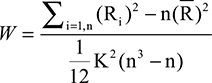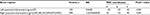Back to Journals » Journal of Pain Research » Volume 11
Modulation of prefrontal connectivity in postherpetic neuralgia patients with chronic pain: a resting-state functional magnetic resonance-imaging study
Authors Li J, Huang X, Sang K, Bodner M , Ma K, Dong XW
Received 26 February 2018
Accepted for publication 24 July 2018
Published 2 October 2018 Volume 2018:11 Pages 2131—2144
DOI https://doi.org/10.2147/JPR.S166571
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Jun Li,1,* Xuehua Huang,2,* Kangning Sang,1 Mark Bodner,3 Ke Ma,2 Xiao-Wei Dong1,4
1Key Laboratory of Brain Functional Genomics (MOE and STCSM), Shanghai Changning ECNU Mental Health Center, Institute of Cognitive Neuroscience, School of Psychology and Cognitive Science, East China Normal University, Shanghai, China; 2Department of Pain Management, Xin Hua Hospital, affiliated with Shanghai Jiao Tong University School of Medicine, Shanghai, China; 3MIND Research Institute, Irvine, CA, USA; 4NYU-ECNU Institute of Brain and Cognitive Science, NYU Shanghai, Shanghai, China
*These authors contributed equally to this work
Background: Although the interaction between pain and cognition has been recognized for decades, the neural substrates underlying their association remain unclear. The prefrontal cortex (PFC) is known as a critical brain area for higher cognitive functions, as well as for pain perception and modulation. The objective of the present study was to explore the role of the PFC in the interaction between chronic pain and cognitive functions by examining the relationship between spontaneous activity in the frontal lobe and pain intensity reported by postherpetic neuralgia (PHN) patients.
Methods: Resting-state functional magnetic resonance imaging data from 16 PHN patients were collected, and regional homogeneity and related functional connectivity were analyzed.
Results: The results showed negative correlations between patients’ pain scores and regional homogeneity values in several prefrontal areas, including the left lateral PFC, left medial PFC, and right lateral orbitofrontal cortex (P<0.05, AlphaSim-corrected). Further analysis revealed that the functional connectivity of some of these prefrontal areas with other cortical regions was also modulated by pain intensity. Therefore, functional connections of the left lateral PFC with both the left parietal cortex and the left occipital cortex were correlated with patients’ pain ratings (P<0.05, AlphaSim-corrected). Similarly, functional connectivity between the right lateral orbitofrontal cortex and bilateral postcentral/precentral gyri was also correlated with pain intensity in the patients (P<0.05, AlphaSim-corrected).
Conclusion: Our findings indicate that activity in the PFC is modulated by chronic pain in PHN patients. The pain-related modulation of prefrontal activity may serve as the neural basis for interactions between chronic pain and cognitive functions, which may link to cognitive impairments observed in chronic pain patients.
Keywords: postherpetic neuralgia, chronic pain, prefrontal cortex, fMRI, regional homogeneity, functional connectivity
Introduction
Patients with chronic pain often show concomitant deficits in attention, memory, and executive functions.1,2 Postherpetic neuralgia (PHN), a chronic neuropathic pain persisting for more than 3 months following herpes zoster infection,3 has been found to affect cognitive abilities of PHN patients, including attention deficits, memory decline, and decision-making impairment.4 However, the neural mechanism underlying the interaction between chronic pain and cognitive functions is largely unknown. There have been very few studies examining how activities in brain areas associated with cognitive functions are modulated by clinical pain.
The prefrontal cortex (PFC) has been regarded as one of the most important brain structures for higher cognitive functions, such as emotional regulation, working memory, decision-making, motor planning, and executive functions.5–8 It is also a critical area in the modulation of chronic pain.9–13 Moreover, studies have indicated that the PFC undergoes both structural and functional changes under chronic pain conditions in both human patients14–18 and animal pain models.19,20 These findings suggest a potential role for the PFC in the interaction between chronic pain and cognitive function.
A number of studies have compared differences in dynamic changes in brain activity between PHN patients and healthy control subjects. It was found that there were changes in functional connectivity between the subregions within the PFC and between the PFC and other cortical areas in PHN patients.21,22 In addition, PHN patients exhibited abnormal regional homogeneity (ReHo), an indicator believed to reflect regional synchronization of spontaneous brain activity,23 in a wide range of cortical and subcortical areas.24 All these findings indicate that both local circuit activity within the PFC and communication between the PFC and other cortical areas are altered in PHN patients. However, much remains to be elucidated about a direct link between these changes in the PFC and PHN pain, as well as the association of the PFC and its network with impairment in cognitive function in PHN patients.
To explore the role of the PFC in the interaction between chronic pain and cognitive function, we first examined whether prefrontal activity was modulated by chronic pain by analyzing the relationship between its local synchronization, measured by ReHo,23 and pain intensity in PHN patients. We then investigated whether pain modulated the functional relationship between the PFC and other brain areas in related networks by analyzing the correlation between functional connectivity25 and pain intensity.
Methods
All procedures used in the present study were approved by the University Committee on Human Research Protection, East China Normal University (approval HR2013/10004). PHN patients were recruited via advertisements and were fully instructed regarding experimental procedures. All patients gave written informed consent in accordance with the Declaration of Helsinki.
PHN patient recruitment
Right-handed patients diagnosed with PHN by two physicians (XH and KM) were recruited at Xin Hua Hospital in Shanghai, China. Patients who reported a history of shingles and associated pain and had had PHN symptoms >3 months were included in the study. Patients of age <50 or >75, with psychiatric diseases, with claustrophobia, current users of opioids, or with metal or electronic implants were excluded. Patients were using only nonsteroidal anti-inflammatory (eg, celecoxib) and/or peripheral neurotrophic drugs when they were recruited.
Pain assessment in PHN patients
To minimize the potential acute analgesic effect of the nonsteroidal anti-inflammatory drug that the PHN patients had taken, patients were asked not to take any medicine for at least 24 hours prior to the experiment, a washout period that allowed about 90% elimination of plasma celecoxib26 but avoided prolonged halt of medicine, as adopted in some other studies with chronic pain patients.27–29 PHN patients were asked to report their perceived spontaneous pain on a commonly used pain numeric rating scale30 of 0–10 (0 for no pain and 10 for worst imaginable pain) right before the functional magnetic resonance imaging (fMRI) scan (Table 1). Pain score over the last 7 days prior to the fMRI experiment was also assessed in the patients.
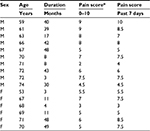  | Table 1 Demographics of PHN patients Note: *Numeric rating scale of 0–10 (0 for no pain, 10 for worst imaginable pain). Abbreviations: PHN, postherpetic neuralgia; F, female; M, male. |
Imaging data acquisition
Imaging was performed on a 3 T Trio scanner (Siemens, Munich, Germany) using an eight-channel birdcage head coil. Each patient lay supine with their head snugly fixed by foam pads. The patient was asked to keep still as long as possible and to keep his/her eyes closed but remain awake. Resting-state fMRIs were obtained using an echo-planar imaging sequence with protocols of 30 axial slices, thickness/gap 4.0/0.8 mm, in-plane resolution 64×64, TR 2000 ms, TE 40 ms, flip angle 90°, FOV 220×220 mm, 240 volumes acquired in 8 minutes.
Imaging data preprocessing
Preprocessing of resting-state fMRI data was conducted using Data Processing Assistant for Resting-State fMRI (DPARSF; http://rfmri.org/dparsf) software (version 2.1) based on statistical parametric mapping (SPM8; http://www.fil.ion.ucl.ac.uk/spm) on the MatLab platform (MathWorks, Natick, MA, USA).31 In brief, the first ten volumes of each functional time series were discarded, as patients were adjusting themselves during that period to the fMRI environment. The remaining 230 images were slice-time-corrected with the 30th slice as the reference and spatially realigned for head motion. Patient head motion was assessed by evaluating three translations and three rotations for each scan. Translational thresholds were set to ±2 mm, while rotational thresholds were limited to ±1°. After head motion correction, functional images were spatially normalized to Montreal Neurological Institute (MNI) space using echo-planar imaging sequence templates (resampled voxel size 3×3×3).
Regional homogeneity
For ReHo measurements,23 normalized images were further analyzed using DPARSF.31 First, we employed it to remove linear trends and reduce low-frequency drift and high-frequency respiratory and cardiac noise using a temporal band-pass (0.01–0.08 Hz) filter. Subsequently, we used it to map regional spontaneous activity across the whole brain, with Kendall’s coefficient of concordance used for assessing in a voxel-wise fashion the similarity of the time series at a given voxel to the time series of its 26 nearest neighbors:
|
where Ri is the sum rank of time point i, 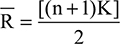 is the mean of Ri values, K is the number of time series corresponding to the spatial voxels contained within a measured cluster (here, K=27, one given voxel plus the number of its neighbors), n is the number of ranks (here, n=230 time points), and i is from 1 to n. Kendall’s coefficient values (W) ranging from 0 to 1 were calculated voxel by voxel across the whole brain, producing an individual ReHo map for each participant. For the purpose of standardization, each individual ReHo map was divided by its own mean ReHo of that entire brain. Then, standardized ReHo maps for all participants were smoothed using a Gaussian filter of 8 mm full width at half maximum to reduce noise and residual differences in normalization.
is the mean of Ri values, K is the number of time series corresponding to the spatial voxels contained within a measured cluster (here, K=27, one given voxel plus the number of its neighbors), n is the number of ranks (here, n=230 time points), and i is from 1 to n. Kendall’s coefficient values (W) ranging from 0 to 1 were calculated voxel by voxel across the whole brain, producing an individual ReHo map for each participant. For the purpose of standardization, each individual ReHo map was divided by its own mean ReHo of that entire brain. Then, standardized ReHo maps for all participants were smoothed using a Gaussian filter of 8 mm full width at half maximum to reduce noise and residual differences in normalization.
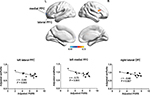
A total of 26 frontal cortical areas were selected from anatomical automatic labeling,32 as listed in Table 2, to compose a mask used for the ReHo analysis (Figure 1). Within the frontal lobe, we calculated Pearson’s correlation between ReHo of each voxel and subjective spontaneous pain reported by patients right before the scan. The threshold for significance and correction for multiple comparisons were calculated using AlphaSim program (https://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). A corrected threshold of P<0.05 was utilized with a combined cutoff threshold of P<0.01 and a minimum cluster size of 1,161 mm3 (43 voxels), determined by 1,000 iterations of Monte Carlo simulation. The resulting r map was overlaid on rendering views with BrainNet Viewer (http://www.nitrc.org/projects/bnv), and the anatomy of surviving brain regions was reported using xjView software (http://www.alivelearn.net/xjview).
  | Table 2 The 26 brain areas of the frontal lobe mask Abbreviations: ALL, anatomical automatic labeling; L, left; R, right. |
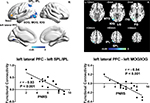
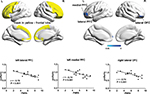  | Figure 1 Frontal brain areas whose ReHo was correlated with PHN pain. Notes: Upper left: mask of frontal lobe. The mask (yellow) consists of 26 anatomical brain areas from anatomical automatic labeling. Names of the 26 anatomical brain areas are listed in Table 2. Upper right: brain areas whose ReHo was correlated with spontaneous pain perceived by PHN patients (P<0.05, corrected). Color bar indicates r-score and cold color indicates negative correlation. Lower: correlations between the ReHo signal from regions of interest and pain scores. Abbreviations: L, left; OFC, orbitofrontal cortex; PFC, prefrontal cortex; PHN, postherpetic neuralgia; PNRS, pain numeric rating scale; R, right; smReHo, smoothened regional homogeneity. |
Functional connectivity
For measurements of functional connectivity,25 fMRI data from preprocessing were further analyzed using DPARSF.31 The preprocessed data were smoothed using a Gaussian filter of 8 mm full width at half maximum to reduce noise and residual differences in normalization. Then, the linear trend was removed and a temporal band-pass (0.01–0.08 Hz) filter applied to reduce low-frequency drift and high-frequency respiratory and cardiac noise. Pearson’s correlation was calculated between averaged time courses of regions of interest (ROIs; clusters where ReHo correlated with patients’ spontaneous pain) for each patient in a cluster-wise manner across the whole brain, with global mean time course, white-matter mean time course, cerebrospinal fluid mean time course, and the six head motion parameters as nuisance covariates. Individual correlation coefficients were converted to z-scores using Fisher’s transformation to improve normality. Pearson’s correlation between functional connectivity of each voxel across the whole brain and patients’ subjective spontaneous pain was calculated. A corrected threshold of P<0.05 was utilized with a combined cutoff threshold of P<0.01 and a minimum cluster size of 1,917 mm3 (71 voxels), determined by 1,000 iterations of Monte Carlo simulation. The resulting r-maps were overlaid on rendering views with BrainNet Viewer and on axial slice views with the Rest slice viewer (http://restfmri.net/forum/index.php), and the anatomy of survived brain regions were reported using xjView software.
Signal extraction from ROI
We extracted averaged signals of ReHo or functional connectivity from ROIs for illustrating correlations with PHN pain with the Resting-State fMRI Data Analysis Toolkit version 1.8.33 Specifically, we first saved surviving clusters from the analysis of ReHo or functional connectivity into binary masks. Then, intersections were examined from these masks and related anatomical regions from the anatomical automatic labeling32 template to obtain particular masks of clusters of interest. Finally, mean values of voxels within each cluster of interest were extracted.
Statistical analysis
Statistical analysis for extracted signals (ReHo or functional connectivity) was performed with SPSS for Windows version 16 and result graphs presented using GraphPad Prism for Windows version 6. Pearson’s correlation was applied to examine whether there existed significant correlations between the extracted ROI signal and subjective spontaneous pain. The Shapiro–Wilk test was used to check whether the pain scores and fMRI data conformed to normal distribution. Average numbers in the present article are presented in the form of means ± standard error.
Results
Demographics and pain scores of PHN patients
The 16 PHN patients (ten males and six females) recruited in the fMRI study had a mean age of 67.1±1.39 (53–74) years. The mean duration of PHN was 25.3±4.63 months. The mean subjective rating of pain intensity in the experiment was 6.1±0.50, with a range of 2–9, which was not significant different from the pain score over the last 7 days prior to the scan (6.6±0.48, range 3–10, paired t-test, t15=1.83; P=0.087).
Relationships between prefrontal local synchronization and pain intensity
To explore whether neural activity in the frontal lobe was associated with PHN pain, we examined the relationship between the degree of local synchronization (measured by ReHo) of the PFC and the intensity of pain experienced by PHN patients. ReHo values within the frontal lobe mask consisting of 26 areas32 were analyzed in these PHN patients. Significant negative correlations were observed between pain scores and ReHo values in several areas (P<0.05, AlphaSim-corrected, Figure 1, Table 3), including the left lateral PFC (BA10, BA46, BA47, peak MNI coordinates, x=–51, y=48, z=0, r=–0.78, cluster P<0.001, ReHo mean 0.859±0.037), left medial PFC (BA10, peak MNI coordinates, x=–15, y=63, z=3, r=–0.74, cluster P=0.001, ReHo mean 0.892±0.045), and right lateral orbitofrontal cortex (BA11, BA47, peak MNI coordinates, x=33, y=51, z=–15, r=–0.74, cluster P=0.001, ReHo mean 0.883±0.038). Patients with higher pain scores exhibited lower ReHo values in these prefrontal areas (Figure 1), and vice versa, which suggested that activity of local circuits in the prefrontal areas was modulated by chronic pain. Both pain scores (W16=0.961, P=0.672) and ReHo values (left lateral PFC, W16=0.974, P=0.901; left medial PFC, W16=0.951, P=0.502; right orbital frontal cortex, W16=0.959, P=0.649) conformed to normal distribution, and thus a parametric method (Pearson’s correlation) was used for the statistical analysis.
In order to exclude the possibility that age and sex might have affected these results, we repeated the analysis with the addition of age and sex as covariates of no interest. The results were similar to those of the original analysis. The P-value remained significant (P<0.01, uncorrected), although it did not pass the correction for multiple comparisons. This might have been due to the small sample size of the present study, and thus statistics may be slightly affected by the reduced number of degrees of freedom. Results of this analysis are summarized in Figure S1.
Correlations between functional connectivity and pain intensity
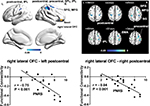
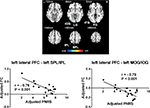
To examine further if pain-induced modulation of local synchronization in the prefrontal region would also result in modulation of activity in its associated networks, functional connectivity between the identified prefrontal areas with modulated ReHo and other brain regions was analyzed. Prefrontal areas where ReHo showed correlations with the degree of pain were chosen as seed regions, and the correlation between seed-based functional connectivity and the intensity of pain was examined. We observed that functional connectivity between the left lateral PFC and two clusters of brain areas was negatively correlated with pain scores reported by the patients (P<0.05, AlphaSim-corrected, Figure 2, Table 4). One of the clusters consisted of the left superior and inferior parietal lobules (BA7, BA40, peak MNI coordinates, x=–24, y=–72, z=60, r=–0.83, cluster P<0.001, mean of functional connectivity 0.254±0.066). The other consisted of the left superior, middle, and inferior occipital gyri, left lingual gyrus, left fusiform gyrus, and left middle and inferior temporal gyri (BA18, BA19, BA37, peak MNI coordinates, x=–42, y=–90, z=–6, r=–0.84, cluster P<0.001, mean of functional connectivity –0.086±0.037). The results with the addition of age and sex as covariates of no interest were similar to those of the original analysis. The P-value and statistical significance remained significant (P<0.01, uncorrected), although it did not pass the correction for multiple comparisons. This might have been due to the reduced number of degrees of freedom. Results of this analysis are summarized in Figure S2.
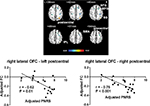
The right lateral orbitofrontal cortex also displayed significant negative correlations between its functional connectivity with two cortical clusters and the degree of pain severity reported by the patients (P<0.05, AlphaSim-corrected, Figure 3, Table 5). One cluster mainly consisted of the left postcentral gyrus, left precentral gyrus, and left inferior parietal lobule (BA2, BA4, BA40, peak MNI coordinates, x=–30, y=–33, z=42, r=–0.75, cluster P<0.001, mean of functional connectivity–0.177±0.052), and the other mainly consisted of the right postcentral gyrus, right precentral gyrus, right superior and inferior parietal lobules, right superior and middle frontal gyri, and right supramarginal gyrus (BA1, BA2, BA3, BA4, BA6, BA8, BA40, peak MNI coordinates, x=33, y=–33, z=45, r=–0.84, cluster P<0.001, mean of functional connectivity –0.182±0.037). Results with the addition of age and sex as covariates of no interest were similar to those of the original analysis. The P-value remained significant (P<0.01, uncorrected), although it did not pass the correction for multiple comparisons. Results of this analysis are summarized in Figure S3.
The left medial PFC did not display any significant correlation between its connectivity with other brain regions and pain intensity. Both pain scores (W16=0.961, P=0.672) and values of functional connectivity conformed to normal distribution (left lateral PFC–left parietal lobe, W16=0.961, P=0.680; left lateral PFC–left occipital lobe, W16=0.956, P=0.591; right orbitofrontal cortex–left post/precentral area, W16=0.930, P=0.245; right orbitofrontal cortex–right post/precentral area, W16=0.945, P=0.410), and thus parametric Pearson’s correlation was used for the statistical analysis.
Discussion
The current study explored the association between chronic pain and the modulation of activity in the PFC using fMRI techniques. We found a unique pattern of alterations in brain activity in PHN patients. Local neural synchronization, measured by ReHo, in left prefrontal/right orbitofrontal cortices was closely associated with chronic pain, as indicated by negative correlations between ReHo and pain scores reported by the PHN patients. Furthermore, when examined at a network level, the strength of functional connections of some of these prefrontal areas with other cortical regions was found to be associated with pain intensity as well. These results suggest that modulation of neural activity in the PFC by chronic pain may constitute the mechanism underlying interactions between chronic pain and cognitive functions and the resultant cognitive impairment observed in chronic-pain patients.1,2
The PFC plays critical roles in many cognitive functions, such as working memory, decision-making, motor planning, and executive functions.5–8 It also plays an important role in the perception and modulation of pain.9,10 Recent studies have demonstrated that the left PFC is involved in both sensory and affective dimensions of painful experience. Transcranial stimulation of the left PFC, but not the right PFC, produces analgesia for both unpleasantness and pain-intensity ratings in acute and chronic pain conditions.9,34–38 In the present study, we observed that ReHo in several subregions of the PFC, including the left lateral PFC, left medial PFC, and right lateral orbital PFC, was negatively correlated with pain. Our finding indicates that activity of the PFC is modulated by the intensity of pain in PHN patients, suggesting the involvement of the PFC in chronic pain in PHN. On the other hand, the modulation could be a result of the cortical plastic change induced by chronic pain. Indeed, a number of previous studies have revealed that under the condition of chronic pain, the PFC undergoes both structural and functional changes, including decreases in gray-matter volume in the PFC, in chronic pain patients,14–18,39 and changes in excitability and activity of prefrontal neurons in the condition of inflammatory and neuropathic pain in animal pain models.19,20 Such alterations in prefrontal activity have been found to be associated with cognitive deficits in various clinical conditions.40–42
Functional networks related to the PFC are known to play critical roles in cognitive functions.43–45 These areas with modulated ReHo observed in our study have been reported to have extensive functional connections with other cortical areas and subcortical structures.46–48 In our study, we found that functional connectivity between the left lateral PFC and left parietal areas was modulated by pain intensity, as indicated by a negative correlation between functional connectivity of these two areas and pain intensity, ie, stronger pain intensity was associated with weaker functional connectivity between prefrontal and parietal areas. The frontoparietal network, composed of the anterior PFC, dorsolateral PFC, anterior cingulate cortex, anterior insular, caudate, and anterior inferior parietal lobule,49,50 has been considered to underpin cognitive functions of attention,51 working memory,52 and executive control.53 This network has been reported to display aberrant connectivity in individuals with cognitive impairment, such as attention deficit/hyperactivity disorder54 and age-related decline in cognitive functions.55 It is thus tempting to assume that the functional decline of this frontoparietal network in association with PHN pain may constitute a neural mechanism for chronic pain-related modulation of higher cognitive functions in PHN patients, such as impairment of attention and working memory.4,56
Interestingly, we also found that functional connectivity between the left lateral PFC and the left occipital cortex was negatively correlated with pain intensity. The occipital lobe has been traditionally thought to be involved in visual information processing. While no visual impairment has been reported in association with chronic pain, a few rodent studies have found that the occipital cortex shows an antinociceptive effect.57,58 In addition, several neuroimaging studies in humans have documented altered signals in occipital lobes of patients with chronic pain disorder.59–61 Along with these findings, our observations suggested that occipital areas participated in the modulation of PHN pain. Further studies are needed to elucidate its role in this modulation.
This study also revealed the modulation of functional connectivity between the right lateral orbitofrontal and bilateral sensorimotor area by PHN pain. The orbitofrontal cortex has been reported to have extensive connectivity to sensory areas of all modalities48,62 and has been proposed to play a critical role in integration of sensory information and representation of the value of multimodal stimulus reinforcers48,63–66 which are used to inform the decision-making process. Our finding that connectivity between the right lateral orbitofrontal cortex and sensorimotor areas gets weakened with increasing PHN pain severity suggests that chronic pain may affect the cognitive functions that depend on connectivity between those two cortical areas. This assumption is in line with clinical observations indicating that chronic pain patients often show deficits in cognitive functions, such as associative learning67 and decision-making.4,28
Conclusion
The present study demonstrates that both ReHo in prefrontal/orbitofrontal areas and their connectivity with parietal, occipital, and sensorimotor areas are highly associated with clinical pain in PHN patients. These findings indicate that there are interactions between neuropathic pain and cognitive functions that involve the PFC. Further studies are needed to verify if there exist direct links between the impairment of cognitive functions and prefrontal subregions, as well as between the impairment and functional networks related to these subregions identified in the present study in PHN patients. A better understanding of the interaction between pain and cognition, especially its underlying neural mechanisms, may help to improve therapeutic strategies for chronic pain patients with pain-related cognitive dysfunction.
Acknowledgments
This work was supported by a research fund from the MIND Research Institute, California, to XWD. This work was also supported by the Key Specialist Projects of Shanghai Municipal Commission of Health and Family Planning (ZK2015B01) and the Programs Foundation of Shanghai Municipal Commission of Health and Family Planning (201540114). We are grateful to Dr Yongdi Zhou for his tremendous contributions to the manuscript. We thank all the volunteers for their participation in this study and thank the Shanghai Key Laboratory of Magnetic Resonance, East China Normal University for materials and technical support.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest and report no conflicts of interest in this work.
References
Berryman C, Stanton TR, Bowering KJ, Tabor A, Mcfarlane A, Moseley GL. Do people with chronic pain have impaired executive function? A meta-analytical review. Clin Psychol Rev. 2014;34(7):563–579. | ||
Moriarty O, Mcguire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93(3):385–404. | ||
Johnson RW, Rice AS. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014;371(16):1526–1533. | ||
Pickering G, Pereira B, Clère F, et al. Cognitive function in older patients with postherpetic neuralgia. Pain Pract. 2014;14(1):E1–E7. | ||
Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 2011;96(3):417–431. | ||
Funahashi S, Andreau JM. Prefrontal cortex and neural mechanisms of executive function. J Physiol Paris. 2013;107(6):471–482. | ||
Dixon ML, Christoff K. The lateral prefrontal cortex and complex value-based learning and decision making. Neurosci Biobehav Rev. 2014;45:9–18. | ||
D’Esposito M, Postle BR. The cognitive neuroscience of working memory. Annu Rev Psychol. 2015;66:115–142. | ||
Brighina F, de Tommaso M, Giglia F, et al. Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. J Headache Pain. 2011;12(2):185–191. | ||
Bogdanov VB, Viganò A, Noirhomme Q, et al. Cerebral responses and role of the prefrontal cortex in conditioned pain modulation: an fMRI study in healthy subjects. Behav Brain Res. 2015;281:187–198. | ||
Zhuo M. Cortical excitation and chronic pain. Trends Neurosci. 2008;31(4):199–207. | ||
Moisset X, Bouhassira D. Brain imaging of neuropathic pain. Neuroimage. 2007;37(Suppl 1):S80–S88. | ||
Lee M, Manders TR, Eberle SE, et al. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J Neurosci. 2015;35(13):5247–5259. | ||
Cauda F, Palermo S, Costa T, et al. Gray matter alterations in chronic pain: a network-oriented meta-analytic approach. Neuroimage Clin. 2014;4:676–686. | ||
Ruscheweyh R, Deppe M, Lohmann H, et al. Pain is associated with regional grey matter reduction in the general population. Pain. 2011;152(4):904–911. | ||
Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29(44):13746–13750. | ||
Obermann M, Rodriguez-Raecke R, Naegel S, et al. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage. 2013;74:352–358. | ||
Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410–10415. | ||
Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009;106(7):2423–2428. | ||
Wu XB, Liang B, Gao YJ. The increase of intrinsic excitability of layer V pyramidal cells in the prelimbic medial prefrontal cortex of adult mice after peripheral inflammation. Neurosci Lett. 2016;611:40–45. | ||
Liu J, Hao Y, du M, et al. Quantitative cerebral blood flow mapping and functional connectivity of postherpetic neuralgia pain: a perfusion fMRI study. Pain. 2013;154(1):110–118. | ||
Jiang J, Gu L, Bao D, et al. Altered homotopic connectivity in postherpetic neuralgia: a resting state fMRI study. J Pain Res. 2016;9:877–886. | ||
Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394–400. | ||
Cao S, Li Y, Deng W, et al. Local brain activity differences between herpes zoster and postherpetic neuralgia patients: a resting-state functional MRI study. Pain Physician. 2017;20(5):E687–E699. | ||
Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. | ||
Paulson SK, Vaughn MB, Jessen SM, et al. Pharmacokinetics of celecoxib after oral administration in dogs and humans: effect of food and site of absorption. J Pharmacol Exp Ther. 2001;297(2):638–645. | ||
Angst MS, Brose WG, Dyck JB. The relationship between the visual analog pain intensity and pain relief scale changes during analgesic drug studies in chronic pain patients. Anesthesiology. 1999;91(1):34–41. | ||
Apkarian AV, Sosa Y, Krauss BR, et al. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108(1-2):129–136. | ||
Abbas AM, Abdellah MS, Khalaf M, et al. Effect of cervical lidocaine-prilocaine cream on pain perception during copper T380A intrauterine device insertion among parous women: a randomized double-blind controlled trial. Contraception. 2017;95(3):251–256. | ||
Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798–804. | ||
Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. | ||
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. | ||
Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6(9):e25031. | ||
Naylor JC, Borckardt JJ, Marx CE, et al. Cathodal and anodal left prefrontal tDCS and the perception of control over pain. Clin J Pain. 2014;30(8):693–700. | ||
Short EB, Borckardt JJ, Anderson BS, et al. Ten sessions of adjunctive left prefrontal rTMS significantly reduces fibromyalgia pain: a randomized, controlled pilot study. Pain. 2011;152(11):2477–2484. | ||
Borckardt JJ, Smith AR, Reeves ST, et al. A pilot study investigating the effects of fast left prefrontal rTMS on chronic neuropathic pain. Pain Med. 2009;10(5):840–849. | ||
Umezaki Y, Badran BW, Gonzales TS, George MS. Daily left prefrontal repetitive transcranial magnetic stimulation for medication-resistant burning mouth syndrome. Int J Oral Maxillofac Surg. 2015;44(8):1048–1051. | ||
Maeoka H, Matsuo A, Hiyamizu M, Morioka S, Ando H. Influence of transcranial direct current stimulation of the dorsolateral prefrontal cortex on pain related emotions: a study using electroencephalographic power spectrum analysis. Neurosci Lett. 2012;512(1):12–16. | ||
Cao S, Qin B, Zhang Y, et al. Herpes zoster chronification to postherpetic neuralgia induces brain activity and grey matter volume change. Am J Transl Res. 2018;10(1):184–199. | ||
Ji G, Sun H, Fu Y, et al. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci. 2010;30(15):5451–5464. | ||
Janetsian SS, Linsenbardt DN, Lapish CC. Memory impairment and alterations in prefrontal cortex gamma band activity following methamphetamine sensitization. Psychopharmacology. 2015;232(12):2083–2095. | ||
Devilbiss DM, Spencer RC, Berridge CW. Stress degrades prefrontal cortex neuronal coding of goal-directed behavior. Cereb Cortex. 2016;8:bhw140. | ||
Beckmann CF, Deluca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–1013. | ||
Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. | ||
de Luca M, Beckmann CF, de Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29(4):1359–1367. | ||
Reid AT, Bzdok D, Langner R, et al. Multimodal connectivity mapping of the human left anterior and posterior lateral prefrontal cortex. Brain Struct Funct. 2016;221(5):2589–2605. | ||
Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–219. | ||
Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. | ||
Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. | ||
Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. | ||
Ptak R. The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. Neuroscientist. 2012;18(5):502–515. | ||
Shen J, Zhang G, Yao L, Zhao X. Real-time fMRI training-induced changes in regional connectivity mediating verbal working memory behavioral performance. Neuroscience. 2015;289:144–152. | ||
Harding IH, Yücel M, Harrison BJ, Pantelis C, Breakspear M. Effective connectivity within the frontoparietal control network differentiates cognitive control and working memory. Neuroimage. 2015;106:144–153. | ||
Lin HY, Tseng WY, Lai MC, Matsuo K, Gau SS. Altered resting-state frontoparietal control network in children with attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc. 2015;21(4):271–284. | ||
Müller NG, Knight RT. Age-related changes in fronto-parietal networks during spatial memory: an ERP study. Brain Res Cogn Brain Res. 2002;13(2):221–234. | ||
Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD, Pain CPD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain. 2005;6(6):356–363. | ||
Reis GM, Dias QM, Silveira JW, del Vecchio F, Garcia-Cairasco N, Prado WA. Antinociceptive effect of stimulating the occipital or retrosplenial cortex in rats. J Pain. 2010;11(10):1015–1026. | ||
Rossaneis AC, Reis GM, Prado WA. Stimulation of the occipital or retrosplenial cortex reduces incision pain in rats. Pharmacol Biochem Behav. 2011;100(2):220–227. | ||
Klug S, Stefanie K, Anderer P, Saletu-Zyhlarz G, et al. Dysfunctional pain modulation in somatoform pain disorder patients. Eur Arch Psychiatry Clin Neurosci. 2011;261(4):309–275. | ||
Karibe H, Arakawa R, Tateno A, et al. Regional cerebral blood flow in patients with orally localized somatoform pain disorder: a single photon emission computed tomography study. Psychiatry Clin Neurosci. 2010;64(5):476–482. | ||
Cauda F, Sacco K, Duca S, et al. Altered resting state in diabetic neuropathic pain. PLoS One. 2009;4(2):e4542. | ||
Cavada C, Compañy T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suárez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10(3):220–242. | ||
Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–1107. | ||
Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. | ||
Walton ME, Behrens TE, Noonan MP, Rushworth MF. Giving credit where credit is due: orbitofrontal cortex and valuation in an uncertain world. Ann N Y Acad Sci. 2011;1239:14–24. | ||
Schoenbaum G, Esber GR, You Hdo. How do you (estimate you will) like them apples? Integration as a defining trait of orbitofrontal function. Curr Opin Neurobiol. 2010;20(2):205–211. | ||
Walteros C, Sánchez-Navarro JP, Muñoz MA, Martínez-Selva JM, Chialvo D, Montoya P. Altered associative learning and emotional decision making in fibromyalgia. J Psychosom Res. 2011;70(3):294–301. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.