Back to Journals » International Journal of Women's Health » Volume 12
Modulating Anxiety and Functional Capacity with Anodal tDCS Over the Left Dorsolateral Prefrontal Cortex in Primary Dysmenorrhea
Authors Dutra LRDV , Pegado R , Silva LK , da Silva Dantas H, Câmara HA, Silva-Filho EM , Correia GN , Micussi MTABC
Received 8 August 2019
Accepted for publication 3 January 2020
Published 5 April 2020 Volume 2020:12 Pages 243—251
DOI https://doi.org/10.2147/IJWH.S226501
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Larissa Ramalho Dantas Varella Dutra,1 Rodrigo Pegado,2 Luana Karyne Silva,2 Hégila da Silva Dantas,2 Hialison Andrade Câmara,2 Edson Meneses Silva-Filho,2 Grasiéla Nascimento Correia,2 Maria Thereza Albuquerque Barbosa Cabral Micussi1
1Health Science Center, Federal University of Rio Grande do Norte, Rio Grande do Norte, Brazil; 2Graduate Program in Rehabilitation Science, Federal University of Rio Grande do Norte, Rio Grande do Norte, Brazil
Correspondence: Edson Meneses Silva-Filho
Faculdade de Ciências da Saúde do Trairi - Universidade Federal do Rio Grande do Norte, FACISA/UFRN, Rua Teodorico Bezerra, Santa Cruz, RN 59200-000, Brazil
Tel +55 81 99470 6661
Email [email protected]
Background: Primary dysmenorrhea is a common and often debilitating condition affecting 40– 90% of menstruating women. This condition reduces functionality, quality of life, and social activities. Transcranial direct current stimulation (tDCS) has been used in many chronic pain syndromes, with evidence of improved pain, functionality, and mood in women with primary dysmenorrhea. The objective of this study was to determine whether tDCS could offer clinical benefits on pain, anxiety, affectivity, and functionality in women with primary dysmenorrhea.
Methods: This parallel, sham, randomized, double-blind trial was conducted with 26 women randomized into sham tDCS and active tDCS. Anodal tDCS was applied for 5 consecutive days over F3 corresponding to the left dorsolateral prefrontal cortex (DLPFC) and the cathode electrode over Fp2 for 20 min with an intensity of 2 mA. A numeric rating scale (NRS) was used to assess pain, anxiety, positive and negative affect, and submaximal aerobic performance during two consecutive menstrual cycles.
Results: No significant interaction was found between intervention and time on the NRS [F(2,44) = 1.358, p = 0.26], and a significant main effect of time [F(2,44) = 4.446, p = 0.01] was found. The active group showed a significant reduction in anxiety (p = 0.03) with a mean difference of 5.12 (95% CI 0.79 to 11.05). No significant differences in positive and negative affect were found (p = 0.95 and p = 0.15, respectively). Submaximal aerobic performance was significantly greater in the active group [F(2,21) = 5.591, p = 0.02], with a mean difference of 70.87 (95% CI 8.53 to 133.21).
Conclusion: Anodal tDCS over the DLPFC seems to be an effective therapeutic approach for improving anxiety and functionality in women with primary dysmenorrhea.
Keywords: non-invasive brain stimulation, menstrual cycle, pain, anxiety, functional capacity
Introduction
Primary dysmenorrhea is defined as painful cramps in the lower abdominal or pelvic area with or without radiation to the back or legs.1 This is a common condition affecting adolescents and young women of reproductive age, which starts with menarche, and lasts for 24–72 h.2 Studies suggest a prevalence of primary dysmenorrhea between 40% and 90% of female adolescents, and 10–20% of young adults describe their suffering as so severe and distressing that it requires absence from school or work.3 Primary dysmenorrhea reduces motor functionality, quality of life social activities, and sometimes it is accompanied by nausea, backache, fatigue, diarrhea, sleeplessness, and nervousness.4,5 Studies have shown that many factors are associated with this syndrome; however, its etiology is still unknown.5–7 Significant relationships between some mental health components, such as depression, anxiety, and stress, and primary dysmenorrhea were related in some studies and may contribute toward increased pain and low functionality.8
Dysmenorrhea is considered to be one of the leading causes of pain, in the absence of underlying pathology, and can be considered part of the medically unexplained syndromes.9 This condition could be considered a genuine chronic pain syndrome with long intermittent periods of pain.3 Findings in women with primary dysmenorrhea revealed changes in functional connectivity of the anterior cingulate cortex, alterations in brain metabolism, and disturbance in pain modulatory systems.2,3 Pain-related regions, including the medial prefrontal cortex, posterior cingulate cortex, and insula, exhibit abnormal functional and structural changes in otherwise healthy women with primary dysmenorrhea.3 These regions are part of the pain neuromatrix and are associated with cognitive and emotional processing of pain. Patients with chronic pain possibly have altered cross-network connectivity and imbalance between the systems.3
Furthermore, recurrent and chronic menstrual pain causes psychological distress, and the most prevalent conditions are anxiety disorders with or without depression.8 Anxiety fluctuations are associated with menstrual symptoms and can increase dysmenorrhea and menstrual cycle problems.10 Anxiety and loss of social support networks may increase the feeling of menstrual pain and have an important impact on quality of life.8
Medications and physical therapy have been recommended for the management of primary dysmenorrhea; however, the data supporting their effectiveness are limited.1 Transcranial direct current stimulation (tDCS) has been used in many chronic pain syndromes, showing improvements in pain, functionality, and mood state.11 tDCS uses an electrical load transmitted through the skull, where it alters the load of the cell membrane and thereby changes cortical excitability and apparently even mental processes.12 This is an easy and safe therapy with no serious side effects.13 The primary effect of tDCS on neurons is a subthreshold shift of resting membrane potentials toward depolarization or hyperpolarization, depending on current flow direction.13
For chronic pain syndromes, tDCS is commonly used with an anodal electrode over the primary motor cortex (M1), left dorsolateral prefrontal cortex (DLPFC), or primary visual cortex.13 The left DLPFC is stimulated because this area has been implicated in affective, cognitive, and attentional aspects of pain, and is a primary target of neuromodulation for affective disorders, including depression.13 The DLPFC is a superficial part of the medial pain pathway, which includes subjective pain processing, anticipated pain, and pain control.14 Studies have reported that anodal tDCS over the DLPFC reduced rated pain intensity in patients with chronic pain and enhanced mood in a series of psychiatric disorders.11,15,16
Considering these assumptions, we expect anodal tDCS targeting the left DLPFC to improve pain, positive and negative affectivity, anxiety, and functional capacity. Although evidence exists to support the use of tDCS in people with chronic painful symptoms, mood disturbance, and motor impairment, to date no clinical trial has analyzed the effects of tDCS in women with dysmenorrhea. If our hypothesis is confirmed, tDCS may be an alternative, low-cost, and safe treatment option that can be used by home-based or in-clinic therapy for women with primary dysmenorrhea. Moreover, owing to the direct influences of the DLPFC on the symptoms of primary dysmenorrhea, tDCS could help to improve it. In this study, we aimed to analyze the effects of anodal tDCS over the DLPFC on pain, functionality, and mood in women with primary dysmenorrhea.
Materials and Methods
Participants and Setting
This parallel, sham, randomized, double-blind trial followed the CONSORT recommendations.17 This study was approved by the local institutional ethics committee (Federal University of Rio Grande do Norte) (number 2.932.953), and registered on Rebec (Brazilian platform of clinical trials) (identifier RBR-77Z6Q8). All participants gave written informed consent according to the Declaration of Helsinki and to resolution no. 466/12 of the National Health Council. The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
From June 2017 to June 2018, patients were recruited in Natal, Brazil, and the study was conducted at the Federal University of Rio Grande do Norte. The diagnosis of primary dysmenorrhea was defined according to No. 345 – Primary Dysmenorrhea Consensus Guideline.10 Patients were selected from a specialized outpatient service and regarded as suitable to participate in this study if they fulfilled the following inclusion criteria: aged from 18 to 40 years; presented a mean pain score of at least 3 on the numeric rating scale (NRS) during the menstrual cycle preceding the evaluation; had a regular menstrual cycle; not lactating; no history of brain surgery, tumor, dizziness, or epileptic disease; chronic genitourinary infections, alcohol or drug abuse; and did not have metal implants in the head. Women who became pregnant and/or started taking analgesic medicines were excluded.
Sample size was estimated based on statistical considerations for a parallel trial and on a previous study with tDCS and primary dysmenorrhea (NRS was used).11 The effect size was calculated using G-Power 3.1.9.2. Sample size was estimated based on the assumption of significance of 0.05, power of 95%, with 0.5 effect size and two groups. According to this methodology, the sample size resulted in 22 participants. We decided to add four more patients to prevent any reduction in power in case of patient dropout.
Study Design
A total of 67 individuals were recruited for the verification of inclusion and exclusion criteria. Thirty-seven individuals were excluded for not meeting the inclusion criteria and four declined to participate. Randomization was done with 26 individuals, through a numerical sequence generated by an allocated computer using appropriate software (www.randomization.com) to assign each participant to either the active tDCS group or the sham tDCS group, by an independent researcher who was not involved with either stimulation or assessments. Each person was equally likely to belong to either group. Allocation was concealed through opaque envelopes. Participants and researchers involved in assessments and interventions were blind to group allocation throughout the trial (Figure 1). Patients were considered dropouts if they missed 1 day of treatment or failed to provide all baseline or post-intervention data.
 |
Figure 1 CONSORT flowchart for the study. |
Intervention
Direct current stimulation was administered using a continuous electric stimulator, with three energy batteries (9 V) connected in parallel. The maximum energy output was 10 mA and was controlled by a professional digital multimeter (DT832; WeiHua Electronic Co. Ltd, China) with a standard error of ±1.5%. Silicone electrodes were placed into a 35 cm2 (5 cm × 7 cm) square sponge soaked in saline solution (150 mMol of NaCl diluted in water Milli-Q). Rubber bandages were used to hold electrodes in place for the duration of stimulation. For electrode placement, the 10/20 EEG system with the anode electrode was used, placed over F3 for DLPFC stimulation, and the cathode electrode was placed over the contralateral supraorbital area (Fp2). For both sham and active stimulation, one 20 min session was delivered each day for 5 consecutive days. For the sham tDCS, electrodes were placed at the same positions as for the active tDCS, but the current was turned off after 30 s of stimulation, according to methods of clinical studies in brain stimulation.14 These methods provide the same initial sensory feelings of active tDCS conditions, specifically, itching, and tingling feelings on the scalp, for the first few seconds of tDCS. Both groups related the same sensation during the 30 s period. Stimulation was always performed at the same time of day, according to the participant’s preference.
Outcomes
Baseline demographic data, including age, body mass index, menstrual cycle duration, and sociodemographic factors, of all participants were recorded. The primary outcome measure was pain, and the secondary outcome measures were positive and negative affect, anxiety, and submaximal aerobic performance.
Outcome measures were assessed during two consecutive menstrual cycles (Figure 2). Baseline data were assessed on the first day of menstruation over the first menstrual cycle. Intervention was performed about 4 days before beginning the second menstrual cycle (the period when volunteers informed researchers of the onset of pelvic pain-related symptoms). For all participants, the intervention overlapped by 1 day with the onset of menses. The post-evaluation was conducted on the first day of menstruation of the second menstrual cycle, and all physical and mood parameters were assessed. All subjects completed several questionnaires and underwent physical examinations.
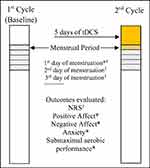 |
Figure 2 Research protocol.Notes: ƚOutcome evaluated on first, second, and third days of menstruation. *All outcomes evaluated only on the first day of menstruation. |
The NRS was used to assess the primary outcome. This straight 10-cm scale is numbered from 0 to 10, where 0 represents no pain and 10 is the most pain.19 Subjects were instructed to mark the number that best reflected the symptoms of pain at that moment.19
The severity of anxiety symptoms was measured using the Brazilian version of the Hamilton Anxiety Scale (HAS). The HAS was administered by an interviewer who asked a series of semi-structured questions related to symptoms of anxiety. The interviewer rated the individuals on a five-point scale for each of the 14 items. Seven of the items specifically address psychic anxiety, and the remaining seven somatic anxieties. The values on the scale range from 0 to 4: 0 means that there is no anxiety, 1 indicates mild anxiety, 2 indicates moderate anxiety, 3 indicates severe anxiety, and 4 four indicates very severe or grossly disabling anxiety. The total anxiety score ranges from 0 to 56.20
Positive affect (PA) and negative affect (NA) were measured using the Positive and Negative Affect Schedule.21 This questionnaire has 20 items, 10 on PA and 10 on NA, and participants responded to each item on a five-point Likert-type scale (1: very slightly or not at all; 2: a little; 3: moderately; 4: quite a bit; and 5: extremely). The time-frame adopted in this study was “in general.” The scores range is 10–50 for both PA and NA.21
The Six-Minute Walk Test (6MWT) was used to assess the submaximal level of functional capacity, indicating endurance. This aerobic submaximal test reflects the activities of daily life and better indicates the functional level of daily physical activities.22 The 6MWT has also been used as a one-time measure of functional status of patients with many clinical conditions, including patients with chronic pain such as fibromyalgia and lower back pain. The 6MWT measures the maximum distance that subjects can walk, as quickly as possible, during 6 min.22
Data Analyses
Analyses were performed using SPSS software (version 19.0; Chicago, USA) and GraphPad Prism 5. Quantitative variables were expressed as means and standard deviations. The Shapiro–Wilk and Levene’s tests were applied to assess the normality of the distribution and homogeneity of variance of the data, respectively. Missing data were treated by intention-to-treat analysis. An unpaired t-test was used to compare numerical characteristics between groups. Differences in sociodemographic characteristics between groups were calculated using the chi-squared test.
The effects of stimulation on pain were calculated using a mixed ANOVA in which the dependent variable was the score of pain, and the independent fixed variables were the time (day 1, day 2, and day 3), the group of stimulation (active and sham), and the interaction term group vs time. To determine the difference between groups for each category of time and vice versa, three separate between-subjects ANOVAs were performed. When appropriate, post hoc comparisons were carried out using Bonferroni correction for multiple comparisons. Greenhouse–Geisser correction was applied when the assumption of sphericity was violated. To assess psychological outcomes, an unpaired t-test or paired t-test was used. One-way ANCOVA was used to determine the effect of tDCS on post-intervention for functional capacity. The main purpose of running the one-way ANCOVA was to establish whether there were any statistically significant group differences on the dependent variable after adjusting for the time (before and after). Partial η2 was used to calculate the effect size, where η2 = 0.01 was considered small, η2 = 0.06 moderate, and η2 = 0.14 large effect. Statistical significance was set at p ˂ 0.05.
Results
Table 1 shows the demographic and clinical characteristics of the two groups. All patients tolerated tDCS well and there were only minor related adverse effects, such as skin tingling. Losses during the period of treatment occurred due to incompatibility with the intervention period.
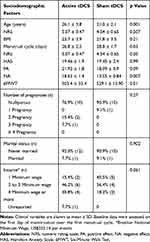 |
Table 1 Sociodemographic and Clinical Variables |
Effect of Stimulation on Pain Intensity
To assess pain, the first analyses were made using the NRS on the first, second, and third days of the first menstrual cycle (without tDCS), and in the first, second, and third days of the second menstrual cycle (with tDCS).
In the first menstrual cycle, there were no statistically significant interactions between groups and time [F(2,44) = 0.26, p = 0.766, partial η2 = 0.01]. Pain levels did not show significant differences between groups on the first (p = 0.508), second (p = 0.673), and third days (p = 0.886) during the first menstrual cycle, suggesting that pain changed equally in each group. In fact, it is normal that pain decreases along these days after menstruation. This is demonstrated with a significant main effect of time [F(2,44) = 35.724, p ˂ 0.0001, partial η2 = 0.35].
In the second menstrual cycle, we assessed the interaction time vs group of stimulation. There was no statistically significant interaction between intervention and time on pain [F(2,44) = 1.358, p = 0.26, partial η2 = 0.05]. There was a significant main effect of time [F(2,44) = 4.446, p = 0.01, partial η2 = 0.168] (Figure 3). When a one-way ANOVA was used, the active group showed a significant decrease in pain (p = 0.01) and day 1 had a significant difference compared with day 3 (p = 0.03). A reduction of 63.16% was found in the active group when day 1 was compared with day 3 after tDCS (45.36% before tDCS) (Figure 3). The sham group did not show significant differences. Intergroup comparison did not show significant differences on day 1 (p = 0.95), day 2 (p = 0.79), or day 3 (p = 0.15) after stimulation.
Effects of Stimulation on Psychological and Functional Outcomes
Figure 3 shows the results for psychological and functional outcomes. Baseline anxiety did not show significant differences between groups (p = 0.99). The active group showed a significant reduction in anxiety (p = 0.03), with a mean difference of 5.12 (95% CI 0.79 to 11.05). The active and sham groups did not show significant differences in PA before vs after tDCS (p = 0.95 and p = 0.15 respectively), with mean difference of 5.12 in the active group (95% CI 3.37 to 4.10) and in intergroup analysis (p = 0.32).
NA showed a significant difference between groups at baseline (p = 0.007). When we compared before vs after tDCS, no differences were seen in intragroup (p = 0.15) and intergroup (p = 0.26) analyses, with a mean difference of 0.9 (95% CI 2.85 to 4.66) in the active group.
After adjustment for pre-intervention distance in submaximal aerobic performance (TC6ʹ), there was a significant difference in post-intervention distance between the interventions [F(2,21) = 5.591, p = 0.02, partial η2 = 0.21]. TC6ʹ was significantly greater in the active group (584.18 ± 120.16 meters) than in the sham group (531.18 ± 56.4 m), with a mean difference of 70.87 m (95% CI 8.53 to 133.21 m) (p = 0.028).
Discussion
The present study aimed to assess the effects of tDCS applied over the DLPFC (F3/Sp2 montage) on pain, mood, and functionality in women with primary dysmenorrhea. The results showed no significant effect on pain improvement and affectivity. However, the average decrease in the NRS was 20.05% and 63.16% after applying tDCS on the DLPFC in the sham and active groups, respectively. Enhancements in anxiety and functional capacity were observed only in the active group.
In recent years, several studies have investigated the use of tDCS in analgesia, functionality, and mood disturbance, with good results, but meta-analysis suggests further studies including different montages and numbers of sessions.12,14,23,24 Nevertheless, motor cortex stimulation seems to be the best target to improve pain in various chronic pain syndromes.12,24 tDCS has been used to address a variety of pathological pain conditions, including fibromyalgia, chronic post-stroke pain, and complex regional pain syndrome.23 Fregni et al studied women with fibromyalgia and showed a significant reduction in pain using anodal tDCS over the primary motor cortex, whereas no pain improvement was found when anodal tDCS was applied over the DLPFC.25 Other authors suggest a positive effect of DLPFC stimulation on pain-related emotions.26 DLPFC stimulation probably mediates analgesic effects by modulating affective–emotional networks related to pain.26 Furthermore, stimulation over the DLPFC was significantly associated with upregulated positive reactions to positive emotional stimuli and downregulated negative reactions to emotional stimuli.26 tDCS application over the DLPFC probably operates by increasing prefrontal regulation of limbic responses to negative stimuli, including negative emotional processing.28
Mental disorders including depression and anxiety have also been explored.29 Several review articles suggest the therapeutic efficacy of tDCS in treating these psychiatric disorders, showing evidence that excitatory stimulation of the left prefrontal cortex can reduce symptom severity in anxiety and depression.27 Also, depression and anxiety are strongly associated with menstrual pain in primary dysmenorrhea.29 Emotional distress in women who experience cyclical primary dysmenorrheic pain has been reported, with poorer mood state during menstruation than in pain-free women.29 Pain experiences in females are usually more sophisticated than in males, and primary dysmenorrhea subjects are vulnerable to anxiety.3 This study showed a significant enhancement in anxiety, only in the active group. Primary dysmenorrhea in women exhibited abnormal function in the ventromedial prefrontal cortex (according to trait-related regional homogeneity), part of the default mode network, during the periovulatory phase.3 These alterations may be caused by cumulative menstrual pain, and an inverse correlation between primary dysmenorrhea and anxiety was found.3 tDCS over the DLPFC could improve anxiety and functionality specifically during menstruation. No previous studies have evaluated the effects on anxiety of anodal tDCS over the left DLPFC.
Women with dysmenorrhea present scores significantly lower in physical and functional domains.30 Pain and mood disturbance were associated with low functionality during some days before and during menstruation.30 Functional capacity was assessed using the 6MWT. A mean difference of 70.87 m was shown between groups with an improvement only in the active group. The 6MWT is easy to administer, is well tolerated, reflects activities of daily living, and estimates the submaximal level of functional capacity.22 Improvement in functional capacity during the period of menstruation can positively affect personal lives, including family relationships, friendships, school/work performance, and social and recreational activities.2,31,32
For treatment of menstrual pain intensity and to enhance quality of life, physiotherapists could consider using heat, transcutaneous electrical nerve stimulation, and yoga in the management of primary dysmenorrhea.33 No other rehabilitation treatments showing significant effects were found for primary dysmenorrhea.33 According to Kannan and Claydon, among women who sought treatment, 73% took analgesics and 58% had physiotherapy management.33 Exploring new avenues of rehabilitation and maintenance of functionality through non-pharmacological and/or non-invasive intervention is necessary to prevent dysfunction and poor quality of life in women with primary dysmenorrhea. tDCS could be a safe, low-cost, and promising non-invasive technique for relieving symptoms in women with primary dysmenorrhea and other pelvic pain disturbance.
This study had several limitations. The evaluation period of two consecutive cycles was relatively short and observation of more cycles is needed to evaluate the long-term effects of 5 days of tDCS. Some sociodemographic factors, including age, days of menstrual cycle, and NA were different between groups at baseline.
These preliminary results provide initial evidence that anodal tDCS over the DLPFC reduces anxiety and improves functionality in women with primary dysmenorrhea. Future investigations involving more tDCS sessions and different montages could prove the benefits not only in mood and physical activity but also in pain relief and quality of life. Long-term follow-up with three or more menstrual cycles will also clarify whether relief of symptoms is possible.
Conclusion
Anodal tDCS over the left DLPFC seems to be an effective therapeutic approach for improving anxiety and functional capacity in patients with primary dysmenorrhea. Although painful symptomatology decreased, no significant effects were seen between groups.
Ethics Approval and Informed Consent
This study was approved by the local institutional ethics committee from the Federal University of Rio Grande do Norte (number 2.932.953) and Brazilian platform of clinical trials (identifier RBR-77Z6Q8). Informed consent was provided by all participants according to the Declaration of Helsinki.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Osayande AS, Mehulic S. Diagnosis and initial management of dysmenorrhea. Am Fam Physician. 2014;89(5):341–346.
2. Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain. 2011;152(9):1966–1975. doi:10.1016/j.pain.2011.03.029
3. Wu T-H, Tu C-H, Chao H-T, et al. Dynamic Changes of Functional Pain Connectome in Women with Primary Dysmenorrhea. Sci Rep. 2016;6:24543. doi:10.1038/srep24543
4. Bajalan Z, Moafi F, MoradiBaglooei M, Alimoradi Z. Mental health and primary dysmenorrhea: a systematic review. J Psychosom Obstet Gynaecol. 2018:1–10. doi:10.1080/0167482X.2018.1470619
5. Li WC, Tu CH, Chao HT, Yeh TC, Chen LF, Hsieh JC. High prevalence of incidental brain findings in primary dysmenorrhoea. Eur J Pain (United Kingdom). 2015;19(8):1071–1074. doi:10.1002/ejp.639
6. Liu P, Y L, Wang G, et al. Changes of functional connectivity of the anterior cingulate cortex in women with primary dysmenorrhea. Brain Imaging Behav. 2017:1–8. doi:10.1007/s11682-017-9730-y.
7. Liu P, Yang J, Wang G, et al. Altered regional cortical thickness and subcortical volume in women with primary dysmenorrhoea. Eur J Pain (United Kingdom). 2016;20(4):512–520. doi:10.1002/ejp.753
8. Balik G, Üstüner I, Kağitci M, Şahin FK. Is there a relationship between mood disorders and dysmenorrhea? J Pediatr Adolesc Gynecol. 2014;27(6):371–374. doi:10.1016/j.jpag.2014.01.108
9. Faramarzi M, Salmalian H. Association of psychologic and nonpsychologic factors with primary dysmenorrhea. Iran Red Crescent Med J. 2014;16(8). doi:10.5812/ircmj.16307
10. Burnett M, Lemyre M. No. 345-primary dysmenorrhea consensus guideline. J Obstet Gynaecol Can. 2017;39(7):585–595. doi:10.1016/j.jogc.2016.12.023
11. Pegado R, Silva LK, da Silva Dantas H, et al. Effects of Transcranial Direct Current Stimulation for Treatment of Primary Dysmenorrhea: Preliminary Results of a Randomized Sham-Controlled Trial. Pain Med. 2019;pii:pnz202. doi:10.1093/pm/pnz202
12. Lefaucheur JP. A comprehensive database of published tDCS clinical trials (2005—2016). Neurophysiol Clin. 2016;46(6):319–398. doi:10.1016/j.neucli.2016.10.002
13. Luedtke K, Rushton A, Wright C, Geiss B, Juergens TP, May A. Transcranial direct current stimulation for the reduction of clinical and experimentally induced pain: a systematic review and meta-analysis. Clin J Pain. 2012;28(5):452–461. doi:10.1097/AJP.0b013e31823853e3
14. Lefaucheur J-P, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56–92. doi:10.1016/j.clinph.2016.10.087
15. Mariano TY, van’t WM, Garnaat SL, Rasmussen SA, Greenberg BD. HHS public access. Pain Med. 2016;131(20):1796–1803. doi:10.1161/CIRCULATIONAHA.114.010270.Hospital
16. Iannone A, Cruz AP de M, Brasil-Neto JP, Boechat-Barros R. Transcranial magnetic stimulation and transcranial direct current stimulation appear to be safe neuromodulatory techniques useful in the treatment of anxiety disorders and other neuropsychiatric disorders. Arq Neuropsiquiatr. 2016;74(10):829–835. doi:10.1590/0004-282X20160115
17. Kuo MF, Chen PS, Nitsche MA. The application of tDCS for the treatment of psychiatric diseases. Int Rev Psychiatry. 2017;29(2):146–167. doi:10.1080/09540261.2017.1286299
18. Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials (Chinese version). J Chin Integr Med. 2010;8(7):604–612. doi:10.3736/jcim20100702
19. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF). Arthritis Care Res. 2011;63(SUPPL. 11):240–252. doi:10.1002/acr.20543
20. Hamilton M. Hamilton Anxiety Rating Scale (HAM-A). J Med. 1959;61(4):81–82.
21. Galinha IC, Pais-Ribeiro JL. Contribuição para o estudo da versão portuguesa da Positive and Negative Affect Schedule (PANAS): II – estudo psicométrico. Análise Psicológica. 2005;2(XXIII):219–227.
22. Crapo RO, Casaburi R, Coates AL, et al. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi:10.1164/rccm.166/1/111
23. Ne OC, Marston L, Spencer S, Lh D, Bm W. Non-invasive brain stimulation techniques for chronic pain (Review) summary of findings for the main comparison. Cochrane Database Syst Rev. 2018;3. doi:10.1002/14651858.CD008208.pub4.www.cochranelibrary.com.
24. Zhu CE, Yu B, Zhang W, Chen WH, Qi Q, Miao Y. Effectiveness and safety of transcranial direct current stimulation in fibromyalgia: a systematic review and meta-analysis. J Rehabil Med. 2017;49(1):2–9. doi:10.2340/16501977-2179
25. Fregni F, Gimenes R, Valle AC, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54(12):3988–3998. doi:10.1002/art.22195
26. Maeoka H, Matsuo A, Hiyamizu M, Morioka S, Ando H. Neuroscience letters influence of transcranial direct current stimulation of the dorsolateral prefrontal cortex on pain related emotions: a study using electroencephalographic power spectrum analysis. Neurosci Lett. 2012;512(1):12–16. doi:10.1016/j.neulet.2012.01.037
27. Vicario CM, Salehinejad MA, Felmingham K, Martino G, Nitsche MA. A systematic review on the therapeutic effectiveness of non-invasive brain stimulation for the treatment of anxiety disorders. Neurosci Biobehav Rev. 2019;96(2018):219–231. doi:10.1016/j.neubiorev.2018.12.012
28. Ironside M, Browning M, Ansari TL, et al. Effect of prefrontal cortex stimulation on regulation of amygdala response to threat in individuals with trait anxiety a randomized clinical trial. JAMA Psychiatry. 2019;02478(1):71–78. doi:10.1001/jamapsychiatry.2018.2172
29. Palm U, Hasan A, Strube W, Padberg F. tDCS for the treatment of depression: a comprehensive review. Eur Arch Psychiatry Clin Neurosci. 2016;266(8):681–694. doi:10.1007/s00406-016-0674-9
30. Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015;21(6):762–778. doi:10.1093/humupd/dmv039
31. Rodrigues AC, Gala S, Â N, et al. [Dysmenorrhea in adolescents and young adults: prevalence, related factors and limitations in daily living]. Acta Med Port. 2011;24(S2):383–392. Portuguese.
32. Tomás-Rodríguez MI, Palazón-Bru A, Martínez-St John DRJ, Navarro-Cremades F, Toledo-Marhuenda JV, Gil-Guillén VF. Factors associated with increased pain in primary dysmenorrhea: analysis using a multivariate ordered logistic regression model. J Pediatr Adolesc Gynecol. 2017;30(2):199–202. doi:10.1016/j.jpag.2016.09.007
33. Kannan P, Claydon LS. Some physiotherapy treatments may relieve menstrual pain in women with primary dysmenorrhea: a systematic review. J Physiother. 2014;60(1):13–21. doi:10.1016/j.jphys.2013.12.003
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

