the primers listed in Table 1. We obtained the genetic background of blaKPC gene in E. coli A59 for further visualized genetic environment comparisons of blaKPC-positive strains with Easyfig.
Reverse Transcription-Quantitative PCR
Total RNAs from clinical isolates were extracted using TaKaRa MiniBEST Universal RNA Extraction Kit and cDNA synthesis was performed with PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa). The transcription levels of the blaKPC gene were measured with FastStart Universal SYBR Green Master (ROX)(Roche) as recommended by the manufacturers. The mdh housekeeping gene was used as the internal reference. Primer sequences are listed in Table 1.
Results
Molecular Characteristics of 207 E. coli Isolates Causing BSI
During 2010 to 2015, a total of 207 E. coli isolates causing BSI were collected. The overall E. coli isolates causing BSI is on the rise, especially in 2015 (Figure 1). The antibiotic resistance rates were listed as follows: 67.1% (cefotaxime), 36.2% (cefepime), 42.5% (ceftazidime), 24.2% (cefoxitin), 87.4% (ampicillin), 45.9% (aztreonam), 59.9% (piperacillin), 5.8% (piperacillin-tazobactam), 0% (meropenem) and 0% (imipenem), 5.3% (ertapenem), 19.8% (fosfomycin), 76.8% (ciprofloxacin), 9.2% (amikacin), 61.4% (gentamicin) (Table 2). Low susceptibility rates were observed for piperacillin (25.1%) in contrast to increased susceptibility when combined with β-lactamase inhibitors, namely piperacillin-tazobactam (90.8%).
 |
Table 2 Antimicrobial Susceptibility of Escherichia coli |
 |
Figure 1 The isolation numbers of Escherichia coli causing bloodstream infections according to the year. |
The positive rates of β-lactamase resistant genes were 0.48% (blaKPC), 57% (blaTEM), 23.67% (blaCTX-M-1), 18.84% (blaCTX-M-9), 1.93% (blaSHV). The rates of blaESBLs by year were shown in Figure 2. The most common blaESBLs was blaCTX-M-1, followed by blaCTX-M-9. Only one E. coli strain A59 was discovered harboring blaKPC-2 gene. Genes blaCTX-M-2, blaCTX-M-8, blaCTX-M-25 and blaNDM, blaIMP, blaSPM, blaAIM, blaVIM, blaOXA, blaGIM, blaBIC, blaSIM, blaDIM were not detected in this study.
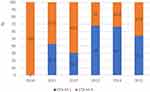 |
Figure 2 The distribution of ESBLs genes in ESBLs-producing Escherichia coli. |
OMP Profile of blaKPC-Positive Strain
SDS-PAGE analysis revealed different OMP profiles among the two isolates (Figure 3). OmpC loss was observed in isolate E. coli A59 compared to E. coli A49.
Conjugation and Transformation Experiments of the blaKPC Positive Strain
The blaKPC-2-carrying plasmid named pKP12-100 was extracted from K. pneumoniae 12–100 and transformed into E. coli DH5α. The MIC of the transformant E. coli DH5α (pKP12-100) can be seen in Table 3. Conjugation and transformation of E. coli A59 were failed with at least three repeats.
Carbapenems’ Susceptibility and Efflux Pump Inhibition Test of blaKPC Positive Strains
The MICs of KP12-100 to imipenem, meropenem, and ertapenem were 128μg/mL, 128μg/mL and ≥256μg/mL respectively while the transformant of KP12-100 was 8μg/mL, 4μg/mL and 128μg/mL. The MICs of E. coli A59 to imipenem, meropenem, and ertapenem were 2μg/mL, 1μg/mL and 16μg/mL respectively. Efflux pump inhibitor carbonyl cyanide 3-chlorophenyl-hydrazone resulted in at least 8-fold decrease in the MIC of imipenem, meropenem and ertapenem for E. coli DH5α (pKP12-100). And an 8-fold decrease in the MIC of ertapenem was observed in DH5α-P12-100 (Table 3).
Genetic Environment of blaKPC-Positive Strain
Combining next-generation sequencing with a PCR mapping approach, genetic environment of blaKPC in E. coli A59 was ISKpn6-blaKPC-2-ISKpn27-IS26 sharing the same core structure as that from the chromosome of ECO3385 (CP029420.1). Since blaKPC-2 was not able to be transferred to the recipient we supposed that the blaKPC in E. coli A59 may be located on the chromosome (Figure 4).
 |
Figure 4 Comparisons between the structures of ECO3385 and EC-A59. ISKpn6-blaKPC-2-ISKpn27-IS26 is shown in green. |
RT-qPCR of blaKPC Gene
Compared with the imipenem resistant control strain, E. coli DH5α (pKP12-100), the transcription levels of blaKPC gene were five-fold lower for isolate E. coli A59 (Figure 5). At least 280-bp sequence upstream of the blaKPCs in the two strain was identical, see Supplementary Data 5, indicating that they shared the same promoter region of blaKPC.
Discussion
Data from the CHINET Antimicrobial Surveillance Program showed that the proportion of E. coli in BSI pathogens is 22.2%, which is a leading cause of BSI in China. The antimicrobial resistance rates of E. coli isolated from 35 hospitals in 2017 throughout China were as follows: 25.2% (cefepime), 25.2% (ceftazidime), 12.2% (cefoxitin), 86.5% (ampicillin), 4.1% (piperacillin-tazobactam), 1.5% (meropenem) and 1.5% (imipenem), 2% (ertapenem), 5.2% (fosfomycin), 57.8% (ciprofloxacin), 2.3% (amikacin) (http://www.chinets.com/). In our research, the antimicrobial resistance rates were almost consistent with these data. Although all isolates were susceptible to meropenem and imipenem, there were 11 E. coli isolates resistant to ertapenem. According to the existing research, the expression of β-lactamases such as an AmpC β-lactamase or an ESBL combined with porin loss participated in ertapenem resistance in Enterobacteriaceae isolates.19
ESBLs are often encoded by plasmids that are transferable from strain to strain and between bacterial species.20,21
In our study, blaCTX-M was the dominant genotype among the ESBLs-producing E. coli which is consistent with the situation in China.22 The occurrence of ESBLs is increasing.8 Data from rural Thailand showed very high rates, reaching 69.3% in 2010. The great majority of CTX-M alleles identified in Thailand belonged to group 9.23
Carbapenem-resistant E. coli is posing great challenges to human health.12,24 The plasmid-mediated horizontal transmission of carbapenemase genes is the main cause of the surge in the prevalence of CRE. NDM, one of the metallo-β-lactamases, is the predominant carbapenemase in E. coli while KPC carbapenemases are the most prevalent ones among class A carbapenemase group and found mostly on plasmids in K. pneumoniae.15,25 K. pneumoniae are the predominant carriers of blaKPC, mainly associated with the clonal group 258 (CG258) including ST258, ST11, ST340, ST512, and others.26,27 One study indicated that type I-E CRISPR-Cas system targeting the backbone regions of blaKPC-bearing IncF plasmids influences the acquisition of blaKPC plasmid in K. pneumoniae. The absence of type I-E CRISPR-Cas in CG258 contributes to the dissemination of IncF epidemic resistance plasmids in this clonal complex.28 Until now, reports about KPC-producing E. coli have been rare and the low detection rate of blaKPC in E. coli remains obscure.
From the results of the national surveillance of CRE strains in China, it was shown that the core structure of ISKpn6-blaKPC-2-ISKpn27 was conservative in KPC-producing K. pneumoniae and E. coli strains.29 In this study only the chromosomes of ECO3385 (CP029420.1) and E. coli A59 shared the same core structure. In addition to the conservative sequences, they still hold another transposable element IS26, and this kind of structure was a little bit different from the previously reported pK048 (IncFIIK5) harboring non-Tn4401 elements in China.30 Whether certain divergences between K. pneumoniae and E. coli resulted in the intergeneric diversity of transposable genetic elements should be taken into consideration.
Previous research showed that KPC enzymes contribute to the carbapenem resistance in K. pneumoniae.26 Compared with the KP12-100, the MIC of carbapenem for its transformation strain E. coli DH5α (pK12-100) had decreased at least 16-fold, which indicated the existence of other resistance mechanisms. E. coli A59 was a blaKPC positive strain with OmpC loss which was not resistant to meropenem and imipenem. Considering the low expression level and the failed conjugation and transformation experiments, we propose that the decreased MIC of meropenem and imipenem may be due to the low expression level of blaKPC located on the chromosome of E. coli A59. Only one KPC-producing E. coli was detected in this study. More strains will be included to clarify the overall detection rate of blaKPC in E. coli in the future.
Ethical Statement
The strains we used in this study were obtained from the biological sample and strains bank of the Institute of Antibiotics, Huashan Hospital, Shanghai, China. They came from normal clinical testing and were stored in the strains bank. The ethics committee of Huashan Hospital authorized our study and written informed consent was not required. This study would not do harm to rights, benefits, and health of the subjects, and the privacy and personal identity information of the subjects will not be included in this study.
Acknowledgments
We thank Leilei Wang and Li Ding at Institute of Antibiotics for assistance in laboratory work.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81402976 and 81673479) and Science and Technology Commission of Shanghai Municipality (grant number 18411950601).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vila J, Sáez-López E, Johnson JR, et al. Escherichia coli: an old friend with new tidings. FEMS Microbiol Rev. 2016;40(4):437–463. doi:10.1093/femsre/fuw005
2. Kern WV, Rieg S. Burden of bacterial bloodstream infection-a brief update on epidemiology and significance of multidrug-resistant pathogens. Clin Microbiol Infect. 2020;26(2):151–157. doi:10.1016/j.cmi.2019.10.031
3. Berger J, Diab-Elschahawi M, Blacky A, et al. A matched prospective cohort study on Staphylococcus aureus and Escherichia coli bloodstream infections: extended perspectives beyond resistance. Am J Infect Control. 2010;38(10):839–845. doi:10.1016/j.ajic.2010.04.212
4. Bou-Antoun S, Davies J, Guy R, et al. Descriptive epidemiology of Escherichia coli bacteraemia in England, April 2012 to March 2014. Euro Surveill. 2016;21(35). doi:10.2807/1560-7917.ES.2016.21.35.30329
5. Abernethy JK, Johnson AP, Guy R, et al. Thirty day all-cause mortality in patients with Escherichia coli bacteraemia in England. Clin Microbiol Infect. 2015;21(3):
6. Wang H, Liu J, Huang Z, et al. Clinical characteristics and risk factors for shock and death from E. coli bacteremia in pediatric hematological patients. J Infect Dev Ctries. 2019;13(5):365–373. doi:10.3855/jidc.11099
7. Bush K, Bradford PA. Epidemiology of β-lactamase-producing pathogens. Clin Microbiol Rev. 2020;33(2).
8. Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657–686. doi:10.1128/CMR.18.4.657-686.2005
9. Peirano G, Pitout J. Extended-spectrum β-lactamase-producing Enterobacteriaceae: update on molecular epidemiology and treatment options. Drugs. 2019;79(14):1529–1541. doi:10.1007/s40265-019-01180-3
10. Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159–166. doi:10.1016/S1473-3099(08)70041-0
11. Tamma PD, Aitken SL, Bonomo RA, et al. Infectious diseases society of America antimicrobial resistant treatment guidance: gram-negative bacterial infections. Clin Infect Dis. 2020.
12. Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med. 2012;18(5):263–272. doi:10.1016/j.molmed.2012.03.003
13. Potter RF, D’Souza AW, Dantas G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist Updat. 2016;29:30–46. doi:10.1016/j.drup.2016.09.002
14. Queenan AM, Bush K. Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev. 2007;20(3):440–458. doi:10.1128/CMR.00001-07
15. Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–796. doi:10.1016/S1473-3099(13)70190-7
16. Dallenne C, Da Costa A, Decré D, et al. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65(3):490–495. doi:10.1093/jac/dkp498
17. Portnoy DA, Wolf-Watz H, Bolin I, et al. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984;43(1):108–114. doi:10.1128/IAI.43.1.108-114.1984
18. Yi H, Cho Y-J, Yong D, et al. Genome sequence of Escherichia coli J53, a reference strain for genetic studies. J Bacteriol. 2012;194(14):3742–3743. doi:10.1128/JB.00641-12
19. Chung HS, Yong D, Lee M. Mechanisms of ertapenem resistance in Enterobacteriaceae isolates in a tertiary university hospital. J Investig Med. 2016;64(5):1042–1049. doi:10.1136/jim-2016-000117
20. Rupp ME, Fey PD. Extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae: considerations for diagnosis, prevention and drug treatment. Drugs. 2003;63(4):353–365. doi:10.2165/00003495-200363040-00002
21. Bevan ER, Jones AM, Hawkey PM. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother. 2017;72(8):2145–2155. doi:10.1093/jac/dkx146
22. Hawkey PM. Prevalence and clonality of extended-spectrum β-lactamases in Asia. Clin Microbiol Infect. 2008;14(Suppl 1):159–165. doi:10.1111/j.1469-0691.2007.01855.x
23. Woerther PL, Burdet C, Chachaty E, et al. Trends in human fecal carriage of extended-spectrum β-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev. 2013;26(4):744–758. doi:10.1128/CMR.00023-13
24. Bonomo RA, Burd EM, Conly J, et al. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis. 2018;66(8):1290–1297. doi:10.1093/cid/cix893
25. Wu W, Feng Y, Tang G, et al. NDM metallo-β-lactamases and their bacterial producers in health care settings. Clin Microbiol Rev. 2019;32(2).
26. Chen L, Mathema B, Chavda KD, et al. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol. 2014;22(12):686–696. doi:10.1016/j.tim.2014.09.003
27. Chen L, Mathema B, Pitout JDD, et al. Epidemic Klebsiella pneumoniae ST258 is a hybrid strain. mBio. 2014;5(3):e01355–14. doi:10.1128/mBio.01355-14
28. Tang Y, Fu P, Zhou Y, et al. Absence of the type I-E CRISPR-Cas system in Klebsiella pneumoniae clonal complex 258 is associated with dissemination of IncF epidemic resistance plasmids in this clonal complex. J Antimicrob Chemother. 2020;75(4):890–895. doi:10.1093/jac/dkz538
29. Zhang R, Liu L, Zhou H, et al. Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBioMedicine. 2017;19:98–106. doi:10.1016/j.ebiom.2017.04.032
30. Liu P, Li P, Jiang X, et al. Complete genome sequence of Klebsiella pneumoniae subsp. pneumoniae HS11286, a multidrug-resistant strain isolated from human sputum. J Bacteriol. 2012;194(7):1841–1842. doi:10.1128/JB.00043-12
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



