Back to Journals » Cancer Management and Research » Volume 13
miR-4284 Promotes Cell Proliferation, Migration, and Invasion in Non-Small Cell Lung Cancer Cells and is Associated with Postoperative Prognosis
Authors Yang H, Zhang W, Luan Q, Liu Y
Received 20 February 2021
Accepted for publication 18 May 2021
Published 28 July 2021 Volume 2021:13 Pages 5865—5872
DOI https://doi.org/10.2147/CMAR.S305379
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Hanbing Yang,1 Wenjing Zhang,2 Qingxia Luan,3 Yanchao Liu2
1Department of Interventional Thoracic Oncology, Affiliated Hospital of Weifang Medical University, Weifang, Shandong, 261031, People’s Republic of China; 2Department of Hematology, Affiliated Hospital of Weifang Medical University, Weifang, Shandong, 261031, People’s Republic of China; 3Department of Pediatrics, Affiliated Hospital of Weifang Medical University, Weifang, Shandong, 261031, People’s Republic of China
Correspondence: Hanbing Yang
Department of Interventional Thoracic Oncology, Affiliated Hospital of Weifang Medical University, Weifang, Shandong, 261031, People’s Republic of China
Tel +86-0536-3081293
Email [email protected]
Purpose: MicroRNA-4284 (miR-4284) was demonstrated to be aberrantly expressed and affected cell activities in some types of diseases, including cancer. However, the role of miR-4284 in non-small cell lung cancer (NSCLC) is largely unknown. The aim of this study was to investigate the expression and biological role of miR-4284 in NSCLC.
Patients and Methods: The qRT-PCR assay was applied to detect the expression of miR-4284 in NSCLC tissues and cell lines. Kaplan–Meier curve method and multiple Cox regression analyses were used to explore the prognostic factors for postoperative NSCLC patients. The CCK-8 assay was carried out to measure the proliferative abilities of A549 and H1299 cells. Transwell migration and invasion assays were used to determine the cell migratory and invasive capabilities of NSCLC cells.
Results: miR-4284 expression was upregulated in NSCLC tissues and cell lines. High expression of miR-4284 was correlated with poor differentiation, positive lymph node metastasis, and advanced TNM stages. In addition, postoperative patients with higher expression of miR-4284 exhibited a shorter overall survival time than those with lower expression of miR-4284. Moreover, the upregulation of miR-4284 accelerated cell proliferative, migratory, and invasive abilities of A549 and H1299 cells, while the downregulation of miR-4284 inhibited these cellular capabilities.
Conclusion: miR-4284 was noticeably upregulated in NSCLC and associated with a poor prognosis of postoperative NSCLC patients. miR-4284 promoted the proliferation, migration, and invasion of A549 and H1299 cells. This study indicated that miR-4284 might serve as a prognostic biomarker and a potential therapeutic target for postoperative NSCLC patients.
Keywords: miR-4284, malignant activities, non-small cell lung cancer, prognosis, biomarker
Introduction
Lung cancer is one of the most common tumors of the respiratory tract in the world with the highest morbidity and mortality.1 The occurrence of lung cancer is a complex process of multi-gene involvement and multi-stage development.2 The high incidence and high morbidity rate of lung cancer have made it a global public health problem.3 According to histological characteristics, lung cancer can be roughly divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), among which NSCLC accounts for about 85–90%.4,5 There are multiple risk factors for the pathogenesis of NSCLC, such as smoking, environmental pollution, and genetic factors (such as genetic mutations).6–8 Surgery, radiotherapy, and chemotherapy are the main therapeutic strategies for NSCLC. Because most of the cases are diagnosed in the middle and late stages, and after surgical resection usually occur relapse, the survival outcome of NSCLC patients is still poor.9 Thus, exploring new molecular therapeutic strategies is still imperative to improve the prognosis of NSCLC patients.
In recent years, microRNAs (miRNAs) have attracted more and more attention from many scientists, especially their roles in various diseases.10–12 miRNAs are a class of endogenous, small, highly conserved non-coding molecule RNAs with a length of about 19–23 nucleotides.13,14 The aberrant expression of miRNAs is involved in various cellular processes, such as differentiation, proliferation, metastasis, invasion, and apoptosis.15,16 In addition, it has been demonstrated that miRNAs play crucial roles in the pathogenesis and progression of cancers through functioning as oncogenic or tumor suppressor.17 For instance, upregulation of miR-1323,18 miR-212-5p,19 and miR-671-3p20 was found in NSCLC tissues and cells and participated in the proliferation, migration, invasion, and apoptosis as oncogenes. In addition, the downregulation of miR-144,21 and miR-140-3p22 functioned as a tumor suppressor in the development of NSCLC. miR-4284 has been reported to be abnormally expressed in several diseases, including cancer.23–25 Microarray analysis identified several recurrence-associated differentially expressed miRNAs in lung adenocarcinoma, including miR-4284.26 However, the expression pattern and potential role of miR-4284 in NSCLC is still exclusive.
In the present study, the expression of miR-4284 in NSCLC tissues and cell lines was evaluated. Then the relationship between miR-4284 expression and clinical characteristics of NSCLC patients, as well as its clinical prognostic significance for post-operative NSCLC patients was analyzed. In addition, to explore the potential functional role of miR-4284 in NSCLC, we further analyzed its effects on proliferation, migration, and invasion of NSCLC cells.
Materials and Methods
Patients and Tissue Specimens
A total of 125 NSCLC patients who underwent surgery from January 2012 to June 2014 in the Affiliated Hospital of Weifang Medical University was enrolled in this study. All the patients did not receive anti-tumor therapy before surgical resection. The 125 pairs of NSCLC tissues and adjacent non-tumor tissues were obtained from these NSCLC patients and immediately frozen in liquid nitrogen for further RNA extraction. The clinicopathological characteristics of the patients were collected, recorded, and listed in Table 1. In addition, a 5-year follow-up survival information of all the patients was obtained for subsequent analyses. All the studies were carried out under the approval of the Ethics Committee of Affiliated Hospital of Weifang Medical University and the guidelines outlined in the Declaration of Helsinki were followed. Written informed consent was obtained from each individual.
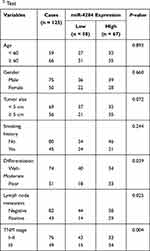 |
Table 1 The Association Between miR-4284 Expression and Clinical Characteristics of NSCLC Patients Was Analyzed Using χ2 Test |
Cell Culture and Transfection
Human NSCLC cell lines A549, SK-MES-1, H358, H1299, and normal epithelium cell line BEAS-2B were obtained from the Cell Bank of the Chinese Academy of Science (Shanghai, China). All cells were cultured in DMEM (Gibco, Carlsbad, CA) medium added with 10% FBS (Gibco, Carlsbad, CA) in an incubator with a humidified atmosphere containing 5% CO2 at 37°C.
The NSCLC cells were seeded in 6-well plates before transfection. miR-4284 mimic, miR-4284 inhibitor, and miRNA negative control (miR-NC) were synthesized from GenePharma (Shanghai, China) to upregulate or downregulate the expression of miR-4284 in vitro. Then Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) was used for cell transfection assay following the manufacturer’s instructions.
RNA Isolation and Quantitative Reverse-Transcription PCR (qRT-PCR)
Total RNA from the frozen tissue specimens and cell lines was isolated with TRIzol reagent as per the manufacturer’s instructions. The concentration and quality of RNA were determined by measuring the optical density (A260/A280: 1.8–2.0) with a Nanodrop ND-2000 (Thermo Fisher Scientific, Waltham, MA, USA). Then the RNA was reverse transcribed into cDNA by PrimeScriptTM RT reagent Kit (TaKaRa, Otsu, Shiga, Japan). The qRT-PCR was performed using the cDNA as targeted transcripts with SYBR Green Real-Time PCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) on an ABI 7500 Real-Time PCR system. The relative expression of miR-4284 was calculated using the 2−ΔΔCt method normalized to U6.
Cell Proliferation Assay
The cell proliferation of A549 and H1299 cells was measured using cell counting kit-8 (CCK-8; Dojindo, Japan) following the manufacturer’s instruction. The NSCLC cells (2000 cells/well) were seeded into 96-well plates and incubated for 0, 1, 2, 3 days. Then, 10 μL CCK-8 solution was added to each well and further incubated for 2 h. A microplate reader was used to measure the absorbance at 450 nm.
Cell Migration and Invasion Assays
The transwell assays were used to access cell migration and invasion of transfected cells. The 24-well transwell upper chambers with 8 μm pore size membrane (Millipore, MA, USA) that coated without (migration assay) or with (invasion assay) Matrigel (BD Bioscience, CA, USA) was added transfected cells at a density of 1×105 cells/well in serum-free medium. The lower chambers were added with medium supplemented with 10% FBS. After 24 h of incubation, the cells that migrated or invaded into the lower membranes were stained and counted by a microscope (Olympus, Japan).
Statistical Analysis
All experiments were repeated at least three times independently and all the data were presented as mean ± SD. The statistical analysis was performed using SPSS20.0 software (IBM, Armonk, NY, USA) and GraphPad 7.0 software (GraphPad, San Diego, CA, USA). Comparisons between groups were analyzed using the Students’ t-test, whereas data among three or more groups were compared using one-way ANOVA. The χ2 test was used to analyze the relationship between miR-4284 expression and clinical characteristics of NSCLC patients. Then, Kaplan–Meier curve with Log rank test and multiple Cox regression analysis was used to analyze the clinical prognostic significance of miR-4284. Differences of P value less than 0.05 were considered statistically significant.
Results
miR-4284 Was Upregulated in NSCLC Tissues and Cell Lines
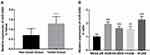
The expression of miR-4284 in 125 pairs of NSCLC tissue specimens and matched non-tumor tissue specimens was detected using qRT-PCR. As presented in Figure 1A, miR-4284 expression in NSCLC tissues was markedly upregulated compared with that in adjacent non-tumor tissues (P < 0.001). Furthermore, miR-4284 expression was measured in the human NSCLC cell lines A549, SK-MES-1, H358, and H1299, as well as the normal epithelium cell line BEAS-2B. As shown in Figure 1B, miR-4284 expression in NSCLC cell lines was increased relative to that in the normal lung epithelial cell line BEAS-2B (P < 0.01). Among these NSCLC cell lines, A549 and H1299 exhibited higher expression levels of miR-4284, which were chosen for subsequent experiments.
Association Between miR-4284 Expression and Clinicopathological Characteristics of NSCLC Patients
To explore whether miR-4284 expression was associated with the development of NSCLC, we analyzed the association between miR-4284 expression and clinicopathological characteristics of NSCLC patients. The NSCLC patients were divided into two groups (low miR-4284 expression group and high miR-4284 expression group) according to their miR-4284 expression. The results in Table 1 showed that miR-4284 expression was significantly associated with differentiation (P = 0.039), lymph node metastasis (P = 0.025), and TNM stage (P = 0.004). However, no significant difference was found between miR-4284 expression and other clinical characteristics of NSCLC patients, including age, gender, tumor size, and smoking history (all P > 0.05).
miR-4284 Expression Was Associated with Prognosis of Post-Operative NSCLC Patients
The 5-year follow-up survival information of the patients was recorded for survival analysis. The Kaplan–Meier curve was plotted and the result (Figure 2) implied that the patients with high miR-4284 expression levels had a poor survival compared to those with low miR-4284 expression levels (Log rank test, P = 0.021). In addition, combining the clinical characteristics into the multiple Cox regression analysis, we observed that miR-4284 expression, lymph node metastasis, and TNM stage were independent prognostic factors for the post-operative NSCLC patients (P < 0.05, Table 2).
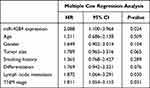 |
Table 2 Multiple Cox Regression Analysis Analyzed Risk Factors to Survival Using Patients’ Characteristics and miR-4284 Expression |
miR-4284 Promoted Proliferation, Migration, Invasion Abilities in A549 and H1299 Cells
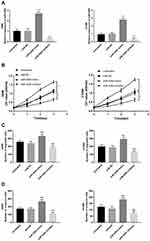
To explore the functional role of miR-4284 in NSCLC cells, we transfected miR-4284 mimic or miR-4284 inhibitor into A549 and H1299 cells to upregulate or downregulate miR-4284 expression, respectively. After 48 h of transfection, qRT-PCR analysis was used to measure the expression levels of miR-4284 in these cells. The results in Figure 3A revealed that miR-4284 expression was upregulated by miR-4284 mimic, while miR-4284 expression was downregulated by miR-4284 inhibitor, compared with untreated cells (P < 0.001). Then, CCK-8 assay was used to detect the effects of miR-4284 on cell proliferation and the results revealed that the proliferation of A549 and H1299 cells were promoted due to miR-4284 upregulation, while that was inhibited due to miR-4284 downregulation (P < 0.05, Figure 3B). The Transwell migration and invasion assays showed that the increased miR-4284 expression promoted cell migration and invasion, while knockdown of miR-4284 suppressed cell migration and invasion (P < 0.001, Figure 3C and D).
Discussion
The miRNA in this study, miR-4284, was reported to be aberrantly expressed in some types of diseases, including cancer.27,28 Tamaddon G and co-workers assessed the deregulation of miRNAs profiles in diffuse large B-cell lymphoma (DLBL) and indicated that miR-4284 and miR-4484 can act as a diagnostic biomarker for DLBL.23 In clear cell papillary renal cell carcinoma and radiotherapy prostate cancer, miR-4284 was abnormally expressed and it might be candidate miRNA for the development of novel prognostic biomarkers.29,30 However, no study excavates its expression, clinical significance, and function in NSCLC. Firstly, we observed that miR-4284 was upregulated in NSCLC tissue specimens when compared with adjacent non-tumor tissue specimens. Moreover, miR-4284 was upregulated in all NSCLC cell lines compared to normal epithelial cell lines. These results suggest that miR-4284 might play an oncogenic role in NSCLC. Additionally, high expression of miR-4284 was correlated with poor differentiation, positive lymph node metastasis, and advanced TNM stage, suggesting that miR-4284 expression might be involved in the development of NSCLC.
Considering the abnormal expression of miR-4284 in NSCLC tissues, furthermore, the potential prognostic significance of miR-4284 in NSCLC was explored. The result showed that postoperative NSCLC patients with higher miR-4284 expression levels exhibited a shorter survival time than those with lower miR-4284 expression levels. What’s more, multiple Cox regression analyses indicated that miR-4284 expression was an independent prognostic factor for postoperative NSCLC patients. The above results suggest that miR-4284 expression might be a prognostic marker for postoperative NSCLC patients.
The abnormal expression of miRNAs in NSCLC can regulate tumor cell proliferation and metastasis, thus affecting the development of lung cancer. A previous study has demonstrated that miR-4284 expression was increased in gastric cancer and its upregulation promoted cell growth, migration, and invasion by targeting ten-eleven translocation 1(TET1).31 In the present study, the results showed that overexpression of miR-4284 promoted cell proliferation, migration, and invasion of A549 and H1299 cells, while downregulation of miR-4284 exhibited the opposite activities. Thus, miR-4284 might play an oncogenic role in the progression of NSCLC.
The present study still has some limitations, and the results are preliminary. Firstly, the sample size of the patients was small. Secondly, although these results provided a novel insight into the role of miR-4284 in NSCLC, the detailed mechanisms remain unclear. Previous studies indicated that miR-4284 might play a role in the development of tumors by targeting TET131 or CXCL5.24 However, whether the molecules mentioned above were also involved in the detailed mechanism of miR-4284 remains unclear, which will be investigated in future researches.
Conclusion
In conclusion, these findings support that miR-4284 was upregulated in NSCLC tissues and cell lines. The upregulation of miR-4284 in NSCLC might be a candidate prognostic factor. Furthermore, an increase of miR-4284 expression might play an oncogenic role in the progression of NSCLC by promoting cell proliferation, migration, and invasion. Thus, miR-4284 may be a novel prognostic biomarker and therapeutic target for the treatment of postoperative NSCLC patients.
Ethics Statement
All the studies were carried out under the approval of the Ethics Committee of Affiliated Hospital of Weifang Medical University and the guidelines outlined in the Declaration of Helsinki were followed. Written informed consent was obtained from each individual.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wong MCS, Lao XQ, Ho KF, Goggins WB, Tse SLA. Incidence and mortality of lung cancer: global trends and association with socioeconomic status. Sci Rep. 2017;7(1):14300. doi:10.1038/s41598-017-14513-7
2. Liu X, Li Z, Zhang Z, et al. Meta-analysis of GSTM1 null genotype and lung cancer risk in Asians. Med Sci Monit. 2014;20:1239–1245.
3. Fidler MM, Bray F, Soerjomataram I. The global cancer burden and human development: a review. Scand J Public Health. 2018;46(1):27–36. doi:10.1177/1403494817715400
4. Brambilla E. Lung cancer: multidisciplinary approach for management: cell and molecular biology assembly contribution to the celebration of 20 years of the ERS. Eur Respir J. 2010;35(4):717–720. doi:10.1183/09031936.00018810
5. Tsim S, O’Dowd CA, Milroy R, Davidson S. Staging of non-small cell lung cancer (NSCLC): a review. Respir Med. 2010;104(12):1767–1774.
6. Boffetta P, Nyberg F. Contribution of environmental factors to cancer risk. Br Med Bull. 2003;68(1):71–94. doi:10.1093/bmp/ldg023
7. Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol. 2013;30(2):93–98. doi:10.1055/s-0033-1342949
8. de Groot PM, Wu CC, Carter BW, Munden RF. The epidemiology of lung cancer. Transl Lung Cancer Res. 2018;7(3):220–233. doi:10.21037/tlcr.2018.05.06
9. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016:1–19. doi:10.1007/978-3-319-24223-1_1.
10. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi:10.1038/nrd.2016.246
11. Wojciechowska A, Braniewska A, Kozar-Kaminska K. MicroRNA in cardiovascular biology and disease. Adv Clin Exp Med. 2017;26(5):865–874.
12. Jafarzadeh-Esfehani R, Mostafa Parizadeh S, Sabeti Aghabozorgi A, et al. Circulating and tissue microRNAs as a potential diagnostic biomarker in patients with thrombotic events. J Cell Physiol. 2020;235:6393–6403.
13. Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–524.
14. Tian S, Guo X, Yu C, Sun C, Jiang J. miR-138-5p suppresses autophagy in pancreatic cancer by targeting SIRT1. Oncotarget. 2017;8(7):11071–11082. doi:10.18632/oncotarget.14360
15. Wang H, Song T, Qiao Y, Sun J. miR-940 inhibits cell proliferation and promotes apoptosis in esophageal squamous cell carcinoma cells and is associated with post-operative prognosis. Exp Ther Med. 2020;19(2):833–840.
16. Jia Y, Gao Y, Dou J. Effects of miR-129-3p on biological functions of prostate cancer cells through targeted regulation of Smad3. Oncol Lett. 2020;19(2):1195–1202.
17. Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009;28(3–4):369. doi:10.1007/s10555-009-9188-5
18. Zhao H, Zheng C, Wang Y, et al. miR-1323 promotes cell migration in lung adenocarcinoma by targeting Cbl-b and is an early prognostic biomarker. Front Oncol. 2020;10:181. doi:10.3389/fonc.2020.00181
19. Chen F, Sun N, Wang Y, et al. miR‐212‐5p exerts tumor promoter function by regulating the Id3/PI3K/Akt axis in lung adenocarcinoma cells. J Cell Physiol. 2020;235:7273–7282.
20. Li ZY, Zhang ZZ, Bi H, et al. Upregulated microRNA‑671‑3p promotes tumor progression by suppressing forkhead box P2 expression in non‑small‑cell lung cancer. Mol Med Rep. 2019;20(4):3149–3159.
21. Pu R, Pu M, Huang H, Cui Y. MicroRNA 144 inhibits cell migration and invasion and regulates inflammatory cytokine secretion through targeting toll like receptor 2 in non-small cell lung cancer. Arch Med Sci. 2020;17. doi:10.5114/aoms.2020.93084
22. Hu C, Zou Y, Jing LL. miR-140-3p inhibits progression of non-small cell lung cancer by targeting Janus kinase 1. J Biosci. 2020;45(1):1–11.
23. Tamaddon G, Geramizadeh B, Karimi MH, Mowla SJ, Abroun S. miR-4284 and miR-4484 as putative biomarkers for diffuse large B-cell lymphoma. Iran J Med Sci. 2016;41(4):334–339.
24. Koukos G, Polytarchou C, Kaplan JL, et al. A microRNA signature in pediatric ulcerative colitis: deregulation of the miR-4284/CXCL5 pathway in the intestinal epithelium. Inflamm Bowel Dis. 2015;21(5):996–1005. doi:10.1097/MIB.0000000000000339
25. He XM, Zheng YQ, Liu SZ, Liu Y, He YZ, Zhou XY. Altered plasma microRNAs as novel biomarkers for arteriosclerosis obliterans. J Atheroscler Thromb. 2016;23(2):196–206. doi:10.5551/jat.30775
26. Sim J, Kim Y, Kim H, et al. Identification of recurrence-associated microRNAs in stage I lung adenocarcinoma. Medicine. 2018;97(25):e10996. doi:10.1097/MD.0000000000010996
27. Palmieri O, Creanza TM, Bossa F, et al. Functional implications of microRNAs in Crohn’s disease revealed by integrating microRNA and messenger RNA expression profiling. Int J Mol Sci. 2017;18(7):1580.
28. Liu W, Wang P, Xie Z, et al. Abnormal inhibition of osteoclastogenesis by mesenchymal stem cells through the miR-4284/CXCL5 axis in ankylosing spondylitis. Cell Death Dis. 2019;10(3):188. doi:10.1038/s41419-019-1448-x
29. McDermott N, Meunier A, Wong S, Buchete V, Marignol L. Profiling of a panel of radioresistant prostate cancer cells identifies deregulation of key miRNAs. Clin Transl Radiat Oncol. 2017;2:63–68. doi:10.1016/j.ctro.2017.01.005
30. Munari E, Marchionni L, Chitre A, et al. Clear cell papillary renal cell carcinoma: micro-RNA expression profiling and comparison with clear cell renal cell carcinoma and papillary renal cell carcinoma. Hum Pathol. 2014;45(6):1130–1138. doi:10.1016/j.humpath.2014.01.013
31. Li Y, Shen Z, Jiang H, et al. MicroRNA4284 promotes gastric cancer tumorigenicity by targeting ten-eleven translocation 1. Mol Med Rep. 2018;17;5:6569–6575.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.