Back to Journals » Psychology Research and Behavior Management » Volume 12
miR-195-Sirt3 Axis Regulates Angiotensin II-Induced Hippocampal Apoptosis and Learning Impairment in Mice
Authors Fan X, Xiao M, Zhang Q, Li N, Bu P
Received 28 June 2019
Accepted for publication 11 October 2019
Published 6 December 2019 Volume 2019:12 Pages 1099—1108
DOI https://doi.org/10.2147/PRBM.S221209
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Igor Elman
Xiaosheng Fan, 1, 2 Ming Xiao, 1 Qinghai Zhang, 1 Na Li, 1 Peili Bu 1
1The Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education and Chinese Ministry of Health, The State and Shandong Province Joint Key Laboratory of Translational Cardiovascular Medicine, Qilu Hospital of Shandong University, Jinan, Shandong 250012, People’s Republic of China; 2Department of Cardiology, Laiwu People’s Hospital, Laiwu, Shandong 271100, People’s Republic of China
Correspondence: Peili Bu
The Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education and Chinese Ministry of Health, The State and Shandong Province Joint Key Laboratory of Translational Cardiovascular Medicine, Qilu Hospital of Shandong University, Jinan, Jinan 250012, People’s Republic of China
Email [email protected]
Objective: Apoptosis plays an essential role in cell development and aging, which is associated with a series of diseases, such as neurodegeneration. MircoRNAs exert important roles in the regulation of gene expression. As a stress-responsive deacetylase in mitochondria, sirtuin-3 (sirt3) is a key regulator for mitochondrial function and apoptosis. Also, miR-195 has been demonstrated to be involved in cell cycle and apoptosis. Therefore, this study aimed to investigate the effects of miR-195-sirt3 axis on angiotensin II (ANG II)-induced hippocampal apoptosis and behavioral influence.
Materials and methods: ANG II infusion was used to establish the hypertensive model in HT22 cells and 129S6/SvEvTac mice, respectively. TUNEL assay was used to evaluate the apoptosis level. Mitochondrial membrane potential (MMP) was measured to evaluate the mitochondrial property. Immunohistochemistry, RT-PCR, Western blotting, and luciferase reporter assay were conducted to determine the underlying molecular mechanism.
Results: The results revealed that ANG II treatment promoted apoptosis in the hippocampal cells and tissues, along with increased sirt3 and decreased miR-195 expression. Silencing sirt3 by genetic engineering or siRNA reversed ANG II-induced hippocampal apoptosis. Sirt3 was identified as a direct target gene of miR-195. Forced expression of miR-195 could play counteractive roles in hippocampal apoptosis induced by ANG II. Furthermore, the behavioral assay demonstrated that ANG II-induced hippocampal apoptosis impaired the performance in the spatial navigation task, but not in the spatial memory task.
Conclusion: The results suggested that miR-195-sirt3 axis plays an important role in the ANG II-induced hippocampal apoptosis via altering mitochondria-apoptosis proteins and mitochondria permeability and that hippocampal apoptosis is associated with impaired learning capability in hypertensive mice. This study provides insights into the molecular architecture of apoptosis-related neurodegenerative diseases.
Keywords: Sirt3, miR-195, hippocampus, apoptosis, hypertension, mitochondria, learning
Corrigendum for this paper has been published
Introduction
Apoptosis is a common type of programmed cell death that is a homeostatic regulator that maintains the cell population in multicellular organisms.1 The mechanisms of apoptosis are highly complicated and involve sophisticated molecular and cellular cascades. There are two interactive pathways mediating apoptosis, namely, the extrinsic pathway associated with the ligand-receptor pattern and the intrinsic pathway that is highly related to the involvement of mitochondria.2 To date, apoptosis has been demonstrated to be highly associated with a series of neurodegenerative diseases, such as Alzheimer’s disease,3 Parkinson’s disease,4 and ischemia.5
Furthermore, as an important risk factor, the correlation between hypertension and apoptosis has also been uncovered in neurodegenerative diseases.6,7 Growing evidence demonstrated that hypertension is involved in aberrant cognition ability and dementia in both humans and rodents.8,9 For example, hypertension-associated aberrant synaptic plasticity and synapse loss in the hippocampus lead to cognitive impairment in mice, displaying aging-like phenotypes.10 Moreover, Moonga et al reported that hypertension also links to declined cognition and hippocampal glucose hypometabolism, worsening the phenotype of Alzheimer’s disease.11 Therefore, exploring the mechanism of hypertension-associated apoptosis would open an important window for understanding the underlying mechanisms for these diseases.
MicroRNAs (miRNAs), 21–24 nucleotides, are a group of small non-coding RNAs that play important roles in the post-transcriptional regulation.12 As an important regulator of gene expression, miRNAs bind 3ʹUTR of target genes, thereby silencing gene expression through inducing mRNA degradation or inhibiting subsequent translation.13 It has been demonstrated that miR-195 exerts essential roles in various diseases, including schizophrenia, cardiovascular disease, and cancer.14 Also, numerous studies suggest that miR-195, along with its target genes including WEE1, CDK6, and Bcl-2, regulate cell cycle and apoptosis, in particular, miR-195 promotes apoptosis while suppresses cell proliferation.15
Sirtuin-3 (sirt3), belonging to the sirtuin family, is primarily distributed in the mitochondria.16 Sirt3, a highly conserved deacetylase, is critical for the regulation of mitochondrial function and the anti-oxidative process.17 Many lines of evidence reveal that sirt3 is associated with several neuronal apoptosis-related diseases.18 So far, the functions of sirt3 in neuronal apoptosis are still not completely understood. Thus, this study aimed to investigate the effects of miR-195-sirt3 axis on angiotensin II (ANG II)-induced hippocampal apoptosis in mice and elucidate the underlying mechanism.
Materials and Methods
Animals
In this study, experimental protocols were approved by the Ethical Review Board of Shandong University. All animal procedures and care were performed according to the Animal Care and Treatment Administration of the National Ministry of Health and the requirement of the Ethics Committee in Qilu Hospital of Shandong University (No. 2017047). Male sirt3-knockout (sirt3-KO) 129S6/SvEvTac mice aging 6–7 weeks were purchased from the Jackson Laboratory (Bar Harbor, USA). Male wild-type (WT) 129/SvlmJ mice at the same age (6 weeks) were purchased from the Animal Center Laboratory at Peking University (Beijing, China). All mice were individually housed at 20–26°C with 40–70% relative humidity and a 12:12-h light-dark cycle (6:00 AM to 6:00 PM). Water and food were given ad libitum.
Establishment of the Hypertensive Mouse Model
The hypertensive mouse model was established by systemic ANG II (Sigma Chemical, Co., St. Louis, USA) infusion (1500 ng/kg/min) into wild-type mice via subcutaneous osmotic minipumps (Alzet, Cupertino, USA) for 21 days. Blood pressure and heart rate were measured daily from one day before the infusion to one-day post-infusion, and the procedures followed those described in previous reports.19,20
Cell Lines
The hippocampal cell line HT22 was a gift obtained from the Cardiovascular Remodeling and Function Research Key Laboratory in Qilu Hospital of Shandong University (Jinan, China) and the usage of HT22 was approved by the Ethical Review Board of Shandong University. Cells were cultured in DMEM medium containing 10% fetal bovine serum (Gibco BRL, Grand Island, USA) under a condition of 5% CO2 at 37 °C. The ANG II treatment was performed at a concentration of 5–10 nmol/L for 48 hrs.
Three candidate sirt3-siRNAs (si-sirt3-340, si-sirt3-752 and si-sirt3-1613) were synthesized by GenePharma Co., Ltd (Shanghai, China). The transfection of sirt3-siRNAs into HT22 cells was conducted according to the manufacturer’s instructions of Lipofectamine™ 2000 Transfection Reagent (Invitrogen, Waltham, USA). The sirt3-siRNA with the highest transfection efficiency was selected for subsequent experiments.
Quantification of Apoptosis
Mouse hippocampal tissues were collected and fixed in 4% paraformaldehyde in PBS containing 0.12 mM sucrose for 15 mins. Apoptosis was evaluated by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) apoptosis detection kit (Beyotime Institute of Biotechnology, Nantong, China) according to the manufacturer’s instructions. The apoptotic cells were observed by fluorescence microscopy (Carl Zeiss, Germany).
Detection of the Mitochondrial Membrane Potential (MMP)
The MMP was detected by the MitoProbe JC-1 Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer’s instructions. The ratio of decreased red to increased green fluorescence intensity was evaluated. Red color represents mitochondrial membrane potential indicator (m-MPI) accumulates in the mitochondria in healthy cells while green color indicates m-MPI accumulates that are converted to green fluorescent monomers when mitochondrial membrane potential (MMP) depolarizes and cells in a less healthy state. The cells were observed via the confocal laser scanning microscope (Nikon Instruments, Melville, USA) and the changes of mitochondrial membrane potential were evaluated by the red/green fluorescence intensity ratio.
Immunohistochemistry (IHC) Analysis for Expressions of Apoptosis-Associated Factor
Mouse hippocampal tissues were fixed in 4% paraformaldehyde for 48 h, embedded in paraffin, and cut into 3–5 μm sections (5 sections for each animal). The detailed procedures followed those in the report.21 The primary antibodies for caspase-3, Bcl-2, bax, and CytC (Santa Cruz, Shanghai, China) were applied. Three random regions in the sections were selected to analyze.
Real-Time PCR
Total RNA was extracted from HT22 cells or hippocampal tissues following the manufacturer’s instructions for the RNeasy Mini kit (Qiagen, Hilden, Germany). Then, cDNA was synthesized by using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, USA) following the manufacturer’s instructions. Real-time PCR reactions were performed on a Lightcycler (Roche, Mannheim, Germany). Gene expression data were analyzed using the 2−ΔΔCt method,22 and β-Actin was used as a constitutive control. The primers were used as followed: miR-195(Forward, 5ʹ-CCTAGCAGCACAGAAA-3ʹ; Reverse, 5ʹ- GAGCAGGCTGGAGAA-3ʹ), sirt3 (Forward, 5ʹ-CTGGATGGACAGGACAGATAAG-3ʹ; Reverse, 5ʹ-TCTTGCTGGACATAGGATGATC-3ʹ), and β-Actin (Forward, 5ʹ-CAGGGCGTGATGGTGGGCA-3ʹ; Reverse, 5ʹ- CAAACATCATCTGGGTCATCTTC-3ʹ).
Western Blot Analysis
Apoptosis-related proteins in cells and tissues were measured by Western blotting (n=6 for each group). Total protein was isolated from hippocampal tissues and HT22 cells by using cell lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China). Protein extracts were separated by 8%–12% SDS-PAGE electrophoresis and electrically transferred to PVDF membranes, which were blocked with 5% skim milk in PBS-0.05% Tween 20 for 2 hrs at room temperature. Subsequently, the primary antibody for β-actin (Catalog #: sc-47778), caspase-3 (Catalog #: sc-56053), Bcl-2 (Catalog #: sc-509), bax (Catalog #: sc-20067), CytC (Catalog #: sc-13560), and sirt3 (Catalog #: sc-365175) (Santa Cruz, Shanghai, China) respectively, was applied overnight at 4°C. The membranes were then incubated with their respective secondary antibody for 1 hr at 37°C and visualized by enhanced chemiluminescence assays (Thermo Fisher Scientific, Rockford, Ill). Optical densities of bands were normalized to the abundance of β-actin and analyzed by using ImageJ software.23
miR-195 Mimics Transfection in vitro and Delivery in vivo
MiR-195 mimics and negative control (20 nmol/l) were obtained from RiboBio (Guangzhou, China). Transfection procedure was performed by using cardiomyocytes by using Lipofectamine™ 2000 Transfection Reagent (Invitrogen, Waltham, USA) following the manufacturer’s instructions. MiR-195 Mimics and Negative control were mixed with Invivofectamine® 2.0 reagent (Thermo Fisher Scientific, Waltham, USA) and injected into tail veins of ANG II-treated mice (7 mg/kg body weight) on three consecutive days. Twenty-four hours post-injection, expression of miR-195 was evaluated by RT-PCR.
Luciferase Reporter Assay
The target gene of miR-195 was analyzed by several online tools, including TargetScan (www.targetscan.org), MiRcode (http://www.mircode.org/index.php), and miRDB (http://www.mirdb.org/). A putative binding site of miR-195 was predicted in the 3ʹUTR of sirt3. The luciferase reporter assay kit (Promega, Fitchburg, USA) was applied to verify the online prediction that there is a miR-195 binding site in 3ʹUTR of sirt3. The protocol was performed in accordance with the manufacturers’ instructions.
Behavioral Analysis
The Morris Water Maze assay was applied to evaluate the spatial learning and memory performances of mice and the detailed procedures were described in another study.24 The spatial navigation task was conducted in the first five days and the spatial memory task was performed on the sixth day.
Statistical Analysis
Data were analyzed by SPSS 19.0 software (SPSS Inc, Chicago, USA) and were expressed as mean ± S.D. Mean differences between groups were analyzed using the Tukey’s test. In this study, differences were regarded to be significant at P < 0.05.
Results
ANG II Treatment Enhanced the Expression of Sirt3 and Hippocampal Apoptosis
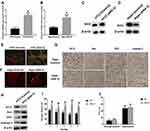
In this study, HT22 cells and wide-type mice were subjected to ANG II treatment to establish the hypertensive cell and mouse model, respectively.19,20 The average blood pressures and heart rates of hypertensive mice were 160/100 mmHg and 644 beats per minute (BPM), respectively. The results revealed that sirt3 mRNA (Figure 1A and B) and protein (Figure 1C and D) expression were increased in HT22 cells and hippocampal tissues of hypertensive mice, respectively. Meanwhile, MMP assay indicated that ANG II was associated with decreased MMP in HT22 cells (Figure 1E), suggesting the apoptosis level of HT22 was elevated by ANG II. Also, the TUNEL assay demonstrated that more apoptotic cells were found in the hippocampal tissues treated with ANG II compared with the control group (Figure 1F). Collectively, these results together indicated ANG II exerted the pro-apoptotic effect on both hippocampal cells and tissues. To further investigate the pro-apoptotic effect of ANG II, the expressions of apoptosis-associated factors were evaluated by Western blot and IHC in HT22 cells and hippocampal tissues, respectively (Figure 1H and G). The results showed that the level of Bcl-2 was reduced while the levels of Bax, CytC, and caspase-3 were increased in both hippocampal cells and tissues, indicating the increased apoptotic level was promoted by ANG II. Furthermore, we tested the behavioral impact of ANG II in hypertensive mice. In the spatial navigation task, mice treated with ANG II displayed longer latency time to reach the platform in all five testing days (Figure 1I), indicating the learning was, at least in part, impaired by ANG II treatment. However, ANG II did not cause the behavioral difference in the spatial memory test (Figure 1J).
The Effect of Sirt3 on ANG II-Induced Hippocampal Apoptosis
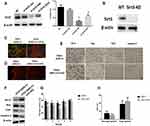
To investigate the role of sirt3 in ANG II-induced hippocampal apoptosis, we applied sirt3-siRNAs to silence sirt3 in HT22 cells while established sirt3-KO mouse model. The efficiency of siRNA and sirt3 protein expression were evaluated by Western blot, and the result indicated that sirt3 was successfully silenced in both HT22 cells and the hippocampal tissues (Figure 2A and B). Meanwhile, HT22 cells treated with both ANG II and si-sirt3 showed higher MMP than those treated with only ANG II (Figure 2C). Also, less apoptotic cells were found in the hippocampal tissues of sirt3-KO mice compared with those of wide-type mice (Figure 2D). In addition, both Western blot and IHC assay revealed that Bcl-2 was increased while Bax, CytC, and caspase-3 were reduced in both sirt3-siRNA treated HT22 cells and hippocampal tissues of sirt3-KO mice (Figure 2F and E). These results together suggested that “sirt3-silencing” may attenuate the pro-apoptotic effect of ANG II in hippocampal cells and tissues. This hypothesis was also verified in the behavioral response of sirt3-KO mice. In the spatial navigation task, sirt3-KO mice treated with performed shorter latency time to reach the platform on the first, third, and fourth testing day (Figure 2G). We did not observe the behavioral difference in the spatial memory assay (Figure 2H)
miR-195 Was Increased in ANG II-Induced Hippocampal Apoptosis
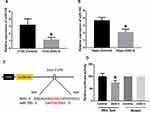
MiRNAs play important roles in neurodegeneration-associated apoptosis.25 By reviewing previous studies, miR-195 has been demonstrated to be essential to the regulation of apoptosis.26 To investigate if miR-195 is associated with ANG II-induced hippocampal apoptosis, we first detected the expression of miR-195 in ANG II-treated HT22 cells and hippocampal tissues. The results suggested that miR-195 expression significantly decreased by ANG II treatment (Figure 3A and B), implying the potential roles of miR-195 in hippocampal apoptosis induced by ANG II. By online prediction tools, a binding site of miR-195 was found in 3ʹ UTR of sirt3 (Figure 3C). Then, we performed a luciferase reporter assay and results showed that luciferase activity was reduced in miR-195-transfected HT22 cells with wild-type 3ʹUTR reporter while there was no difference of luciferase activity in HT22 cells transfected with the mutant 3ʹUTR reporter (Figure 3D). Collectively, these results suggested that miR-195 was involved in the ANG II-induced apoptosis and sirt3 was a direct target gene of miR-195.
Effect of miR-195 on ANG II-Induced Hippocampal Apoptosis
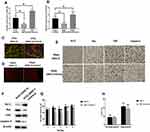
To investigate the effect of miR-195 on ANG II-induced hippocampal apoptosis, we delivered miR-195 mimics to ANG II-treated HT22 cells and hypertensive mice. The RT-PCR results indicated that miR-195 expression was enhanced in both HT cells and hippocampal tissues of hypertensive mice (Figure 4A and B). In addition, HT22 cells treated with both ANG II and miR-195 mimics displayed higher MMP than those only treated with ANG II (Figure 4C). Hippocampal tissues from miR-195 mimics-treated hypertensive mice showed less apoptotic cells than those of hypertensive mice without miR-195 mimics treatment (Figure 4D). For apoptosis-associated factors, miR-195 mimics significantly promoted expression of Bcl-2 while inhibited expressions of Bax, CytC, and caspase-3 in ANG II-treated HT22 cells (Figure 4F) and hippocampal tissues (Figure 4E). Furthermore, miR-195 mimics-treated hypertensive mice displayed shorter time latency time to reach the platform on the second, third, and fourth testing day (Figure 4G). Similarly, the performance in the spatial memory assay was not affected by enhanced expression of miR-195 (Figure 4H). Accumulatively, these results demonstrated that miR-195 mimics reversed the effect of ANG II on the hippocampus.
Discussion
The mitochondria working with a group of apoptosis-related proteins play important roles in neurodegenerative diseases, such as Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease.27 The hippocampus is a key neural region involved in these diseases.28 In addition, previous studies suggest that hypertension is one of primary factors leading to apoptosis and neurodegeneration.29,30 Therefore, the ANG II-induced hypertensive mouse model was used in this study to investigate the functions of miR-195 and sirt3 in hippocampal apoptosis and associated behavioral influence.
In this study, the apoptosis level was enhanced by ANG II treatment in both HT22 cells and hippocampal tissues along with the upregulation of sirt3. Also, silencing sirt3 via either genetic engineering or siRNA displayed counteractive roles to hippocampal apoptosis. To date, the function of the mitochondrial sirt3 in the apoptosis regulation is controversial. In Bcl2-related apoptosis, sirt3 displays a pro-apoptotic effect,31 and cells lacking sirt3 is associated with reduced stress-induced apoptosis.32 On the other hand, sirt3 plays anti-apoptotic roles in preventing cell death in response to cellular DNA damage.33 In this study, we also found that the effect of sirt3 on apoptosis is related to a group of apoptosis-associated factors, such as Bcl-2, Bax, CytC, and caspase-3. It is reported that overexpression of sirt3 leads to apoptosis and elevates the expressions of Bcl-2 and Bax in lung adenocarcinoma cells.34 Sirt3 regulates apoptosis induced by Bcl-2 silencing in epithelial cancer cells.35 Thus, these results collectively demonstrated that sirt3 exerts pro-apoptotic roles in ANG II-induced apoptosis in the hippocampus.
As a multifunctional factor, miR-195 is involved in various cell activities and diseases.15 Liu et al reported that miR-195 inhibits tumorigenicity and enhances apoptosis in colorectal cancer cells.36 MiR-195 has also observed to promotes palmitate-induced apoptosis in cardiomyocytes by silencing Sirt1.26 Guo et al reported that miR-195-EGR3 axis is the core regulatory network for schizophrenia.37 In this study, the downregulation of miR-195 was found to be associated with enhanced hippocampal apoptosis induced by ANG II while forced expression of miR-195 reversed the pro-apoptotic effect of ANG II the hippocampus. Sirt3 was also identified as a direct target gene of miR-195 in hippocampal apoptosis. Thus, these results together imply that ANG II attenuated the inhibitory effect of miR-195 on sirt3, leading to the amplified pro-apoptotic function of sirt3 in hippocampal apoptosis. This hypothesis was also verified by results from silencing sirt3 and enhancing miR-195 experiments, in which miR-195 acts as a sponge of sirt3 in hippocampal apoptosis.
Mitochondrial dysfunction is a major characteristic of apoptosis, which is involved in the intrinsic apoptotic pathway.2 In this study, MMP was downregulated by ANG II treatment while increased apoptosis level was detected by the TUNEL assay. It is well known that the initiation of the intrinsic apoptotic pathway results in changes in mitochondrial membrane properties via the regulation of the Bcl-2 family, such as the inhibition of Bcl-2 and translocation of Bax. In turn, cytochrome c (CytC) leads to the generation of intracellular “apoptosome” which causes the activation of caspase-9. Thus, the signaling from both the intrinsic and extrinsic apoptosis pathways converges on caspase-3/7, eventually triggering terminal pathways of apoptosis.38 In this study, ANG II-induced apoptosis was observed to be associated with these intrinsic pathway regulators mentioned above, suggesting that ANG II-induced apoptosis may mainly rely on the intrinsic pathway of apoptosis regulation.
Beyond the molecular and cellular studies, the Morris Water Maze test was applied in this study to evaluate the effects of apoptosis on memory and learning performance. The results suggested that hypertensive mice showed a longer latency time to navigate the platform. Whereas either sirt3-KO mice treated with ANG II or hypertensive mice treated with miR-195 mimics displayed better performance in navigation test. Furthermore, it is interesting that the performance in spatial memory assay was not affected by hippocampal apoptosis. These results indicated that hippocampal apoptosis impaired learning capability hypertensive mice, and there may be two distinct regulatory pathways regulating behavioral response in these two behavior tests.
Conclusion
In conclusion, the results suggested that sirt3 exerts pro-apoptotic roles in ANG II-induced hippocampal apoptosis while ANG II attenuates the inhibitory effect of miR-195 on sirt3, collectively promoting apoptosis level. Also, activities of apoptosis-associated factors demonstrated that ANG II-induced hippocampal apoptosis might mainly replay on the intrinsic apoptotic pathway. Furthermore, hippocampal apoptosis is associated with impaired learning capability in mice treated with ANG II. This study provides insights into the molecular mechanism and therapeutic strategy of apoptosis-related neurodegenerative diseases.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516.
2. Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288.
3. Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989.
4. Barcia C, Ros C, Annese V, et al. IFN-γ signaling, with the synergistic contribution of TNF-α, mediates cell specific microglial and astroglial activation in experimental models of Parkinson’s disease. Cell Death Dis. 2011;2:e142. doi:10.1038/cddis.2011.17
5. Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi:10.1146/annurev.physiol.010908.163111
6. Goel R, Bhat SA, Hanif K, Nath C, Shukla R. Angiotensin II receptor blockers attenuate lipopolysaccharide-induced memory impairment by modulation of NF-κB-mediated BDNF/CREB expression and apoptosis in spontaneously hypertensive rats. Mol Neurobiol. 2018;55:1725–1739.
7. Goel R, Bhat SA, Rajasekar N, Hanif K, Nath C, Shukla R. Hypertension exacerbates predisposition to neurodegeneration and memory impairment in the presence of a neuroinflammatory stimulus: protection by angiotensin converting enzyme inhibition. Pharmacol Biochem Behav. 2015;133:132–145.
8. Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. 2017;19:24. doi:10.1007/s11906-017-0724-3
9. Iadecola C, Yaffe K, Biller J, et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension. 2016;68:e67–e94.
10. Tucsek Z, Valcarcel-Ares MN, Tarantini S, et al. Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment. GeroScience. 2017;39:385–406.
11. Moonga I, Niccolini F, Wilson H, Pagano G, Politis M. A.s.D.N. Initiative, Hypertension is associated with worse cognitive function and hippocampal hypometabolism in Alzheimer’s disease, European. J Neurol. 2017;24:1173–1182.
12. Flynt AS, Lai EC. Biological principles of microRNA-mediated regulation: shared themes amid diversity. Nat Rev Genet. 2008;9:831–842.
13. Cai Y, Yu X, Hu S, Yu J. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics. 2009;7:147–154. doi:10.1016/S1672-0229(08)60044-3
14. Yang X, Yu J, Yin J, Xiang Q, Tang H, Lei X. MiR-195 regulates cell apoptosis of human hepatocellular carcinoma cells by targeting LATS2. Die Pharmazie Int J Pharm Sci. 2012;67:645–651.
15. He JF, Luo YM, Wan XH, Jiang D. Biogenesis of MiRNA‐195 and its role in biogenesis, the cell cycle, and apoptosis. J Biochem Mol Toxicol. 2011;25:404–408. doi:10.1002/jbt.20396
16. Jablonski RP, Kim SJ, Cheresh P, et al. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. FASEB J. 2017;31:2520–2532. doi:10.1096/fj.201601077R
17. Zhai M, Li B, Duan W, et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT 3‐dependent regulation of oxidative stress and apoptosis. J Pineal Res. 2017;63:e12419. doi:10.1111/jpi.2017.63.issue-2
18. Salvatori I, Valle C, Ferri A, Carri MT. SIRT3 and mitochondrial metabolism in neurodegenerative diseases. Neurochem Int. 2017;109:184–192. doi:10.1016/j.neuint.2017.04.012
19. Widder JD, Guzik TJ, Mueller CF, et al. Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27:762–768. doi:10.1161/01.ATV.0000259298.11129.a2
20. Carlson SH, Wyss JM. Long-term telemetric recording of arterial pressure and heart rate in mice fed basal and high NaCl diets. Hypertension. 2000;35:E1–5. doi:10.1161/01.HYP.35.2.e1
21. Liu W, Xia P, Feng J, et al. MicroRNA-132 upregulation promotes matrix degradation in intervertebral disc degeneration. Exp Cell Res. 2017;359:39–49. doi:10.1016/j.yexcr.2017.08.011
22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi:10.1006/meth.2001.1262
23. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675.
24. Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 2015;129:540–548.
25. Nelson PT, Wang WX, Rajeev BW. MicroRNAs (miRNAs) in neurodegenerative diseases. Brain Pathol. 2008;18:130–138.
26. Zhu H, Yang Y, Wang Y, Li J, Schiller PW, Peng T. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res. 2011;92:75–84. doi:10.1093/cvr/cvr145
27. Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi:10.1038/nature05292
28. Laviola G, Hannan AJ, Macri S, Solinas M, Jaber M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol Dis. 2008;31:159–168. doi:10.1016/j.nbd.2008.05.001
29. Zhu H, Tan L, Li Y, et al. Increased apoptosis in the paraventricular nucleus mediated by AT1R/Ras/ERK1/2 signaling results in sympathetic hyperactivity and renovascular hypertension in rats after kidney injury. Front Physiol. 2017;8:41. doi:10.3389/fphys.2017.00041
30. Karimian G, Buist-Homan M, Mikus B, Henning RH, Faber KN, Moshage H. Angiotensin II protects primary rat hepatocytes against bile salt-induced apoptosis. PLoS ONE. 2012;7:e52647. doi:10.1371/journal.pone.0052647
31. Allison SJ, Milner J. SIRT3 is pro-apoptotic and participates in distinct basal apoptotic pathways. Cell Cycle. 2007;6:2669–2677. doi:10.4161/cc.6.21.4866
32. Kim H-S, Patel K, Muldoon-Jacobs K, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52. doi:10.1016/j.ccr.2009.11.023
33. Yang H, Yang T, Baur JA, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi:10.1016/j.cell.2007.07.035
34. Xiao K, Jiang J, Wang W, et al. Sirt3 is a tumor suppressor in lung adenocarcinoma cells. Oncol Rep. 2013;30:1323–1328. doi:10.3892/or.2013.2604
35. Kim SH, Lu HF, Alano CC. Neuronal Sirt3 protects against excitotoxic injury in mouse cortical neuron culture. PLoS ONE. 2011;6:e14731. doi:10.1371/journal.pone.0014731
36. Liu L, Chen L, Xu Y, Li R, Du X. microRNA-195 promotes apoptosis and suppresses tumorigenicity of human colorectal cancer cells. Biochem Biophys Res Commun. 2010;400:236–240. doi:10.1016/j.bbrc.2010.08.046
37. Guo AY, Sun J, Jia P, Zhao Z. A novel microRNA and transcription factor mediated regulatory network in schizophrenia. BMC Syst Biol. 2010;4:10. doi:10.1186/1752-0509-4-10
38. Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov. 2017;16:273–284. doi:10.1038/nrd.2016.253
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.