Back to Journals » OncoTargets and Therapy » Volume 11
Modulation of human melanoma cell proliferation and apoptosis by hydatid cyst fluid of Echinococcus granulosus
Authors Gao XY, Zhang GH, Huang L
Received 13 July 2017
Accepted for publication 31 October 2017
Published 15 March 2018 Volume 2018:11 Pages 1447—1456
DOI https://doi.org/10.2147/OTT.S146300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
Xiang-Yang Gao,1,* Guang-Hui Zhang,2,* Li Huang3
1Department of Laboratory Medicine, Pu’er People’s Hospital, Pu’er, 2Department of Clinical Laboratory, Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, 3Department of General Surgery, Shanghai General Hospital, Shanghai, China
*These authors contributed equally to this work
Objective: The objective of this paper was to assess the effects of hydatid cyst fluid (HCF) of Echinococcus granulosus on melanoma A375 cell proliferation and apoptosis.
Methods: A375 cells were classified into five groups by in vitro culture: normal group, control group, 10% HCF group, 20% HCF group and 30% HCF group. Trypan blue staining method was employed to detect the toxicity of HCF. Effects of different concentrations of HCF on melanoma A375 cell proliferation at different time points were evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Flow cytometry and propidium iodide (PI) staining were used to detect cell cycle, and Annexin-V/PI double staining method was used to determine A375 cell apoptotic rate. Western blotting was applied to detect the expression of phosphorylated extracellular regulated protein kinases, proliferating cell nuclear antigen (PCNA), cell-cycle-related proteins (cyclin A, cyclin B1, cyclin D1 and cyclin E) and apoptosis-related proteins (Bcl-2, Bax and caspase-3).
Results: HCF with a high concentration was considered as atoxic to A375 cells. HCF promoted A375 cell proliferation, and the effects got stronger with an increase in concentrations but was retarded after reaching a certain range of concentrations. HCF increased phosphorylation level and expression of extracellular regulated protein kinase, as well as PCNA expression. HCF also promoted the transferring progression of A375 cells from the G0/G1 phase to the S phase to increase the cell number in S phase and increased the expression of cyclin A, cyclin D1 and cyclin E. HCF increased the expression of procaspase-3 (the precursor of apoptosis-related protein caspase-3) and antiapoptotic protein-Bcl-2, and decreased the expression of proapoptotic factor Bax, thereby inhibiting cell apoptosis.
Conclusion: As a result, this study confirmed that HCF promotes proliferation and inhibits apoptosis of melanoma A375 cells.
Keywords: hydatid cyst fluid, melanoma, A375 cell, proliferation, apoptosis, cell cycle
Corrigendum for this paper has been published
Introduction
Melanoma, also known as black evil, is a malignant skin cancer originating from neuroectoderm, known by its characteristics of serious malignancy, easy to metastasize and relapse, and has a poor prognosis.1 It is more prevalent in the Caucasian population with an incidence of about 5.1 cases per million populations per year in the USA.2 Earlier reports have showed that the survival rates decrease with an increase in tumor thickness in every millimeter; for example, the 10-year survival rate declined from 92% for thickness ≤1 mm to 50% with melanoma thickness >4 mm.3 The two main pathogenic factors for melanoma are genetic predisposition and environmental factors.4 Diagnostic imaging showed that metastatic tumors of melanoma frequently occur in the liver, lumbar vertebrae and bilateral lung.5 Therefore, inhibiting the proliferation of melanoma cells in these easily infected parts could serve as a target for the treatment of melanoma.
Echinococcus granulosus is one of the causes of hydatid disease, and the main characteristic is endemic, which affects the liver and lungs generally.6 There are four kinds of well-known Echinococcus that were considered to infect humans: E. multilocularis, E. granulosus, E. vogeli and E. oligarthrus.7 As proven by previous research, the parasite can develop larvae enveloping themselves in a cyst that forms in the organs (such as brain, heart, liver, lung, spleen and spinal cord) of the intermediate hosts for >20 years, thereby making the diagnosis markedly difficult and is usually based on paraclinical methods like serology.8 Hydatid cyst fluid (HCF) is secreted by the soluble components of the germinal layer in metacestode and provides the essential nutrients necessary for the growth and development of the larval cyst and of the protoscoleces that get collected within the HCF.9 A study highlighted the importance of a mutual cross-reaction existing between the antigens of hydatid cyst and excretory secretory products of cancer cells.10 Moreover, extracellular-regulated protein kinase (ERK) signaling pathway, as a kind of signal transduction pathway, stimulates the activation of mitogen-activated protein kinase (MAPK) signaling pathway, which primarily promotes cell proliferation and differentiation.11,12 Proliferating cell nuclear antigen (PCNA) is responsible for its indispensable role in cellular DNA replication and repair by the organization of numerous protein components of ERK/MAPK signaling pathway.13 HCF has been observed to activate the MAPK signaling pathway in rat liver cells, thereby achieving phosphorylation and a high expression of ERK signaling pathway.14 Besides, alveolar echinococcosis is a severe chronic helminthic disease that mimics slow-growing liver cancer,15 and Aghayan et al found that melanoma could transfer into the liver,16 which indicated that HCF and melanoma have the common action site. Therefore, in this study we used melanoma A375 cell as the subject to explore the concrete effects of different concentrations of HCF on melanoma, which could serve as experimental basis, and we hypothesized that HCF could contribute to the treatment of melanoma in a certain concentration.
Materials and methods
Preparation of HCF
Sheep liver with cystic echinococcosis was selected and scrubbed with 75% ethanol in order to extract HCF using a 20-mL single-use syringe. Following that, the HCF was centrifuged at a speed of 3,000 r/min for 20 min at 4°C (semi-diameter was 8 cm) in order to remove the hydatid sand. Filtration sterilization was conducted with a 0.22-μm aperture, and then stored for further experimentation.
Cell culture and grouping
Human malignant melanoma cell line A375 was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum (FBS) (Evergreen, Hangzhou, Zhejiang, China) and 1% double-antibiotics (100 U/L penicillin and 100 mg/L streptomycin; Gibco Company, Grand Island, NY, USA) for conventional incubation at 37°C in a homothermal incubator with 5% CO2, followed by digestion and subculture every 2–3 days. The cells in the logarithmic growth phase were selected and were digested by 0.25% trypsin for cell counting using a cell counting plate. The cells were then inoculated into a 6-mm culture dish with 1×106 cells per dish, followed by incubation for 24 h via the conventional method. The culture medium was replaced by serum-free medium for another 24 h of incubation after cells adhered to the plate walls, followed by medium replacement after cell synchronization. On completion of incubation, A375 cells were assigned into five groups according to different conditions – the normal group (without FBS), the control group (containing 10% FBS) and different concentrations (10%, 20% and 30%) of HCF (different concentrations of cyst fluid were added to cultures containing 10% FBS).
Trypan blue staining
In biosciences, trypan blue is used as a vital stain to selectively color dead tissues or cells blue. Namely, live cells or tissues with intact cell membranes are not colored, while trypan blue traverses the membrane in a dead cell. Hence, dead cells are shown in a distinctive blue color under a microscope. To determine the toxicity of HCF on A375 cells in vitro, we performed trypan blue staining to calculate living cells. After 12 h of culturing, cells in the logarithmic phase of growth were selected and resuspended in a cell growth medium, followed by inoculation in a 24-well plate with 2×105 cells/mL in each well. Then 0.2 mL trypsin was added into the 24-well plate and stirred several times after which it was discarded. Cells were digested with 0.25% of 0.4 mL trypsin for 30 s and terminated by medium containing 10% FBS. That was followed by addition of 0.1 mL trypan blue solution (4 g/L) diluted by 0.1 M phosphate-buffered saline (PBS) to the plates and mixed evenly in order to count the number of living cells (each well was counted three times). The inhibition rate of cell growth formula was as follows: (cell number in the control group – cell number in the treatment group)/cell number in the control group ×100%. Cell morphology was observed and photographed under an inverted microscope.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
MTT, a yellow tetrazole, is absorbed by mitochondria where it is reduced to purple formazan by succinate dehydrogenase. To determine the effect of HCF on A375 cell viability in vitro, we performed MTT assay to reflect the number of viable cells present under defined conditions in different treatment groups. The A375 cells in the logarithmic growth phase were selected and inoculated in a 96-well plate with 1×105/mL in each well. According to the grouping, eight wells were labeled for each group with 100 μL in each well. After that, the wells were incubated at 37°C in a humidified incubator with 5% CO2 in air. On completion of 24-h incubation, after cells adhered to the well, the RPMI 1640 culture medium (Gibco 31800-014; Gibco Company) containing 1% FBS was added into the medium for starvation culture for 12 h. After incubated for 3, 6, 12 and 24 h, cells in each well were added with 10 μL MTT (0.5 mg/mL; Sigma Chemical Co., St Louis, MO, USA) devoid of light. Thereafter, the cell culture in the 96-well plate was extracted, and 100 μL dimethyl sulfoxide (Sigma Chemical Co.) was added in dark. Microplate reader was employed to detect cell concentration when vibrating and dissolving for 15 min in dark. The optical density (OD) value at 570 nm in each well was measured under a spectrophotometer. Meanwhile, after 24 h the cell morphology was observed and photographed under an inverted microscope.
Propidium iodide (PI) staining
PI is a fluorescent intercalating agent that can be used to stain cells. PI is used as a DNA stain in flow cytometry to evaluate the cell viability or DNA content in cell-cycle analysis, or in microscopy to visualize the nucleus and other DNA-containing organelles. As the PI cannot cross the membrane of live cells, it is employed to differentiate between necrotic, apoptotic and healthy cells. In this experiment, we performed flow cytometry analysis of PI staining to reflect DNA content in each cell cycle, thereby indicating the effect of HCF on A375 cell-cycle progression in vitro. After 12-h cell culture, the A375 cells were digested by 0.25% trypsin without ethylenediaminetetraacetic acid (EDTA) (avoiding excessive digestion). A portion of the cells were collected and centrifuged at 2,500 r/min for 5 min, after which the suspension was discarded. Cells were rinsed in precooling PBS two times and cell collection. After that, the cells were fixed by about 3 mL of 70% precooling ethanol at 4°C overnight. The next day, cells were rinsed with PBS and centrifuged at 1,000 r/min for 5 min, followed by discarding the supernatant. Cells were incubated with 10 μL of RNase at 37°C for 5 min. Subsequently, cells were stained with 1% PI solution (40710ES03; Shanghai Qcbio Science & Technologies Co., Ltd., Shanghai, China) devoid of light for 30 min. After staining, the cells were filtrated with a cell strainer, and flow cytometry (FACSCalibur, BD Biosciences, Franklin Lakes, NJ, USA) was employed to record the cell cycle at the wavelength of 480 nm through red fluorescence light and in order to detect cell proportion in the G0/G1, S and G2/M phases. All experiments were repeated three times.
Annexin-V/PI double staining
Annexin A5 (or annexin V) is used as a nonquantitative probe for the detection of cells expressing phosphatidylserine on their cell surface, an event observed in apoptosis and other forms of cell death processes. It based on a combination of the staining of DNA in the cell nucleus with PI, thereby distinguishing viable cells from apoptotic cells and necrotic cells. The flow cytometry analysis of Annexin-V/PI staining was employed to determine the effect of HCF on A375 cell apoptosis in vitro. After 12 h of culturing, the same results unfolded with digestion of cells by 0.25% trypsin (without EDTA, avoiding excessive digestion). Some cells were collected, and they were centrifuged at 2,500 r/min for 5 min, followed by two cycles of PBS rinsing and cell collection, and then the supernatant was discarded. The collected cells were subsequently transferred to Eppendorf (EP) tubes (1×106 cells per tube), and 500 μL binding buffer was added to each tube. Each EP tube contained 5 μL PI and 5 μL Annexin V dye solution (Nanjing KeyGen Biotech, Co., Ltd., Nanjing, China) after mixing. All tubes were incubated devoid of light at room temperature for 10–15 min after addition of the dyes. A 400-mesh screen filter was used to filter the incubated cell suspension. After filtration, the suspension was placed on ice. The cells were excited at 488 nm, and fluorescein isothiocyanate fluorescence was detected using a 520-nm band-pass filter, while PI was detected using a 610-nm band-pass filter within 30 min. Among the four quadrants, AnnexinV+PI− was apoptosis in the early stage and AnnexinV+PI+ was apoptosis in the late stage, while apoptosis rate was calculated with early apoptosis rate in the early stage plus apoptosis rate in the late stage.17
Western blotting
Cells were collected 1, 3, 6 and 12 h after incubation, and the adherent cells were digested with trypsin. After centrifugation, cells were rinsed twice with cold PBS and were allowed to react with precooling lysis buffer for 30 min and then centrifuged at 12,000 r/min for 20 min at 4°C. The fully lysed cells were further centrifuged, and the supernatant in the EP tube was regarded as the total protein extracted from cells. Bicinchoninic Acid Protein Assay Kit was adopted to determine the protein concentrations, and then the concentration of each group was adjusted into equal amount. After quantitation, the extracted protein was added with 5× sodium dodecyl sulfate loading buffer and was denaturated at 95°C for 5 min for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After SDS-PAGE, transmembrane was conducted and stored at 4°C overnight with 5% skimmed milk powder. The membranes were washed with Tris-buffered saline with Tween 20 (TBST) and were then incubated with monoclonal antibodies: rabbit anti-sheep phosphorylated extracellular regulated protein kinases (p-ERK) (1:2,000), PCNA (1:1,000), cyclin A (1:3,500), cyclin B1 (1:2,000), cyclin D1 (1:1,000), cyclin E (1:3,000), Bcl-2 (1:1,000), Bax (1:2,000), caspase-3 (1:3,000) and the internal reference glyceraldehyde-3-phosphate dehydrogenase (1:5,000) (Cell Signaling Technology, Inc., Danvers, MA, USA) overnight. The membranes were then rewashed with TBST and incubated with second antibody labeled with horseradish peroxidase (HRP) at 37°C for 1 h. After incubation, the membranes were washed with TBST and then developed by HRP electrochemiluminescence. Following the development, the film was washed, dried, scanned and recorded.
Statistical analysis
The statistical analyses were conducted with SPSS21.0 (SPSS; IBM Corp., Armonk, NY, USA). All data were calculated using the mean ± SD from the results. Differences between groups were highlighted and analyzed using a paired t-test. Multiple sets of data were analyzed using one-way analysis of variance. p<0.05 was considered as statistically significant.
Results
HCF had little toxicity on melanoma cells A375
Initially, we performed trypan blue staining to calculate living cells, further for evaluating the toxicity of HCF on A375 cells in vitro. After 12 h of culturing, in comparison with the control group (cells were treated with 10% FBS), the cell survival rate was recorded to be elevated in the 10%, 20% and 30% HCF groups (the 20% HCF group > the 30% HCF group), indicating no obvious inhibitory effect (all p>0.05); these results indicate that HCF had no toxicity on melanoma A375 cells. Besides, no significant differences were observed among the normal and control groups (p>0.05) (Figure 1).
A375 cells grow significantly after HCF treatment
As shown in Figure 2, cells in the normal group had general status, while cells in the control group had regular morphology and clear outline as well as translucent soma. In comparison with the control group, cells treated with Echinococcus granulosus cyst in the 10% HCF, 20% HCF and 30% HCF groups had increased cell density, and no significant differences were observed in cell morphology among these three groups (all p>0.05).
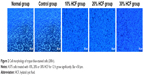  | Figure 2 Cell morphology of trypan blue-stained cells (200×). |
HCF promotes A375 cell viability
MTT assay did not highlight that any significant differences in cell proliferation at 3 h were found among the five groups (all p>0.05). At 6, 12 and 24 h, cells in the 10% HCF, 20% HCF and 30% HCF groups had higher OD values than the control group (p<0.05), as well as higher OD value in cells of the 20% HCF group compared to cells in 30% HCF group (p<0.05), suggesting that HCF promotes the proliferation of melanoma A375 cell, whereas the activating effect was retarded after reaching a certain concentration. Cells in the control group had increased OD values over time, same as cell viability, and no significant difference was observed in the normal group (Figure 3).
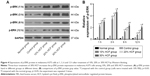
HCF increases expressions of p-ERK and PCNA
In comparison with the control and normal groups, p-ERK and PCNA expressions in cells among the 10% HCF, 20% HCF and 30% HCF groups evidently increased at 1 h interval (all p<0.05). With the increase in HCF concentration, p-ERK and PCNA expressions also increased. The expressions decreased at 6 and 12 h compared to the expressions at 3 h. On comparing with the control group, it was observed that the cells in the 10% HCF, 20% HCF and 30% HCF groups had higher p-ERK and PCNA expressions (p<0.05) (Figures 4 and 5).
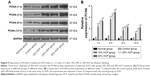
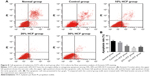
HCF accelerates A375 cell-cycle progression
After 12 h of culturing, HCF induced the transformation from G0/G1 phase to S phase of melanoma A375 cells to increase the S phase. Also, in accordance with the aforementioned statement, a higher HCF concentration corresponded to stronger enhancement (Figure 6). After 3 h of culturing, the expression of cyclin A, cyclin D1 and cyclin E increased in cells in the 10% HCF, 20% HCF and 30% HCF groups compared with the control group (the 20% HCF group > the 30% HCF group). No such increase or difference was observed in cyclin B1 expression between the 10% HCF, 20% HCF and 30% HCF groups and the control group (Figure 7).
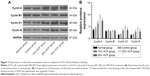
HCF inhibits A375 cell apoptosis
After 12 h of culturing, cell apoptosis and expressions of some related proteins were detected. In comparison with the control group, cells in the 10% HCF, 20% HCF and 30% HCF groups had a significantly decreased cell apoptotic rate (the 30% HCF group > the 20% HCF group), while the normal group had the highest apoptotic rate among the five groups. It was recorded that HCF increased the expression of procaspase-3 (the precursor of apoptosis-related protein caspase-3) and antiapoptotic protein Bcl-2, while simultaneously decreased the expression of proapoptotic protein Bax, thus inhibiting cell apoptosis. Cells in the 20% HCF group had a higher inhibitory effect than cells in the 30% HCF group, and apoptosis-related protein depicted corresponding changes (p<0.05) (Figures 8 and 9).
Discussion
Melanoma is a kind of the severe malignant skin cancers for which very few effective therapies exist.18 Furthermore, its incidence has increased over the course of the last decade.19 As a molecular abnormality, it has been thought to be associated with melanoma progression,20 figuring out that the relevant molecular mechanism in melanoma may play a contributing factor to discovery of a new effective therapeutic target.
The observations of this present study indicated that HCF promoted the expression of ERK and PCNA alongside promoting the proliferation of melanoma A375 cells. Zhang et al demonstrated that HCF could induce LX-2 cells proliferation in cystic echinococcosis.9 Besides, Chookami et al found that antitumor effect of HCF may be related to antigenic similarities existing between hydatid cyst and melanoma cancer cells.21 In addition, the ERK signaling pathways are the chief mechanisms responsible for controlling cell survival, proliferation, differentiation, motility and metabolism in response to extracellular cues.22 The compounds targeting components of ERK signaling, including RAF inhibitors and MEK inhibitors, were reported to result in substantial improvement in clinical outcome of patients suffering with metastatic melanoma.23 A study conducted by Zhang et al showed that in melanoma cell lines, activation of the MAPK family and especially of ERK1/2 was the common cause of resistance for apoptosis.24 Additionally, the expression of activated ERK1/2 in melanocytic lesions has been proven to be associated with malignant potential, suggesting that activated ERK1/2 may play an important role in melanoma progression.25 Also, inhibitors such as ERK1/2 can promote apoptosis, reduce their biological activates, and inhibit the conversion of cellular phenotype by inhibiting the deposition of extracellular matrix in malignant mesothelioma cells.26 A previous study demonstrated that higher concentrations of HCF could lead to higher mRNA levels of ERK1/2,27 which is in consensus with our study. Also, PCNA, a key coordinator of DNA replication, is involved in DNA processes such as DNA repair and chromatin/epigenetic maintenance.28 During these events, PCNA serves as the DNA sliding clamp and binding platform, binding and recruiting other proteins to ensure genome stability.29 As proven by previous evidence, anorectal malignant melanoma patients with lower PCNA level presented a survival advantage over those with higher PCNA level.30 The study by Li et al was consistent with our study and clarified that HepG2 cells exposed to HCF showed a significantly induced expression of PCNA and might stimulate cell proliferation.31
Finally, HCF is considered to upregulate the procaspase-3, the precursor of apoptotic protein caspase-3, and anti-apoptosis protein Bcl-2, while also downregulating proapoptotic protein Bax, indicating that HCF inhibits A375 cell apoptosis. Spotin reported that apoptosis of germinal layer of fertile cysts is possibly one of the suppression mechanisms in hydatidosis patients, in contrast to lymphocytes apoptosis by modulator of hydatid fluid, one of the hydatid cyst survival mechanisms.32 Proteins of the Bcl-2 family regulate the mitochondrial pathway of apoptosis, and Bcl-2 can block apoptosis in response to varieties of insults by preventing the activation and homo-oligomerization of both Bax and Bak.33 Activated caspase-3 is an essential executioner in apoptosis, involved in the growth stimulation.34 The conversion of procaspase-3 to caspase-3 allows induction of apoptosis even in cells possessing defective apoptotic machinery.35 Upon apoptosis induction, Bax is inserted into the outer mitochondrial membrane,36 and neuronal apoptosis is mediated by caspase-3 activated by Bax.37 Mokhtari Amirmajdi et al found that the expression of the Bax as a proapoptotic molecule increased and the expression level of Bcl-2 mRNA as an antiapoptotic molecule reduced in the fertile HCF-treated lymphocytes relative to the infertile HCF-treated lymphocytes and control.38
To conclude, HCF could promote the expression of ERK and PCNA, and the transition from G0/G1 phase to S phase with the proliferation of melanoma A375 cells, and inhibit A375 cell apoptosis by upregulating the procaspase-3 and antiapoptosis protein Bcl-2, and downregulating proapoptotic protein Bax. In this study, we observed that the effect of 20% HCF on cell function was the strongest among 10%, 20% and 30% HCF treatment options. The characteristics of HCF may explain this phenomenon, which need to be further investigated in future studies. However, due to limitation of experimental time and size, the specific interaction mechanism between HCF and melanoma cells remains unclear, therefore prompting requirement for detailed further study.
Acknowledgment
We would like to acknowledge the helpful comments on this paper received from our reviewers.
Disclosure
The authors report no conflicts of interest in this work.
References
Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355(1):51–65. | ||
Meeralakshmi P, Shah PK, Narendran V. Experiences of two different modalities in the management of choroidal melanoma in the Asian Indian population. South Asian J Cancer. 2017;6(3):134–136. | ||
Thalanayar PM, Agarwala SS, Tarhini AA. Melanoma adjuvant therapy. Chin Clin Oncol. 2014;3(3):26. | ||
Lee AY. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 2015;28(6):648–660. | ||
Takeuchi N, Miyazawa S, Ohno Z, et al. A case of spontaneous tumor lysis syndrome in malignant melanoma. World J Oncol. 2016;7(2–3):40–44. | ||
Unal VM, Ozdemir N, Karadag A, Oguzoglu S, Celik H. Primary sacral hydatid cyst causing cutaneous fistula. J Coll Physicians Surg Pak. 2017;27(5):311–312. | ||
McManus DP. Current status of the genetics and molecular taxonomy of Echinococcus species. Parasitology. 2013;140(13):1617–1623. | ||
Ghazani MHM, Valilou MR, Kharati FB, Zirak K. Prevalence of sheep liver hydatid cyst in the northwest region of Iran. Asian J Anim Vet Adv. 2008;3(1):30–35. | ||
Zhang C, Wang L, Ali T, et al. Hydatid cyst fluid promotes peri-cystic fibrosis in cystic echinococcosis by suppressing miR-19 expression. Parasit Vectors. 2016;9(1):278. | ||
Daneshpour S, Bahadoran M, Hejazi SH, et al. Common antigens between hydatid cyst and cancers. Adv Biomed Res. 2016;5(1):9. | ||
Gelmedin V, Caballero-Gamiz R, Brehm K. Characterization and inhibition of a p38-like mitogen-activated protein kinase (MAPK) from Echinococcus multilocularis: antiparasitic activities of p38 MAPK inhibitors. Biochem Pharmacol. 2008;76(9):1068–1081. | ||
Wellbrock C. Mapk pathway inhibition in melanoma: resistance three ways. Biochem Soc Trans. 2014;42(4):727–732. | ||
Evison BJ, Actis ML, Wu SZ, et al. A site-selective, irreversible inhibitor of the DNA replication auxiliary factor proliferating cell nuclear antigen (PCNA). Bioorg Med Chem. 2014;22(22):6333–6343. | ||
Lin RY, Wang JH, Lu XM, et al. Components of the mitogen-activated protein kinase cascade are activated in hepatic cells by Echinococcus multilocularis metacestode. World J Gastroenterol. 2009;15(17):2116–2124. | ||
Bellanger AP, Mougey V, Pallandre JR, Gbaguidi-Haore H, Godet Y, Millon L. Echinococcus multilocularis vesicular fluid inhibits activation and proliferation of natural killer cells. Folia Parasitol (Praha). 2017;64:pii:2017.029. | ||
Aghayan DL, Kazaryan AM, Fretland AA, et al. Laparoscopic liver resection for metastatic melanoma. Surg Endosc. Epub 2017 Sep 15. | ||
Kello M, Drutovic D, Pilatova MB, et al. Chalcone derivatives cause accumulation of colon cancer cells in the G2/M phase and induce apoptosis. Life Sci. 2016;150:32–38. | ||
Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–819. | ||
Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445(7130):851–857. | ||
Dhomen N, Marais R. New insight into BRAF mutations in cancer. Curr Opin Genet Dev. 2007;17(1):31–39. | ||
Chookami MB, Sharafi SM, Sefiddashti RR, et al. Effect of two hydatid cyst antigens on the growth of melanoma cancer in C57/black mice. J Parasit Dis. 2016;40(4):1170–1173. | ||
Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36(6):320–328. | ||
Iwasa H, Kubota K, Hamada N, Nogami M, Nishioka A. Early prediction of response to neoadjuvant chemotherapy in patients with breast cancer using diffusion-weighted imaging and gray-scale ultrasonography. Oncol Rep. 2014;31(4):1555-1560. | ||
Zhang XD, Borrow JM, Zhang XY, Nguyen T, Hersey P. Activation of ERK1/2 protects melanoma cells from TRAIL-induced apoptosis by inhibiting Smac/DIABLO release from mitochondria. Oncogene. 2003;22(19):2869–2881. | ||
Zhuang L, Lee CS, Scolyer RA, et al. Activation of the extracellular signal regulated kinase (ERK) pathway in human melanoma. J Clin Pathol. 2005;58(11):1163–1169. | ||
Jia P, Hu Y, Li G, et al. Roles of the ERK1/2 and PI3K/PKB signaling pathways in regulating the expression of extracellular matrix genes in rat pulmonary artery smooth muscle cells. Acta Cir Bras. 2017;32(5):350–358. | ||
Ren B, Fan HN, Deng Y, Wang HJ, Ren L. [Effect of Echinococcus multilocularis cyst fluid on the expression of five MAPK-pathway genes of rat hepatic stellate cells]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2015;33(2):114–117, 121. | ||
Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129(4):665–679. | ||
Mailand N, Gibbs-Seymour I, Bekker-Jensen S. Regulation of pcna-protein interactions for genome stability. Nat Rev Mol Cell Biol. 2013;14(5):269–282. | ||
Ben-Izhak O, Bar-Chana M, Sussman L, et al. Ki67 antigen and pcna proliferation markers predict survival in anorectal malignant melanoma. Histopathology. 2002;41(6):519–525. | ||
Li CW, Zhao JM, Zhang CS, et al. [Effect of echinococcus granulosus cyst fluid on the proliferation of hepg2 cells]. Zhonghua Gan Zang Bing Za Zhi. 2012;20(12):930–934. | ||
Spotin A, Majdi MM, Sankian M, Varasteh A. The study of apoptotic bifunctional effects in relationship between host and parasite in cystic echinococcosis: a new approach to suppression and survival of hydatid cyst. Parasitol Res. 2012;110(5):1979–1984. | ||
Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122(Pt 4):437–441. | ||
Huang Q, Li F, Liu X, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17(7):860–866. | ||
Putt KS, Chen GW, Pearson JM, et al. Small-molecule activation of procaspase-3 to caspase-3 as a personalized anticancer strategy. Nat Chem Biol. 2006;2(10):543–550. | ||
Nagaraj NS, Anilakumar KR, Singh OV. Diallyl disulfide causes caspase-dependent apoptosis in human cancer cells through a Bax-triggered mitochondrial pathway. J Nutr Biochem. 2010;21(5):405–412. | ||
Kishi T, Hirooka Y, Konno S, Ogawa K, Sunagawa K. Angiotensin II type 1 receptor-activated caspase-3 through ras/mitogen-activated protein kinase/extracellular signal-regulated kinase in the rostral ventrolateral medulla is involved in sympathoexcitation in stroke-prone spontaneously hypertensive rats. Hypertension. 2010;55(2):291–297. | ||
Mokhtari Amirmajdi M, Sankian M, et al. Apoptosis of human lymphocytes after exposure to hydatid fluid. Iran J Parasitol. 2011;6(2):9–16. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.