Back to Journals » Clinical Ophthalmology » Volume 17
MiR-21 Participates in Anti-VEGF-Induced Epithelial Mesenchymal Transformation in RPE Cells
Authors Hao X, Hua Y, Xie C, Xu H
Received 22 July 2023
Accepted for publication 5 October 2023
Published 16 October 2023 Volume 2023:17 Pages 3047—3056
DOI https://doi.org/10.2147/OPTH.S427894
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Xianghui Hao,1 Yingbin Hua,1 Chaohui Xie,1 Haifeng Xu1,2
1State Key Laboratory Cultivation Base, Shandong Provincial Key Laboratory of Ophthalmology, Shandong Eye Institute, Shandong First Medical University & Shandong Academy of Medical Sciences, Qingdao, People’s Republic of China; 2Qingdao Eye Hospital, Shandong First Medical University & Shandong Academy of Medical Sciences, Qingdao, People’s Republic of China
Correspondence: Haifeng Xu, Qingdao Eye Hospital, Shandong Eye Institute, Shandong First Medical University & Shandong Academy of Medical Sciences, 5 Yanerdao Road, Qingdao, Shandong, 266071, People’s Republic of China, Email [email protected]
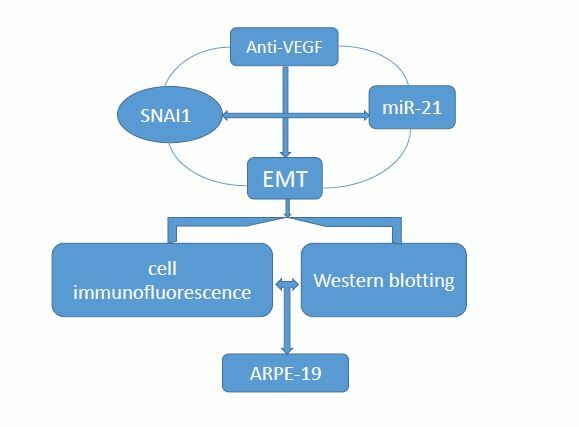
Background: To explore the role and possible mechanism of miR-21 in anti-VEGF drug-induced epithelial-mesenchymal transformation (EMT) in human retinal pigment epithelium (ARPE-19) cells, and to seek more therapeutic targets to improve prognosis vision.
Methods: ARPE-19 cells were exposed to clinical dosage of bevacizumab and miR-21 expression was measured by real-time polymerase chain reaction (RT-PCR) assay. MiR-21 mimic and inhibitor were transfected into bevacizumab-induced ARPE-19, the expression of α-smooth muscle actin (α-SMA), E-cadherin, and SNAI1 were detected by cell immunofluorescence and Western blotting.
Results: Clinical dosage of bevacizumab caused EMT and enhanced miR-21 expression in ARPE-19 cells (P< 0.05). The inhibition of miR-21 attenuated the EMT effect of bevacizumab, while overexpression of miR-21 promoted this activity (P< 0.05). The SNAI1 was up-regulated by bevacizumab and promotion was partially suppressed by the miR-21 inhibitor and aggravated by the miR-21 mimic (P< 0.05).
Conclusion: MiR-21 promotes bevacizumab-induced EMT in ARPE cells which is significantly positively correlated with SNAI1. MiR-21 might be a potential miRNA-based therapeutic target for reducing bevacizumab-induced subretinal fibrosis.
Keywords: bevacizumab, epithelial-mesenchymal transition, MiR-21, SNAI1, therapeutic target
Graphical Abstract:
Introduction
Wet age-related macular degeneration (wAMD), characterized by choroidal neovascularization (CNV) in the macular region, is a major blinding disease with central visual impairment in the elderly population.1 Abnormal neovascularization initially proliferated in the Bruch membrane and retinal pigment epithelium (RPE) layer and gradually invaded the subretinal layer, resulting in subretinal hemorrhage, exudative injury, serous retinal detachment and fibrous scar. CNV fibrosis in the fovea of the macula destroys the structure and function of the macula and eventually leads to permanent visual loss.2 Currently, the first-line treatment of wAMD is intravitreal injection of anti-vascular endothelial growth factor (VEGF) drugs,3,4 which has significantly improved the visual prognosis of patients with wAMD. However, in clinical practice, it can be observed that one of the side effects of long-term injection of anti-VEGF is macular fibrosis; CATT study5 found that after two years of anti-VEGF treatment, 45.3% (480/1059) of wAMD patients had macular fibrosis, and subretinal fibrosis was directly related to the best corrected visual acuity (BCVA). Subretinal fibrosis and atrophy in the outer layer of the retina were also reported to be the main pathological cause of visual loss at the end of wAMD.6 Although fibrosis is a repair reaction of tissue damage, CNV can also develop fibrosis in the natural course of the disease. Studies have shown that anti-VEGF treatment can change the balance between connective tissue growth factor (CTGF) and VEGF, increase the ratio of CTGF/VEGF, and induce angiogenesis to change to fibrosis. VEGF drives angiogenesis and stimulates up-regulation of CTGF. CTGF is the main profibrosis factor in the eye, which can produce negative feedback on angiogenesis by down-regulating VEGF production and increasing the formation of the CTGF-VEGF complex. During anti-VEGF treatment, VEGF antibody reduces the VEGF content by combining with the VEGF receptor. At this time, the excessive CTGF produced continuously activates the switch of vascular fibrosis and stimulates the formation of fibrosis.7 Tissue fibrosis is characterized by the presence of myofibroblasts, which can be generated by the transformation of fibroblast myofibroblasts, and it is produced by epithelial mesenchymal transformation (EMT) in some tissues and cells (including RPE cells).8 Therefore, after anti-VEGF treatment, EMT of ARPE-19 cells plays an important role in the occurrence and development of ocular fibrosis.
MicroRNA (miRNA) is a non-coding RNA composed of 19–24 nucleotides. It can regulate target genes by affecting the stability of mRNA or inhibiting mRNA translation. In addition, it can play a variety of biological functions such as proliferation, differentiation, apoptosis, immune function and angiogenesis.9 Previous studies have shown that miR-21 is closely related to the appearance of fibrosis in organs such as the lungs, liver, gastrointestinal tract, and bronchi.10–14 More research is needed to determine whether miR-21 also mediates EMT of RPE. However, Li H et al15,16 confirmed that overexpression of SNAI1 can trigger EMT in RPE cells. SNAI1 is a zinc finger transcription factor, and one of its main functions is to mediate the regulation of EMT during tumor development and fibrosis. Cheng H-Y et al17 have confirmed the interaction between miR-21 transcription and SNAI1 expression. Therefore, our focus is on whether miR-21 and SNAI1 jointly participate and how they participate in anti-VEGF-induced EMT in RPE cells. The purpose of this study is to investigate the effect of miR-21 on bevacizumab-induced ARPE-19 cell EMT and its correlation with SNAI1.
Methods
Cell Culture and Treatment
The ARPE-19 cells (ATCC, Rockefeller, Maryland, USA) were cultured in DMEM/F-12 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Life Technologies, Carlsbad, USA), 100 U/mL penicillin and 100 μg/mL streptomycin (Sigma-Aldrich, St Louis, MO, USA) at 37 ° C in humidified air with 5% CO2. ARPE-19 cells were exposed to a clinical concentration (0.25 mg/mL) of bevacizumab (Roche Diagnostics, GmbH, Germany) for 2 days. To eliminate the side effects caused by cytokines in FBS, bevacizumab treated cells were cultured in serum free (0% FBS) DF-12 medium.
Cell Transfection
The ARPE-19 cells were subcultured into a six-well plate using a 2 mL volume of DMEM/F-12 medium containing 2% FBS. After the cells adhere to the wall and fuse to 60–70%, the transfection operation is carried out according to the instructions of Ribo FETTM CP reagent (RiboBio Biotechnology, Guangzhou, China): the transfection mixture prepared from four groups (miR-21 mimic, mimic NC, miR-21 inhibitor, inhibitor NC) is added to a six-well plate, so that the concentration of the transfection reagent in the culture medium is 15 pmol. After 24 hours of transfection, replace with DF-12 culture medium without FBS, bevacizumab drug treatment is performed, After 48 hours, cells were collected for subsequent functional tests. The transfection efficiency has been verified through RT-PCR experiments. See Supplementary Material 1 for sequences details of miRNA.
qRT-PCR Assay
The quantitative real-time polymerase chain reaction (qRT-PCR) assay was performed to detect miR-21 expression in ARPE-19 cells. Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA, USA) from ARPE-19 cells and the level of miR-21 was determined using specific primers (RiboBio Biotechnology, Guangzhou, China). U6 was used for normalization. qRT-PCR was performed using the Qiagen Rotor-Gene quantitative PCR system according to the manufacturer’s protocol (Vazyme Biotech, Nanjing, China). Reaction conditions: prechange for 10 seconds at 95 ° C, 5 seconds at 95 ° C, and 20 seconds at 60 ° C for 40 cycles. The expression level of the gene is represented by 2−ΔΔCt (Ct represents a cycle threshold). The relevant miRNA sequences can be found in Supplementary Material 2.
Western Blot Analysis
The total protein was extracted from the lysis of ARPE-19 cells in a radioimmunoprecipitation assay (RIPA) buffer containing a mixture of protease inhibitors and 50 μg of total protein per sample were loaded and separated on 10% SDS-PAGE and then wet transferred using PVDF membranes (Rugby WAR, UK). The membranes were blocked in 5% skim milk powder in TBST and overnight incubated with specific primary antibodies of E-cadherin (ab212059, 1:1000, Abcam, Cambridge, MA, USA), α-smooth muscle actin (ab32575, 1:1000, Abcam, Cambridge, MA, USA), SNAI1 (3879P, 1:1000, Cell Signaling Technology, Trask Lane Danvers, MA, USA), GAPDH (ab216347, 1:1000, Abcam, Cambridge, MA, USA). On the second day, horseradish peroxidase-labeled secondary antibody (ZB-2301, goat anti-rabbit IgG at a concentration of 1:2000, ZSGB-BIO, Beijing, China) was added dropwise for 1 hour at room temperature. After the end of the immunological reaction, cells were illuminated and developed with a Western blot chemiluminescence reagent (Invitrogen, Carlsbad, CA, USA). The images were captured using a Western blot imaging system (Bio-rad ChemiDoc Touch, USA) and analyzed using Image J version software (NIH, Bethesda, MD, USA).
Cell Immunofluorescence Analysis
Cells from different groups were washed three times with PBS and fixed with 4% paraformaldehyde for 20 min at room temperature. After washing 3 times with PBS, permeabilized with permeabilization buffer (0.2% Triton X-100 in PBS) at room temperature, After three washings with PBS, cells were blocked with 10% donkey serum albumin (cat. no. SL050, Solarbio; China) for 30 min at 37 ° C and incubated overnight at 4 ° C with the primary antibodies described above. The dilutions were as follows: Rabbit anti-E-cadherin (1:200), anti-α-SMA (1:200) and anti-SNAI1 (1:200). Cells were stained with FITC-labeled goat anti-rabbit immunoglobulin G (1:200; cat. no. SA00013-2, Proteintech; USA) for 2 h at 37 ° C and DAPI (cat. no. C0065, Solarbio; China) for 5 min at 37 ° C to visualize the nuclei. The images were captured using a confocal microscope (Zeiss GmbH, Jena, Germany, magnification, x200).
Statistical Analysis
Statistical analysis was performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA), and the data was expressed as mean ± standard deviation. Data between two groups were analyzed by the unpaired t-test. The one-way analysis of variance (ANOVA) test was used to determine the statistical difference between three groups. The values of P< 0.05 were considered significant.
Results
Bevacizumab Causes the Epithelial-Mesenchymal Transition(EMT) in ARPE-19 Cells
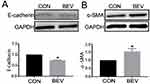
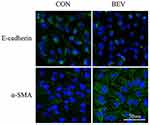
The effect of bevacizumab on EMT in ARPE-19 cells was determined by detecting an epithelial marker (E-cadherin) and a mesenchymal marker (α-smooth muscle actin). Western blot results showed that 0.25 mg/mL of bevacizumab treatment caused loss of E-cadherin expression (Figure 1A) and increased expression of α-smooth muscle actin (Figure 1B), Immunofluorescence results showed that compared to ARPE-19 cells in the blank control group, the bevacizumab(BEV) group significantly decreased E-cadherin expression and upregulated the expression of α-SMA. (Figure 2). These findings significantly indicate that bevacizumab induced EMT in ARPE-19 cells under our experimental conditions.
The Bevacizumab-Induced EMT Was Regulated by miR-21 in ARPE-19 Cells
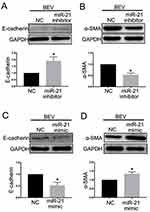
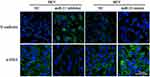
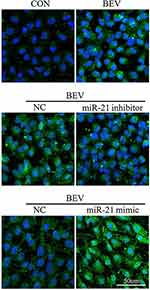
The level of miR-21 in ARPE-19 cells was evaluated by qRT-PCR. The results showed that miR-21 expression in bevacizumab-treated ARPE-19 cells was up-regulated compared to the control group (1.02 ± 0.025 vs 2.43 ± 0.345, P< 0.05) (Figure 3). To determine the role of miR-21 in regulating the EMT induced by bevacizumab, the expression level of miR-21 was decreased by transfected with miR-21 inhibitor and increased by transfected with miR-21 mimic when cells were exposed to bevacizumab. The results of Western blot showed that E-cadherin expression in the miR-21 inhibitor group was obviously higher than in the negative control group and the α-smooth muscle actin was significantly reduced in the miR-21 inhibitor group than in the negative control group (Figure 4A and B). In contrast, E-cadherin expression in the miR-21 mimic group was significantly down regulated compared to the negative control group and the α-smooth muscle actin was up regulated in the miR-21 mimic group than in the negative control group (Figure 4C and D). Immunofluorescence results revealed that the miR-21 inhibitor significantly attenuated the down-regulation of E-cadherin and the up-regulation of α-SMA compared to negative control cells, while miR-21 mimics increased the down-regulation of E-cadherin and the up-regulation of α-SMA(Figure 5). These findings demonstrated that the low expression of E-cadherin and the high expression of α-smooth muscle actin caused by bevacizumab were reversed by decreasing miR-21 expression and enhanced by increasing miR-21 expression, suggesting that miR-21 may play a critical role in the regulation of bevacizumab-induced EMT in ARPE-19 cells.
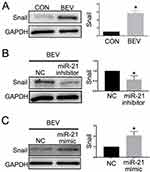
MiR-21 Mediates Bevacizumab-Induced SNAI1 Production in ARPE-19 Cells
SNAI1 is a known mesenchymal marker. In this study, we detected a positive correlation between miR-21 and SNAI1 in the EMT response of ARPE-19 induced by bevacizumab. As expected, bevacizumab significantly increased the expression of the SNAI1 protein in ARPE-19 cells (Figure 6A). Inhibition of miR-21 using the miR-21 inhibitor suppressed SNAI1 production enhanced by bevacizumab (Figure 6B) and promotion of miR-21 using the miR-21 mimic aggravated SNAI1 expression (Figure 6C). Similar results were observed by cell immunofluorescence analysis (Figure 7). The results indicated that miR-21 could promote bevacizumab-induced EMT in ARPE cells, and this process is closely related to the interaction between miR-21 and SNAI1.
Discussion
Bevacizumab and other anti-VEGF drugs are widely used in the treatment of ocular neovascular diseases. After injection of anti-VEGF drugs, when the balance between VEGF and CTGF changes to a certain threshold ratio, the vascular fibrosis switch occurs, and excessive CTGF-driven fibrosis leads to scarring and blindness.7 The EMT of RPE cells plays an important role in the occurrence and development of ocular fibrosis.8,18 In this study, we confirmed the results consistent with clinical trials, that is, bevacizumab can induce EMT in ARPE-19 cells. MiRNA is a small noncoding single-stranded RNA that can be used as a molecular target for disease diagnosis and treatment. MiRNAs, such as miR-148a and miR-124, are associated with EMT in RPE cells.19–21 We found that miR-21 in the anterior chamber of patients with AMD after anti-VEGF treatment was significantly increased, suggesting that anti-VEGF treatment can change the expression level of miR-21, and that miR-21 may be involved in the occurrence of subretinal fibrosis after anti-VEGF treatment (data not shown). This is consistent with the up-regulation of miR-21 in the anti-VEGF-induced EMT process. Furthermore, we found that miR-21 decreased E-cadherin expression and increased α-SMA expression of α-SMA by up-regulating and down-regulating miR-21 content, which had a positive regulatory effect on bevacizumab-induced EMT. Therefore, miR-21 promotes EMT of RPE cells, as well as lung, liver, gastrointestinal, and other organs. SNAI1 is usually maintained at a low level in ARPE-19 cells, but our experiment showed that bevacizumab treatment significantly increased SNAI1 expression in ARPE-19 cells.22 SNAI1 overexpression can directly trigger EMT in ARPE-19 cells. MiR-21 enhanced by miR-21 mimic increased SNAI1 production, while miR-21 inhibited by miR-21 inhibitor decreased SNAI1 production. And SNAI1-induced miR-21 expression in cancer cells has been confirmed by many experts.17,23 Therefore, these results suggest that miR-21 and SNAI1 can interact and jointly regulate EMT in ARPE-19 cells under bevacizumab treatment. Of course, what I have achieved so far is only a preliminary conclusion, and the relevant mechanism needs to be confirmed by further experiments in the future, which is also one of the limitations of this article. Another disadvantage is the lack of animal experiments. In Phase II experiments, we will set up animal experiments to jointly verify the possible pathway mechanism in vivo and in vitro.
We reviewed a large number of literatures. The data of AMD patients and CNV animals showed that CNV-related subretinal fibrosis was mainly through TGF-β1/Smad2/3 signal transduction, leading to SNAI1-mediated EMT changes in RPE cells.24–26 External regulated kinase 1/2 (ERK 1/2) signaling pathway is an important part of the MAPK signaling pathway. The activation of the MAPK signaling pathway can regulate a variety of transcription factors, including SNAI1, which is similar to the typical TGF-β/Smad signaling pathway, has a complex interaction in the regulation of EMT in RPE cells.27,28 Prompt TGF-β/ ERK1/2 signaling pathway may be a potential regulator and therapeutic target of subretinal fibrosis in AMD.29 Because SNAI1 and miR-21 are closely related, they are jointly involved in the process of anti-VEGF-induced EMT process, so the mechanism of the EMT pathway in RPE cells that miR-21 and SNAI1 are jointly involved in is our next focus.
Conclusion
In conclusion, our results show that miR-21 and SNAI1 interact and jointly participate in the regulation of bevacizumab-induced EMT in RPE cells. Inhibition of miR-21 could attenuate bevacizumab-induced EMT. MiR-21 could be a potential miRNA-based therapeutic target in reducing anti-VEGF agent-induced subretinal fibrosis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Menna F, Meduri A, Lupo S, Vingolo EM. Wamd: from pathophysiology to therapeutic treatments. Biomedicines. 2022;11(1):10. doi:10.3390/biomedicines11010010
2. Yu E, Song Y, Gu SM, et al. Alpinumisoflavone ameliorates choroidal neovascularisation and fibrosis in age-related macular degeneration in in vitro and in vivo models. Sci Rep. 2022;12(1):14316. doi:10.1038/s41598-022-18531-y
3. Hussain RM, Ciulla TA. Emerging vascular endothelial growth factor antagonists to treat neovascular age-related macular degeneration. Expert Opin Emerg Drugs. 2017;22(3):235–246. doi:10.1080/14728214.2017.1362390
4. Bakri SJ, Thorne JE, Ho AC, et al. Safety and efficacy of anti-vascular endothelial growth factor therapies for neovascular age-related macular degeneration: a report by the American Academy of Ophthalmology. Ophthalmology. 2019;126(1):55–63. doi:10.1016/j.ophtha.2018.07.028
5. Core JQ, Pistilli M, Hua P, et al. Predominantly persistent intraretinal fluid in the comparison of age-related macular degeneration treatments trials. Ophthalmol Retina. 2022;6(9):771–785. doi:10.1016/j.oret.2022.03.024
6. Lu Y, Huang J, Zhao J, et al. Effects of intravitreal ranibizumab injection on Chinese patients with wet age-related macular degeneration: 5-year follow-up results. J Ophthalmol. 2016;2016:6538192. doi:10.1155/2016/6538192
7. Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, et al. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One. 2008;3(7):e2675. doi:10.1371/journal.pone.0002675
8. Saika S, Yamanaka O, Flanders KC, et al. Epithelial-mesenchymal transition as a therapeutic target for prevention of ocular tissue fibrosis. Endocr Metab Immune Disord Drug Targets. 2008;8(1):69–76. doi:10.2174/187153008783928343
9. Hill M, Tran N. miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech. 2021;4:14.
10. Zhou L, Chen W, Yang H, et al. Calycosin inhibits the malignant behaviors of lung adenocarcinoma cells by regulating the Circ_0001946/Mir-21/Gpd1l/Hif-1alpha signaling axis. Dis Markers. 2022;2022:3969389. doi:10.1155/2022/3969389
11. Dai L, Chen F, Zheng Y, et al. miR-21 regulates growth and emt in lung cancer cells via PTEN/Akt/Gsk3beta signaling. Front Biosci. 2019;24(8):1426–1439. doi:10.2741/4788
12. Liu LH, Fang CK, Ge FC, et al. Jphyd inhibits Mir-21-5p/Smad7-mediated epithelial-mesenchymal transition of hepatocellular carcinoma cells. J Oncol. 2022;2022:7823433. doi:10.1155/2022/7823433
13. Xu G, Meng L, Yuan D, et al. Meg3/Mir‑21 axis affects cell mobility by suppressing epithelial‑mesenchymal transition in gastric cancer. Oncol Rep. 2018;40(1):39–48. doi:10.3892/or.2018.6424
14. Zhang S, Sun P, Xiao X, et al. microRNA-21 promotes epithelial-mesenchymal transition and migration of human bronchial epithelial cells by targeting poly (Adp-Ribose) polymerase-1 and activating Pi3k/Akt signaling. Korean J Physiol Pharmacol. 2022;26(4):239–253. doi:10.4196/kjpp.2022.26.4.239
15. Li H, Wang H, Wang F, et al. Snail involves in the transforming growth factor beta1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells. PLoS One. 2011;6(8):e23322. doi:10.1371/journal.pone.0023322
16. Li H, Li M, Xu D, et al. Overexpression of snail in retinal pigment epithelial triggered epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2014;446(1):347–351. doi:10.1016/j.bbrc.2014.02.119
17. Cheng HY, Hsieh CH, Lin PH, et al. Snail-regulated exosomal microRNA-21 suppresses Nlrp3 inflammasome activity to enhance cisplatin resistance. J Immunother Cancer. 2022;7:10.
18. Subramani M, Ponnalagu M, Krishna L, et al. Resveratrol reverses the adverse effects of bevacizumab on cultured Arpe-19 cells. Sci Rep. 2017;7(1):12242. doi:10.1038/s41598-017-12496-z
19. Jun JH, Joo CK. microRNA-124 controls transforming growth factor beta1-induced epithelial-mesenchymal transition in the retinal pigment epithelium by targeting Rhog. Invest Ophthalmol Vis Sci. 2016;57(1):12–22. doi:10.1167/iovs.15-17111
20. Takayama K, Kaneko H, Hwang SJ, et al. Increased ocular levels of microRNA-148a in cases of retinal detachment promote epithelial-mesenchymal transition. Invest Ophthalmol Vis Sci. 2016;57(6):2699–2705. doi:10.1167/iovs.15-18660
21. Wei Q, Zhang T, Jiang R, et al. Vitreous fibronectin and fibrinogen expression increased in eyes with proliferative diabetic retinopathy after intravitreal Anti-VEGF therapy. Invest Ophthalmol Vis Sci. 2017;58(13):5783–5791. doi:10.1167/iovs.17-22345
22. Li C, Song L, Zhang Z, et al. microRNA-21 promotes Tgf-Beta1-induced epithelial-mesenchymal transition in gastric cancer through up-regulating PTEN expression. Oncotarget. 2016;7(41):66989–67003. doi:10.18632/oncotarget.11888
23. Hsieh CH, Tai SK, Yang MH. Snail-overexpressing cancer cells promote M2-like polarization of tumor-associated macrophages by delivering Mir-21-abundant exosomes. Neoplasia. 2018;20(8):775–788. doi:10.1016/j.neo.2018.06.004
24. Hirasawa M, Noda K, Noda S, et al. Transcriptional factors Associated with epithelial-mesenchymal transition in choroidal neovascularization. Mol Vis. 2011;17:1222–1230.
25. Ishikawa K, Kannan R, Hinton DR. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Exp Eye Res. 2016;142:19–25. doi:10.1016/j.exer.2015.03.009
26. Iwanishi H, Fujita N, Tomoyose K, et al. Inhibition of development of laser-induced choroidal neovascularization with suppression of infiltration of macrophages in Smad3-Null Mice. Lab Invest. 2016;96(6):641–651. doi:10.1038/labinvest.2016.30
27. Cheng X, Wang L, Wen X, et al. Tnap is a novel regulator of cardiac fibrosis after myocardial infarction by mediating Tgf-Beta/Smads and Erk1/2 signaling pathways. EBioMedicine. 2021;67:103370. doi:10.1016/j.ebiom.2021.103370
28. Zhang C, Qin S, Xie H, et al. Ro4929097, a selective gamma-secretase inhibitor, inhibits subretinal fibrosis via suppressing notch and Erk1/2 signaling in laser-induced mouse model. Invest Ophthalmol Vis Sci. 2022;63(10):14. doi:10.1167/iovs.63.10.14
29. Liu D, Zhang C, Zhang J, et al. Molecular pathogenesis of subretinal fibrosis in neovascular AMD focusing on epithelial-mesenchymal transformation of retinal pigment epithelium. Neurobiol Dis. 2023;185:106250. doi:10.1016/j.nbd.2023.106250
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.