Back to Journals » Clinical Ophthalmology » Volume 17
Modified Technique of Ex-PRESS® Filtration Device Combined with a Scleral Pocket for Hereditary Transthyretin Amyloidosis (hATTR) Secondary Open-Angle Glaucoma
Authors Vieira R , Marta A , Ferreira A , Figueiredo A, Reis RF, Sampaio I, Menéres MJ
Received 22 October 2022
Accepted for publication 14 December 2022
Published 31 January 2023 Volume 2023:17 Pages 403—411
DOI https://doi.org/10.2147/OPTH.S394360
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Rita Vieira,1 Ana Marta,1,2 André Ferreira,1,3 Ana Figueiredo,1 Rita Falcão Reis,1 Isabel Sampaio,1 Maria João Menéres1,2
1Ophthalmology Department of Centro Hospitalar Universitário do Porto (CHUPorto), Oporto, Portugal; 2Ophthalmology Teaching Department, Instituto de Ciências Biomédicas Abel Salazar (ICBAS), Oporto, Portugal; 3Anamoty Department of Faculdade de Medicina da Universidade do Porto (FMUP), Oporto, Portugal
Correspondence: Rita Vieira, Ophthalmology Department, Centro Hospitalar Universitário do Porto, Largo do Prof. Abel Salazar, Porto, 4099-001, Portugal, Tel +351913748812, Email [email protected]
Purpose: To evaluate the effectiveness and safety of a modified approach using the Ex-PRESS® implant combined with a scleral pocket in the management of secondary open-angle glaucoma in hereditary transthyretin amyloidosis (hATTR) at our department.
Methods: This was a retrospective analysis. The primary endpoints included Intraocular pressure (IOP) evaluation (baseline, 1st day, 1st week, 1, 3, 6, 12 months and at last follow-up) and number of hypotensive drugs (baseline, 6th, 12th months and at last follow-up). As secondary endpoints surgical complications, the need for additional glaucoma surgery and LogMAR BCVA were evaluated. Qualified and complete success were defined as ≥ 30% IOP decrease from baseline, with or without additional medications, respectively. The minimum follow-up was 12 months.
Results: A total of 32 eyes were included with a mean follow-up of 2.4± 2.9 years. IOP decreased significantly from baseline (27.4± 4.4 mmHg) to 1st day (5.00± 2.9 mmHg), 1st week (6.9± 4.1 mmHg), 1st month (11.7± 7.8 mmHg), 3rd month (11.6± 6.1 mmHg), 6th month (13.1± 6.8 mmHg), 12th month (12.0± 3.5 mmHg) and last visit (11.8± 2.4 mmHg), p< 0.001. There was also a significant reduction in the number of antiglaucoma medications from baseline (3.8± 0.6) and last follow-up (0.4± 0.8), p< 0.001. LogMAR BCVA remained stable (0.25± 0.26 at baseline and 0.25± 0.24 at last follow-up), p=0.767. Transient hypotony occurred in 17 eyes (53.1%), but only 11 (34.4%) exhibited anterior chamber shallowing and needed additional care, namely cycloplegic drops and viscoelastic injection. Complete surgical success was achieved in 22 eyes (68.8%) and qualified success in 6 eyes (18.8%). Four eyes (12.5%) needed additional glaucoma surgery.
Conclusion: The modified ExPRESS® technique appears to be effective, especially when low levels of IOP are required. Additionally, fewer anti-glaucoma drugs were necessary. In the other hand, hypotony was a common side effect with this procedure, although all patients were properly handled, preserving the surgical outcomes.
Keywords: amyloidosis, FAP glaucoma, hereditary transthyretin amyloidosis, hATTR
Introduction
Familial amyloidotic polyneuropathy is caused by mutations in the gene encoding transthyretin, a protein mostly produced by the liver. This is an autosomal dominant disorder with adult-onset and variable penetrance. The Val30Met mutation is the most common TTR variant in Portugal and around the world. Portugal, the site of the disease’s first description, is one of the three primary endemic clusters, along with Sweden and Japan.1,2 Nonetheless, migration has spread this disease to every country. In the north of Portugal, patients with hereditary transthyretin amyloidosis (hATTR) are referred to Centro Hospitalar Universitário do Porto (CHUPorto), a referral facility for this ailment, which explains our expertise with this kind of glaucoma.
Different organs, such as the peripheral nerves, the heart, the kidneys, the ocular tissues, and other organs and tissues become dysfunctional as a result of the disease’s multisystemic extracellular deposition of amyloid fibrils.1–3 Regarding ocular issues, patients with hATTR may experience retinal vascular changes, pupillary light-near dissociation, irregular pupil, optic neuropathy, abnormal conjunctival vessels, loss of corneal sensitivity, neurotrophic corneal ulcers, anterior capsule opacity of the lens, and vitreous opacities.4–6
TTR stabilizing proteins (Tafamidis), gene therapy (RNA silencing—Onpattro, Tegsedi), liver transplantation, and other systemic treatments are presently available for hereditary TTR amyloidosis. By lowering the systemic production of mutant TTR, they all significantly lengthen and improve the quality of life for these patients, but they have no impact on ocular TTR production. Thus, these patients have a higher prevalence of glaucoma, which is explained by the fact that the retinal pigment epithelium (RPE) and ciliary pigment epithelium manufacture TTR locally in the eye in addition to the liver.4,7,8 The difficulty in decreasing IOP and the faster glaucoma progression in hATTR patients, which usually requires rapid surgical intervention, may be caused by this continuing creation of mutant TTR.7,9,10
Hereditary transthyretin amyloidosis secondary open-angle glaucoma pathogenesis is still poorly understood. Some experts proposed mechanisms including amyloid deposition in the intertrabecular space and Schlemm’s canal or the deposition of perivascular amyloid in conjunctival and episcleral tissue contributing to increased episcleral venous pressure and rise intraocular pressure (IOP).9,10
There is a paucity of studies regarding glaucoma related to hATTR, and the majority have short samples. However, Marta et al demonstrated good long-term outcomes and minimal complication rates with Ahmed glaucoma valve (AGV) implantation in the largest reported series with hATTR glaucoma comprising 124 eyes.11 In fact, certain types of refractory glaucoma, such as hATTR-related glaucoma, are now mostly treated using aqueous humour drainage devices.12
The Ex-PRESS Glaucoma Filtration Device is a modified trabeculectomy in which the connection to the anterior chamber (AC) is established through the device itself rather than through a direct fistula. Ex-PRESS implantation is technically easier than trabeculectomy, requiring fewer surgical processes and resulting in fewer problems. As a result, it has been shown to be both safe and successful in the surgical treatment of primary and secondary open-angle glaucoma.13,14 More recently, novel techniques of Ex-PRESS® associated with deep sclerectomy have been described, with good rates of efficacy and safety.15–17 Compared to Ex-PRESS® alone, this modification seemed to be efficient, especially when very low postoperative IOP is needed.17
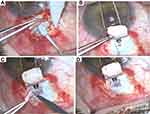
After Ex-PRESS® alone did not appear to be efficient in decreasing IOP in such patients, we began to conduct a modified approach of Ex-PRESS® implant in cases of severe open-angle glaucoma, notably in hATTR secondary glaucoma. The procedure entails creating a small 1.5×2 mm scleral pocket in the sclera, directly below the drainage hole of the Ex-PRESS® implant (Figure 1).
Thus, the purpose of this study was to determine the effectiveness and safety of this modified Ex-PRESS treatment in patients with hATTR secondary glaucoma.
Materials and Methods
Study Design
This is a retrospective analysis of patients with hATTR secondary glaucoma who had undergone an Ex-PRESS® modified procedure at our Ophthalmology Department. This study was carried out in accordance with the principles of the Helsinki Declaration (1964) and its latest amendment (Brazil, 2013). The authors ensured that the patients’ anonymity was carefully protected. All procedures were performed with informed consent and the study protocol complies with the requirements of the institute’s committee on human research (“Departamento de Ensino, Formação e Investigação”) of Centro Hospitalar Universitário do Porto (CHUPorto).
Inclusion Criteria and Population Description
Patients with a hATTR diagnosis, previously confirmed by genetic testing, who showed uncontrolled IOP under maximum topical treatment, or evidence of glaucoma progression seen in functional or anatomical exams, such as visual fields or optic disc retinal nerve fiber layer thickness, met the inclusion criteria. Patients who did not have at least a year of follow-up were not included in this study.
Demographic characteristics [gender, age, systemic treatment (liver transplant, and Tafamidis® therapy)]; previous ocular surgeries (pars plana vitrectomy, phacoemulsification and glaucoma surgeries) were evaluated at baseline data. Follow-up time was recorded.
Surgical Technique
Four skilled glaucoma consultant surgeons (MJM, IS, RFR, and AFig) performed all surgical procedures, under subtenonian anaesthesia with 2% lidocaine and sedation.
The glaucoma filtration system P50 ExPRESS® (Alcon, Fort Worth, Texas, USA) was employed. A traction suture is first used during surgery to expose the top of the globe. The technique comprises the following steps: limbal-based conjunctival incision and dissection of conjunctiva and tenon at 12 o’clock; cauterization of episcleral vessels; creation of the superficial scleral flap (4x4 mm, with 1/3 of depth); mitomycin 0.2 mg/mL application for 3 minutes; corneal paracentesis and viscoelastic introduction in the AC; scleral tunnelling with a 27-gauge needle until AC; implant introduction at 12 o’clock; creation of a small deeper flap (scleral pocket) in the posterior part of the scleral bed (just behind the drainage hole of the ExPRESS device), with 1.5×2 mm and excision of this second flap; suture of the scleral flap with 10–0 nylon sutures; closing the conjunctiva with 10–0 nylon or 8–0 vicryl (by surgeon preference) and subconjunctival injection of cefazolin and dexamethasone are performed. Figures 1A–D show this surgical technique.
All patients received oral and topical steroids upon weaning, topical antibiotics for 8 days, and non-steroidal anti-inflammatory drops for 6 months as postoperative care. As soon as the IOP exceeded 21 mmHg or when progression was confirmed, antiglaucoma medications were introduced.
Outcomes
The main endpoints were intraocular pressure (IOP) which was measured at baseline, the first day, the first week, the first, third, sixth, and twelfth months, and at the last follow-up by Goldmann applanation tonometry and the number of hypotensive eyedrops, which was measured at baseline, the sixth, twelfth months, and at last follow-up.
The secondary endpoints included the best corrected visual acuity (BCVA), endothelial cell count (ECC), and optical disc nerve fiber layer thickness (OD-NFL)]; early (within 1 month) and late (>1 month) surgical complications; and the need for further glaucoma surgeries.
For the purpose of statistical analysis, Snellen visual acuity was converted to logarithm of the minimum angle of resolution (logMAR) values. ECC count was determined using non-contact digital specular microscopy (EM-3000TM Tomey, Germany) and OD-RNFL thickness was assessed using structural optical coherence tomography with the Spectralis® equipment (Heidelberg Engineering, Heidelberg, Germany). In relation to postoperative problems, hypotony was referred to as having IOP < 5 mmHg throughout the course of two visits.
Surgical success was defined as ≥ 30% decrease in IOP from baseline and IOP ≤ 18 mmHg; complete success if any medication was needed and qualified success if anti-glaucoma medication was needed. Surgical failure was defined as final IOP <5 mmHg or >21 mmHg with topical medications and need for other glaucoma surgery during follow-up or loss of light perception.
Statistical analysis was performed with SPSS version 27.0 for MacOS (SPSS Inc., IBM, Somers, NY). The normality of the variables was evaluated by the Kolmogorov–Smirnov test. For the continuous variables an independent sample t-test or one-way repeated measures ANOVA with Bonferroni correction was performed. A p value < 0.05 was considered statistically significant.
Results
Baseline Data
A total of 32 eyes were included. The mean age at surgery was 52.1±8.7 years old and 16 subjects (50%) were female. Systemic symptoms of hATTR started at the average age of 30.4±9.3 years old. 25 eyes (78.1%) belonged to patients who had been submitted to a liver transplant at a mean age of 37.2±8.0 years old. Patients who did not undergo a liver transplant were under Tafamidis®.
Two eyes (6.2%) had previous glaucoma surgeries, including an Ahmed glaucoma valve implant in 1 eye and trabeculectomy in one eye. Fifteen eyes (46.9%) underwent pars plana vitrectomy for vitreous amyloid. Eleven eyes were pseudophakic (34.4%). Two eyes (6.3%) were submitted to a combined surgery with phacoemulsification.
Baseline data is resumed in Table 1.
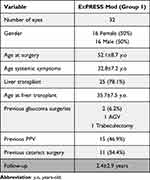 |
Table 1 Baseline Data |
Outcomes
Mean follow-up was 2.4±2.9 years.
IOP decreased significantly from baseline (27.4±4.4 mmHg) to 1st day (5.00±2.9 mmHg), 1st week (6.9±4.1 mmHg), 1st month (11.7±7.8 mmHg), 3rd month (11.6±6.1 mmHg), 6th month (13.1±6.8 mmHg), 12th month (12.0±3.5 mmHg) and until last visit (11.8±2.4 mmHg), p<0.001. The results from the repeated measures ANOVA with Bonferroni correction showed that IOP significantly decrease through all different time points [F (3.75; 116.25) =79.322, p<0.001]. Figure 2 shows the IOP variation.
 |
Figure 2 IOP evolution during follow-up. |
There was also a significant reduction in the number of antiglaucoma medications from baseline (3.8±0.6) to 6th month (0.6±1.0), 12th month (0.4±0.8) and last follow-up (0.4±0.8), p<0.001. The results from the repeated measures ANOVA with Bonferroni correction showed that the number of hypotensive drugs significantly decrease through all different time points [F (1.55; 48.19) =218.127, p<0.001]. Figure 3 shows the number of antiglaucoma medications.
 |
Figure 3 Number of antiglaucoma medications. Number within bars correspond to the mean value. |
LogMAR BCVA remained stable from baseline (0.24±0.26), to 1st month (0.35±0.25, p=0.072) and last visit (0.26±0.24, p=0.767).
Transient hypotony, defined as IOP <5 mmHg in 2 consecutive visits, occurred in 17 eyes (53.1%). From eyes with hypotony, 11 of the 17 eyes (64.7%) had anterior chamber (AC) shallowing and 6 in 17 (35.3%) presented a transient choroidal detachment.
Other early surgical complications were total hyphema in 1 eye (3.1%) and a vitreous haemorrhage (3.1%), which happened in an hypocoagulated patient.
As late complications one eye (3.1%) exhibited a bleb encapsulation and was submitted to a needling and bleb revision 3 months after surgery, with posterior controlled IOP without medication until the last follow-up; one eye (3.1%) with previous Fuchs endothelial dystrophy showed corneal decompensation and is now waiting for endothelial keratoplasty. Table 2 resumes the surgical complications.
 |
Table 2 Early and Late Surgical Complications |
There was a significant decrease in OD-RNFL from baseline (88.6±21.2) and last follow-up (73.6±23.0) p<0.001). ECC also decreased from baseline (2217±669 cells/mm2), until last follow-up (1964±602 cells/mm2), p<0.001.
Conplete surgical success was achieved in 22 eyes (68.8%); Qualified success in 6 eyes (18.8%). Surgical failure, defined by the need for further glaucoma surgeries happened in 4 eyes (12.5%). Those 4 eyes were submitted to AGV implantation. One eye was submitted to a bleb revision and needling with posterior controlled IOP until the last follow-up.
Eyes who underwent a previous PPV did not show a difference in absolute surgical success (X2=3.124, p=0.077), qualified surgical success (X2=2.611, p=0.106) or surgical failure (X2=0.410, p=0.522).
Discussion
Glaucoma, among other ocular manifestations, is the main factor contributing to vision loss in hATTR. Since eye TTR production does not stop, there is a tendency for ocular manifestations, particularly glaucoma, to become more prevalent as life expectancy rises. We also know, from personal experience and published data, that this kind of glaucoma develops early, advances quickly, and that the majority of patients will require immediate surgical intervention.
Trabeculectomy was mentioned in the earliest investigations with hATTR secondary glaucoma.9,18 Kimura et al9 reported that trabeculectomy with mitomycin C (MMC) appeared to be the most promising treatment based on their prior short-term results. With their extended follow-up trial, Kawaji et al18 discovered that trabeculectomy with MMC had only sporadic effectiveness in TTR-FAP glaucoma. Latasiewicz et al also presented non-penetrating deep sclerectomy outcomes, showing that NPDS was a successful therapy for FAP glaucoma.19
Prior to 2010, our glaucoma department’s experience with Trabeculectomy revealed uninspiring outcomes. As a result, the Ahmed glaucoma valve (AGV) implant, an aqueous humour drainage device, became the gold-standard for treating these patients in our department. We have more experience with AGV in refractory glaucomas, making it the preferred tube. However, Kakihara et al20 speculated that alternative tubes, such as the Baerveldt, may be as successful in this type of glaucoma.
AGV implantation in TTR-FAP secondary glaucoma demonstrated positive long-term effects, according to a recent study released by our department.11 That is now the largest series with the longest follow-up in the literature documenting a surgical technique’s success rate for this particular kind of glaucoma. Without a discernible rate of surgical complications, Marta et al11 demonstrated a cumulative probability of success (with or without drugs) of 0.98 at 1 year and 0.72 at 6 years of follow-up.
In our practice, a modified technique of implanting ExPRESS® has been used for the past three years. This updated approach with a posterior scleral pocket appears to be significantly more effective than ExPRESS® alone, which was tested as an alternative in the past but the results were unsatisfactory. Although the fact that this is a bleb-dependent surgery, similar to Trabeculectomy and NPDS, this newly procedure seems to be much more effective.
The Mermoud group published the first description of a new modified non-penetrating deep sclerectomy (NPDS) combined with ExPRESS X-200 implant for open-angle glaucoma in 2010.21 Bissig and Sylvain et al21 described their method, which involved creating a superficial limbus-based scleral flap that was 5×5 mm and 300 μm thick, then creating a deeper flap that was 3×4.5 mm in size at the back of the scleral bed without opening Schlemm’s canal and then removing the first flap. After that, a 21 G paracentesis was done prior to the deep sclerectomy, and the Ex-PRESS X-200 implant was inserted into the anterior chamber through the 21 G paracentesis. With an 18 months follow-up, they revealed cumulative success rates of 69% and 85%, with or without medication, whereas just one patient experienced surgical failure. Antiglaucoma drugs also had a 79% drop. In terms of postoperative complications, they report lower values than our findings regarding hypotony (15% showed transitory hypotony and 8% shallow AC). In contrast, only one eye in our research had bleb encapsulation. The shorter period of MMC in their approach may explain this feature (30–60 seconds versus 3 minutes in our study).
In a subsequent Mermaud group study, Gindroz and Sylvain et al15 published their findings using the same modified DS approach in conjunction with phacoemulsification and the ExPRESS model LR-50 (Optonol Ltd., Neve Ilan, Israel). In addition to a cumulative full success rate of 45.6% and a qualified success rate of 85.2% at 48 months, they also documented minimal rates of complications (4 eyes with hypotony with shallow AC and choroidal effusion). This method15,21 is comparable to ours, notwithstanding the smaller deep flap we use (1.5 x 2 mm) and the fact that our device is the P50 type rather than the X-200 or the LR50, which means it has a smaller inner lumen (50μm vs 200 μm). Although our reported rates of hypotony were higher than those presented in the Mermaud group studies, in theory the creation of a smaller deep flap and the use of a device with a smaller inner lumen would prevent hypotony. Nevertheless, our cases of hypotony were managed properly, with viscoelastic injection in AC on slit-lamp and cycloplegic eyedrops as needed, without affecting the final results in terms of IOP or visual acuity.
With a different technique of a modified NPDS combined to an ExPRESS P-50 implant, Kozobolis et al16 detailed their outcomes. Their procedure entails the creation of a trabeculodescemet membrane using a 4×4 deeper flap, excision of this deeper flap, and insertion of an ExPRESS mini-shunt into the AC through the trabeculodescemet membrane. They compared this method to trabeculectomy, reporting a larger reduction in IOP, fewer anti-glaucoma drugs, and a higher absolute success at 12 months with the modified NPDS combined with ExPRESS. Using a method similar to Kozobolis15, Marietta Fraczkiewicz-Skok et al17 published their findings: a greater decrease in IOP and fewer hypotensive eyedrops when compared to the ExPRESS implant alone in their prospective, randomized control research with a 24-month follow-up. Nevertheless, these surgical methods differ greatly from ours, so a direct comparison cannot be made.
Marta et al,11 reported a lower rate of hypotony after AGV implant in our department comparing to this report (8 eyes had transient hypotony- 6.5%- and 4 eyes- 3–2%- had AC shallowing). This difference can be explained by the fact that AGV is a valvulated device that provides a complex mechanism to control aqueous humor outflow, decreasing the risk of postoperative hypotony-related complications.22 It is crucial to note that temporary hypotony without AC shallowing is typical in the early post-op of a filtering procedure, therefore any further therapy was given in addition to rest. Eyes with a shallow AC, with or without choroidal detachment, were cautiously treated with cycloplegic drops as needed and viscoelastic injection on a slit-lamp.
The more frequent hospital checkups in the initial post-op period as compared to AGV may be a drawback. On the other hand, AGV seems to not achieve IOP levels as low as the modified ExPRESS® technique, being the reason why we started to perform this technique.
One limitation of this study is the relatively short follow-up and short sample. These can be explained by the fact that the modified ExPRESS technique was introduced in our practice in the last 3 years. In the future, we would have more eyes submitted to this novel technique and larger follow-up that can strengthen our results.
In conclusion, the ExPRESS® modified technique seems to be effective, particularly when a lower IOP is required. Also, with this novel technique, a lower number of antiglaucoma medications was necessary. Although hypotony was a common complication, patients with AC shallowing were properly handled, preserving the surgical outcomes.
We believe that the presented study of hATTR glaucoma can help guide the treatment in centers where this diagnosis is less common.
Funding
This work did not receive any funding.
Disclosure
The authors have no conflict of interest to declare for this work.
References
1. Beirão M, Matos E, Reis R, Beirão I, Costa PP, Torres P. Spatial visual contrast sensitivity in liver transplanted Portuguese familial amyloidotic polyneuropathy (ATTR V30M) patients. Amyloid. 2012;19:152–155.
2. Planté-Bordeneuve V, Kerschen P. Transthyretin familial amyloid polyneuropathy. Handb Clin Neurol. 2013;115:643–658.
3. Beirão JM, Moreira LM, Oliveira JC, et al. Aqueous humor erythropoietin levels in open angle glaucoma patients with and without TTR V30M familial amyloid polyneuropathy. Mol Vis. 2014;20:970–976.
4. Martins AC, Rosa AM, Costa E, et al. Ocular manifestations and therapeutic options in patients with familial amyloid polyneuropathy: a systematic review. Biomed Res Int. 2015;2015:282405. doi:10.1155/2015/282405
5. Beirão M, Matos E, Beirão I, Pinho-Costa P, Torres P. No ocular involvement in familial amyloidotic polyneuropathy ATTR V30M domino liver recipientes. Transpl Int. 2012;25:646–651. doi:10.1111/j.1432-2277.2012.01467.x
6. Conceição I, Carvalho M. Clinical variability in type I familial amyloid polyneuropathy (Val30Met): comparison between late- and early-onset cases in Portugal. Muscle Nerve. 2007;35:116–118. doi:10.1002/mus.20644
7. Kawaji T, Ando Y, Nakamura M, et al. Transthyretin synthesis in rabbit ciliary pigment epithelium. Exp Eye Res. 2005;81(3):306–312. doi:10.1016/j.exer.2005.02.003
8. Beirão JM, Malheiro J, Lemos C, et al. Ophthalmological manifestations in hereditary transthyretin (ATTR V30M) carriers: a review of 513 cases. Amyloid. 2015;22(2):117–122. doi:10.3109/13506129.2015.1015678
9. Kimura A, Ando E, Fukushima M, et al. Secondary glaucoma in patients with familial amyloidotic polyneuropathy. Arch Ophthalmol. 2003;121:351–356. doi:10.1001/archopht.121.3.351
10. Beirão NM, Matos ME, Meneres MJ, Beirão IM, Costa PP, Torres PA. Vitreous surgery impact in glaucoma development in liver transplanted familial amyloidosis ATTR V30M Portuguese patients. Amyloid. 2012;19:146–151. doi:10.3109/13506129.2012.710669
11. Marta A, Vieira R, Figueiredo A, et al. Ahmed valve for secondary glaucoma in patients with hereditary transthyretin amyloidosis. Eye. 2022;36(1):111–118. PMID: 33627759; PMCID: PMC8727566. doi:10.1038/s41433-021-01443-y
12. Lee CK, Ma KT, Hong YJ, Kim CY. Long-term clinical outcomes of Ahmed valve implantation in patients with refractory glaucoma. PLoS One. 2017;12:e0187533. doi:10.1371/journal.pone.0187533
13. Chan JE, Netland PA. EX-PRESS glaucoma filtration device: efficacy, safety, and predictability. Med Devices. 2015;8:381–388. PMID: 26366105; PMCID: PMC4562650. doi:10.2147/MDER.S63350
14. Shaarawy T, Goldberg I, Fechtner R. EX-PRESS glaucoma filtration device: review of clinical experience and comparison with trabeculectomy. Surv Ophthalmol. 2015;60(4):327–345. PMID: 25910971. doi:10.1016/j.survophthal.2015.01.001
15. Gindroz F, Roy S, Mermoud A, Schnyder CC. Combined Ex-PRESS LR-50/IOL implantation in modified deep sclerectomy plus phacoemulsification for glaucoma associated with cataract. Eur J Ophthalmol. 2011;21(1):12–19. PMID: 20623471. doi:10.5301/ejo.2010.541
16. Kozobolis V, Panos GD, Konstantinidis A, Teus M, Labiris G. Modified deep sclerectomy combined with Ex-PRESS filtration device versus trabeculectomy for primary open angle glaucoma. Int J Ophthalmol. 2017;10(5):728–732. PMID: 28546928; PMCID: PMC5437459. doi:10.18240/ijo.2017.05.11
17. Frączkiewicz-Skok M, Konopińska J, Mariak Z, Rękas M. Comparison of express implantation and partial deep sclerectomy combined with express implantation and simultaneous phacoemulsification. J Ophthalmol. 2019;2019:7424376. PMID: 31885894; PMCID: PMC6915013. doi:10.1155/2019/7424376
18. Kawaji T, Inoue T, Hara R, Eiki D, Ando Y, Tanihara H. Longterm outcomes and complications of trabeculectomy for secondary glaucoma in patients with familial amyloidotic polyneuropathy. PLoS One. 2014;9:e96324. doi:10.1371/journal.pone.0096324
19. Latasiewicz M, Millá E, Giralt J, Molina JJ, Matas J. Nonpenetrating deep sclerectomy as an effective treatment of glaucoma related to familial amyloid polyneuropathy. J Glaucoma. 2015;24(5):e80–e83. PMID: 25264993. doi:10.1097/IJG.0000000000000126
20. Kakihara S, Hirano T, Imai A, Miyahara T, Murata T. Baerveldt glaucoma drainage implant surgery for secondary glaucoma in patients with transthyretin-related familial amyloid polyneuropathy.Jpn. J Ophthalmol. 2020;64:533–538.
21. Bissig A, Feusier M, Mermoud A, Roy S. Deep sclerectomy with the Ex-PRESS X-200 implant for the surgical treatment of glaucoma. Int Ophthalmol. 2010;30(6):661–668. PMID: 20552258. doi:10.1007/s10792-010-9382-z
22. Riva I, Roberti G, Oddone F, Konstas AG, Quaranta L. Ahmed glaucoma valve implant: surgical technique and complications. Clin Ophthalmol. 2017;11:357–367. PMID: 28255226; PMCID: PMC5322839. doi:10.2147/OPTH.S104220
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.