Back to Journals » Journal of Inflammation Research » Volume 15
MiR-143 Targets IGF-1R to Suppress Autoimmunity in Thyroid-Associated Ophthalmopathy
Authors Tang W, Lv Q, Huang X, Li Y, Zou J, Zheng J, Sun L , Bao Y, Chen H, Li T, Zhang B, Xue S, Song Y, Zhang X, Chen X, Cai J , Shi Y
Received 22 September 2021
Accepted for publication 27 January 2022
Published 1 March 2022 Volume 2022:15 Pages 1543—1554
DOI https://doi.org/10.2147/JIR.S339483
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Wei Tang,1,* Qian Lv,1,* Xiao Huang,2,* Yuzhen Li,2 JunJie Zou,1 Jiaoyang Zheng,1 Liangliang Sun,1 Yi Bao,1 Haiyan Chen,1 Tuo Li,1 Bei Zhang,1 Song Xue,1 Yan Song,1 Xingxing Zhang,1 Xiangfang Chen,1 Jiping Cai,2 Yongquan Shi1
1Department of Endocrinology, Second Affiliated Hospital of Naval Medical University, Shanghai, 200003, People’s Republic of China; 2Department of Ophthalmology, Second Affiliated Hospital of Naval Medical University, Shanghai, 200003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiangfang Chen; Yongquan Shi, Department of Endocrinology, Second Affiliated Hospital of Naval Medical University, No. 415 Fengyang Road, Huangpu District, Shanghai, 200003, People’s Republic of China, Email [email protected]; [email protected]
Objective: Thyroid-associated ophthalmopathy (TAO) is an autoimmune disease that involves the remodeling of orbit and periorbital tissues. Thyroid-stimulating hormone receptor (TSHR) and insulin-like growth factor 1 receptor (IGF-1R) may stimulate the activation of autoimmunity in TAO, but the exact mechanism is unclear. We investigated whether IGF-1R/TSHR modulation in TAO may involve microRNA regulation.
Methods: We conducted microarray analysis using RNA from the orbital connective tissue samples of 3 healthy and 3 patients with TAO. The involvement of differentially regulated microRNA in IGF-1R/TSHR modulation in TAO was evaluated in orbital fibroblasts (OFs) and female BALB/c mice.
Results: Using hierarchical cluster analysis, we identified that miR-143 was downregulated in TAO. The expression levels of miR-143 in OFs were significantly reduced under IL-1B stimulation. However, OF proliferation and inflammatory responses decreased when miR-143 is overexpressed. In contrast, the suppression of miR-143 increased levels of inflammatory markers (IL-6, IL-8, MCP1) and hyaluronan accumulation. Moreover, overexpression of miR-143 significantly lowers levels of IGF-1R and TSHR. A luciferase assay indicated that miR-143 targets the 3′-UTR of IGF-1R. Increases in the expression of IGF-1R increased the expression of the inflammasome marker NLRP3 and apoptotic marker cleaved caspase-1; however, miR-143 overexpression decreased levels of IGF-1R, TSHR, NLRP3, cleaved caspase 1, IL-1B, and IL-18. In a mouse model of TAO, overexpression of miR-143 significantly reduced levels of IGF-1R and attenuated the adipogenesis associated with TAO.
Conclusion: We found that miR-143 directly targets IGF-1R to alleviate the inflammatory response in TAO by indirectly decreasing levels of TSHR and inactivating NLRP3.
Keywords: thyroid-associated ophthalmopathy, miR-143, thyroid-stimulating hormone receptor, insulin-like growth factor 1 receptor, NLRP3
Introduction
Thyroid-associated ophthalmopathy (TAO) is a tissue-specific autoimmune condition.1,2 The distinct characteristics of TAO involve the remodeling of orbit and periorbital tissues and can include eyelid retraction, dysthyroid optic neuropathy, edema, and proptosis.3 The enlargement of orbital tissues results from the deposition of hyaluronan and an increase in adipogenesis.4 Although different mechanisms give rise to Grave’s disease and TAO they both involve the activities of thyroid-stimulating hormone receptor (TSHR) and TSHR-stimulating antibodies (TSAb) in the thyroid and the orbit.5,6 The exogenous expression of TSHR is known to induce both Grave’s disease and TAO in animal models.7 Antibodies against TSHR (TRAb) are found at high levels in patients with Grave’s disease and are believed to correlate with the severity of TAO.8
Recent evidence suggests that interactions between TSHR and insulin-like growth factor 1 receptor (IGF-1R) may stimulate the activation of autoimmunity in TAO but the exact mechanism remains uncertain.9–11 IGF-1R is thought to participate in the activation of orbital fibroblasts (OFs) in TAO because levels of IGF-1R are elevated in the thyroid and orbital tissues of patients with the disease.6,12 TSHR is also found at high levels in OFs and is believed to form a signal complex with IGF-1R.13 When IGF-1R is blocked by teprotumumab, a monoclonal antibody, the expression of TSHR is also lowered.14 IGF-1R is believed to enhance the expression of TSHR but the exact interaction between the two receptors is unclear.15
NLRP3 is expressed in macrophages and forms part of the inflammasome complex.16,17 It is used as an inflammasome marker and contributes to several diseases associated with the inflammatory response.18,19 In TAO, T and B lymphocytes are thought to direct an inflammatory response that stimulates OF activation and leads to hyaluronan accumulation and the physiological characteristics of the disease.20 Several studies have implicated the inflammatory process in the manifestation of TAO.21,22 In fact, the NLRP3 inflammasome is known to activate interleukin (IL) 1B,23 which in turn stimulates an increase of hyaluronan accumulation in OFs24 and also increases levels of IL-6 and prostaglandin E2.20,25
The identification of microRNA (miRNA) that are associated with TAO has been the focus of recent studies.26–28 For instance, fibroblast proliferation in TAO is thought to be stimulated by TSHR through miR-146a and miR-155 and the induction of the PI3K/Akt pathway.29 Therefore, in this study, we searched for miRNAs that were differentially regulated in TAO and identified miR-143 as a miRNA that was significantly downregulated. MiR-143 is known to target IGF-1R in the regulation of the Ras/p38 MAPK signaling pathway and the proliferation and apoptosis of fibroblasts.30 In this study, we assessed whether miR-143 targets IGF-1R in TAO and the impact this has on TSHR expression and the inflammasome by measuring levels of cytokines and NLRP3. Our findings will enhance knowledge on the etiology of TAO and the involvement of miRNA regulation.
Materials and Methods
Subjects
Orbital connective tissue from 8 TAO patients (4 females and 4 males, mean age 44.87 ± 12.44 years) and 8 healthy control subjects (4 females and 4 males, mean age 44.80 ± 11.97 years) with no history of TAO. The clinical activity score (CAS) system was used to assess the activity of TAO.31 Informed written consent was obtained from all patients and healthy control subjects. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Shanghai Changzheng hospital. All the TAO patients had not received steroid treatment or radiation therapy for at least 3 months before the study.
miRNA Microarray Analysis
The total RNA from the orbital connective tissue samples of healthy and TAO patients were obtained using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The quality and quantity of the samples were assessed using a bioanalyzer (Agilent, Santa Clara, CA, USA). The samples were processed as described in a previous study.32 The arrays were scanned using an Affymetrix scanner (Affymetrix Gene Chip Scanner 3000, Affymetrix, Santa Clara, CA, USA). An expression console (Affymetrix) was used to calculate miRNA expression levels. Relative signal intensities were assessed using a multi-array average algorithm. Quantile normalization and log-transformed values were also analyzed using Agilent Technologies. Fold changes were calculated between healthy and TAO samples. Expression changes between healthy and TAO samples were considered significant if the fold change was more than 1.5.
Orbital Fibroblast Cell Culture
Orbital tissue was minced and suspended in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 with 20% fetal bovine serum (FBS) and penicillin-streptomycin (P/S) and cultured as described in a previous study.33 The cells were passaged at 80% confluency. Passaging was achieved using trypsin/EDTA and cultures were continued with 10% FBS and 1% P/S.
Quantitative Real-Time PCR
Total RNA was isolated from samples using Trizol reagent. After quantification, 500 ng of RNA was used for cDNA synthesis with an M-MLV First Strand Kit (Takara, Kyoto, Japan). Then, 1 µL of cDNA was used to perform quantitative real-time PCR (qPCR). The miRNA was initially reverse transcribed using a Go Script Reverse Transcription System Kit (Promega, Madison, WI, USA) with a stem-loop primer. RNU6B (U6) was measured as an internal control for miRNA. The primers for qPCR are listed in Table 1.
 |
Table 1 Primers Used in the Study |
Vectors and Lentiviral Transduction
All the vectors and lentiviruses used in this study were obtained from Hanbio Biotechnology Company (Shanghai, China). The lentivirus packages contained either miR-143 mimic, negative control miRNA mimic, miR-143 inhibitor, or negative control miRNA inhibitor. The sequences used are as follows: miR-143 mimic, sense: 5′-CACAGAUAGAAGGGCCUCGU-3′, antisense: 5′-CCAGGUGAAGCUACUGCAAG-3′; negative control miRNA mimic, sense: 5′-GCUGAUGUCAGAGAAGCAA-3′, antisense: 5′-CAGAGACAUUUCCUGAAGA-3′; miR-143 inhibitor: 5′-CCUGUGCACUGUGACAAUA-3′; negative control miRNA inhibitor: 5′-GAGGCUGGGUCUAAUUAGU-3′.
Cell Counting Kit-8 (CCK-8) Assay
Cell proliferation was assessed using CCK-8 (Sigma-Aldrich, St Louis, MO, USA) following the manufacturer’s instructions. Briefly, cells were seeded onto 96-well plates at a confluency of 5×103 cells/well. CCK-8 solution (10 µL) was mixed with 90 µL of DMEM and then added to each well. The cells were incubated with CCK-8 solution for 2 h and absorbance was measured at 450 nm.
ELISA in Cell Supernatant and Serum
IL-6, IL-8, MCP1, and hyaluronic acid (HA) protein levels were assessed in cell supernatant and thyroxine (T4) and TRAb levels were assessed in the serum using an ELISA kit (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Luciferase Reporter Assay
For the luciferase reporter assay, the 3′-UTR of IGF-1R was amplified using PCR from human cDNA (forward primer: 5′-GCTGGGGCTCTTGTTTACCA-3′, reverse primer, 5′-CCCTCCAAAAACAAGGGCGA-3′). To mutate the miR-143 binding site, the complementary sequence in the 3′-UTR region of IGF-1R was replaced by the mutated sequence. The PCR products were digested with restriction enzymes and inserted into a reporter plasmid. Cells were seeded and co-transfected with IGF-1R reporter plasmids containing either wild type or mutant 3′-UTR along with miR-143 mimic or control.
Western Blot Analysis
Protein samples were isolated from tissue using M-PER tissue protein extraction reagent (Thermo Fisher Scientific), EDTA, and protease inhibitor cocktail. Protein concentration was assessed using Bradford’s method and an equal concentration of protein were loaded onto gels and separated using SDS-PAGE. Proteins were transferred onto a PVDF membrane, which was blocked using 5% skim milk in TBS-0.1% Tween buffer. Membranes were incubated in the presence of primary antibodies (anti-NLRP3, anti-cleaved caspase-1, IL-1B, IL-18, TSHR, IGF-1R, or β-actin) overnight at 4°C. The membrane was then incubated with HRP-conjugated secondary antibody for 1 h at room temperature. The blots were washed thoroughly before incubation with chemiluminescence detection fluid which was further imaged using a chemiluminescent reader. Protein bands were quantified by densitometric analysis using Image J software.
Mice and Immunization
All the mice used in this study were female BALB/c mice, which were provided by Jie Si Jie Lab Animal Ltd., Shanghai, China. All animals were allowed free access to food and water and were used in accordance with the standard of Animal Research Ethics Committee of Second Military Medical University/Naval Medical University (Shanghai, China). Mice received 2–4% isoflurane before euthanasia. TAO was induced by injecting mice intramuscularly with adenovirus expressing the TSHR A-subunit (Ad-TSHR289, 109 particles in 50 μL PBS) as described previously.34 Control mice were injected with empty adenovirus in 50 μL PBS. Three injections were delivered over 3-week intervals and mice were euthanized 4 weeks after the final injection. Serum and orbital tissue were collected for analysis. Serum samples were stored at −80°C until assayed for total serum thyroxine (T4) and TSHR antibodies (TRAb).
Adeno-Associated Virus (AAV) Transfection and Experimental Animal Grouping
AAV was designed and constructed by Hanbio Biotechnology Company (Shanghai, China). Mice in the experimental groups TAO+AAV-NC (n=6), TAO+AAV-miR-143 (n=6), and TAO+AAV-miR-143-antago (n=6) were first injected through the tail vein with either AAV-NC, AAV-miR-143, or AAV-miR-143-antago (1012 vg particles/mouse), respectively. Two weeks later mice were immunized with Ad-TSHR289 (109 particles in 50 μL PBS) to induce TAO as described above.
Histological Examination
The histological examination of sections from the orbital tissue of mice was performed as described previously.35 Briefly, fixed (10% formalin) and paraffin-embedded tissues were stained with hematoxylin and eosin (HE). Images were captured using a compound microscope with dedicated software (Olympus, Tokyo, Japan).
Statistical Analysis
All data are presented as the means and standard deviations of at least three independent experiments. Statistical analyses were performed using GraphPad Prism v6 (GraphPad Software, La Jolla, CA, USA) and ImageJ software was used in the densitometric analysis. Comparisons were assessed with the Student’s t-test and p-values <0.05 were considered significant.
Results
Demographic and Clinical Characteristics
8 TAO patients and 8 control subjects were included in the study. No significant difference was found in age (P = 0.258) or gender (P = 0.991) between the two groups. The CAS was 2.42 ± 1.38 of TAO patients. Compared with control subjects, TAO patients demonstrated significantly decreased visual acuity (control: 0.99 ± 0.21; TAO: 0.78 ± 0.29, P = 0.004).
miR-143 is Downregulated in TAO
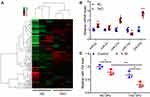
To understand the involvement of miRNAs in TAO, we assessed the differential levels of miRNAs in orbital tissue samples using microarray analysis. Cluster analysis (Figure 1A) clearly illustrated that many miRNAs in TAO tissue samples were highly deregulated compared with the transcriptome of healthy control orbital tissue samples. Among the differential miRNAs, we selected five with altered expression in TAO (miR-22, miR-24, miR-27b, miR-143, and miR-378) and confirmed expression levels with qRT-PCR (Figure 1B). Of these miRNAs, miR-22, miR-27b, and miR-378 were significantly upregulated whereas miR-143 levels were significantly downregulated in TAO samples compared with healthy controls. Next, we selected miR-143 for further assessment. To determine if there was an association between the inflammatory response and the expression levels of miR-143 in TAO, we treated the OFs from TAO and control patient samples with IL-1B stimulation and assessed the levels of miR-143 (Figure 1C). In response to inflammatory stimulation, the expression levels of miR-143 significantly decreased in TAO and control samples. The difference between the decrease in TAO and control samples was also significant. These results indicate a potential role for miR-143 in TAO.
Orbital Fibroblast Proliferation and the Inflammatory Response is Regulated by miR-143 in TAO
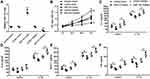
To further understand the role of miR-143 in TAO, we transfected TAO OFs with miR-143 mimics or miR-143 inhibitor with respective controls. We confirmed the efficacy of the transfection using qRT-PCR and that miR-143 mimics increased relative miR-143 levels and a miR-143 inhibitor decreased levels (Figure 2A). Using a CCK-8 assay, we observed that miR-143 mimics decreased OF proliferation, whereas a miR-143 inhibitor significantly increased proliferation (Figure 2B). To assess the response to inflammation, the TAO OFs which were transfected with miR-143 mimics or inhibitors were further stimulated with IL-1B for 24 h. Simultaneously, we assessed the levels of IL-6, IL-18, monocyte chemoattractant protein-1 (MCP1), and HA using ELISA (Figure 2C–F). In the presence of miR-143 mimics, the levels of inflammatory markers (IL-6, IL-8, MCP1) and HA decreased significantly. However, in the presence of a miR-143 inhibitor, the levels of IL-6, IL-8, MCP1, and HA increased (Figure 2C–F). These results indicate that miR-143 is associated with the regulation of proliferation and inflammatory response in TAO.
MiR-143 Binds IGF-1R to Regulate Its Expression
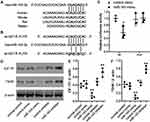
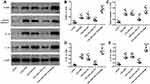
To understand the function of miR-143 in TAO, we assessed its role in regulating its potential target, IGF-1R. We identified that miR-143 potentially binds to the 3′-UTR of IGF-1R and is evolutionarily conserved in humans, mice, rats, and rabbits (Figure 3A). To determine whether miR-143 has a regulatory role with IGF-1R, we designed a luciferase reporter assay with either the wild-type (wt) or mutant (mut) sequence of IGF-1R 3′-UTR. When cells were co-transfected with wt IGF-1R 3′-UTR in the presence of miR-143 mimic, the luciferase activity was significantly decreased compared to the control (Figure 3B). However, such a decrease was not observed in cells transfected with mut IGF-1R 3′-UTR, indicating a loss of miR-143 binding and a reduction in the inhibitory effect on IGF1R (Figure 3C). We additionally confirmed the effect of miR-143 on IGF-1R using Western blotting. Our results indicated that the use of miR-143 mimics significantly decreased IGF-1R and TSHR, however, the use of miR-143 inhibitors significantly reversed this effect and increased the levels of both IGF-1R and TSHR protein levels (Figure 3D–F). Experiments were subsequently performed to understand the role of miR-143. First, we overexpressed IGF-1R in TAO OFs and confirmed its overexpression using Western blotting. IGF-1R overexpression increased TSHR as indicated previously. It was also evident that overexpression of IGF-1R increased the expression of the inflammasome marker NLRP3 and apoptotic marker cleaved caspase-1. Additionally, we observed an increase in levels of proinflammatory markers such as IL-1B and IL-18. However, the use of miR-143 mimics decreased IGF-1R, TSHR, NLRP3, cleaved caspase-1, IL-1B, and IL-18 levels, compared to the control group (Figure 4A–G). These results further confirmed the role of miR-143 in regulating its downstream target IGF-1R and in turn modulating the expression of other inflammatory and apoptotic markers. We next used cells transfected with miR-143 mimics and IGF-1R with their respective controls to assess cell proliferation. The overexpression of IGF-1R increased the proliferation rate, whereas simultaneous use of miR-143 mimics decreased proliferation (Figure 4H). These results indicate that a lack of miR-143 increases IGF-1R which leads to an increase in OF proliferation.
miR-143 Interacts with IGF-1R in TAO in vivo
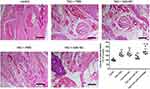
To further explore the role of miR-143 in TAO, we developed an in vivo model, by injecting mice intramuscularly with an adenovirus expressing the TSHR A-subunit (Ad-TSHR289; TAO group). Mice in the TAO group displayed decreased miR-143 levels, compared to the control group (Figure 5A). After 2 weeks, these mice were injected with AAV expressing miR-143. The mice displayed a significant rescue in miR-143 levels. However, anti-miR-143 decreased the levels of miR-143 in TAO mice (Figure 5A). Next, we assessed the levels of IGF-1R using Western blotting. Evidentially, mice in the TAO group displayed a significantly high level of IGF-1R. However, overexpression of miR-143 significantly decreased IGF-1R levels (Figure 5B). The use of anti-miR-143 increased IGF-1R levels. We checked serum T4 and TRAb levels in these mice groups. Interestingly, the serum T4 and TRAb levels were significantly higher in the TAO group than in the control group (Figure 5C and D). Overexpression of miR-143 significantly decreased serum T4 and TRAb levels. However, further use of anti-miR-143 significantly increased serum T4 and TRAb levels (Figure 5C and D). These results further confirm that the lack of miR-143 increases IGF-1R levels leading to an increase of serum T4 and TRAb levels thus contributing to the progression of TAO. Furthermore, we examined the morphological changes in mice orbital tissues either in the presence or absence of miR-143 using HE staining. We observed that TAO models displayed an increased volume of adipose tissue compared to the control. However, overexpression of miR-143 significantly decreased the volume of adipose tissue. Alternatively, the use of anti-miR-143 significantly increased the volume of adipose tissue in TAO mice (Figure 6). These results further confirmed the role of miR-143 in regulating TAO.
Inflammation and Apoptosis are Regulated by miR-143 in a TAO Mouse Model
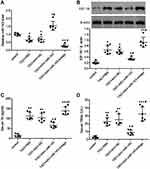
To confirm our results in vitro, we also assessed the levels of NLRP3, cleaved caspase 1, IL-1B, and IL-18 in TAO mice models (Figure 7A–E). We observed that NLRP3, cleaved caspase 1, IL-1B, and IL-18 levels significantly increased in TAO mice. However, when miR-143 is overexpressed, NLRP3, cleaved caspase 1, IL-1B, and IL-18 were significantly decreased. Furthermore, in the presence of anti-miR-143, NLRP3, cleaved caspase 1, IL-1B, and IL-18 increased again. These results further confirmed our in vitro observations, that miR-143 influences TAO by regulating IGF-1R levels, which in turn modulates inflammation and apoptosis, as indicated by changes in the levels of NLRP3, cleaved caspase 1, IL-1B, and IL-18.
Discussion
TAO is a debilitating autoimmune disease that involves the activation of proinflammatory cytokines and increased production of hyaluronan in OFs.4,36 The exact mechanism in the progression of TAO is unclear although several studies implicate TSHR and IGF-1R, which are both upregulated in the OFs of patients with TAO.5,37 The miRNA regulation of genes involved in TAO is gaining interest,38 therefore, in our study, we focused on miRNA that may be involved in the regulation of the inflammasome in TAO using relevant markers such as NLRP3. We found a number of miRNAs that were differentially regulated in the orbital connective tissue of TAO patients and confirmed that three of these were significantly upregulated (miR-22, miR-27b, and miR-378) and one was significantly downregulated (miR-143). Of these, miR-22 and miR-27b have been associated with Grave’s disease and orbitopathy26 and miR-378 has been associated with Sjogren’s syndrome, an autoimmune disease with OF involvement.38 We selected miR-143 for further study because it is known to interact with IGF-1R.30 However, to our knowledge, there are no previous reports on an association between miR-143 and TAO.
In our study, we found that an increase in miR-143 levels using mimics led to a decrease in the levels of IGF-1R and TSHR whereas miR-143 inhibitors reversed this effect and led to increased levels of IGF-1R and TSHR. Additionally, the overexpression of IGF-1R led to increased levels of the inflammasome marker NLRP3. However, increased levels of miR-143 reduced levels of IGF-1R, TSHR, and NLRP3. Overall, our results signify that miR-143 regulates its downstream target IGF-1R to suppress the inflammatory response in TAO. Other studies have also found that the inhibition of IGF-1R can suppress inflammatory responses in TAO.9,39 This has led to the development of teprotumumab, a human IGF-1R inhibiting antibody, which is the only medication currently approved by the US FDA for the treatment of TAO.14,40,41 We obtained similar results to those obtained by teprotumumab in a mouse model of TAO treated with miR-143. HE staining indicated a reduction in the volume of adipose tissue and reduced levels of inflammation in response to the overexpression of miR-143.
From our results, we deduced that the modulation of IGFR1 by miR-143 may, in turn, modulate the inflammasome as demonstrated by NLRP3 levels, this leads to a reduction in TSHR levels and suppresses the characteristics of TAO. We did not examine the interaction between TSHR and IGF-1R in detail however our results suggest that they may interact indirectly rather than directly. Several studies have arrived at similar conclusions, the two receptors share interacting signal pathways but may not necessarily activate each other.5,9 However, immunoprecipitation results in some studies have suggested that TSHR and IGF-1R may colocalize.13
To conclude, our study showed that miR-143 directly targets IGF-1R alleviating the inflammatory response in TAO via a decrease in TSHR and inactivation of NLRP3. Further studies are required on the exact interactions between TSHR and IGF-1R, but our results signify that miR-143 could have potential in the alleviation of TAO and may increase understanding in disease etiology.
Funding
The Demonstration Project for Three-Year Public Health Action Plan (2020–2022) in Shanghai (Grant No. GWV-10.2-XD25). Project of young science foundation of national natural science foundation of China (81800865). National Natural Science Foundation of China (Grant No. 81170728).
Disclosure
Authors declare that there is no conflict of interest.
References
1. Huang Y, Fang S, Zhang S, Zhou H. Progress in the pathogenesis of thyroid-associated ophthalmopathy and new drug development. Taiwan J Ophthalmol. 2020;10:174–180. doi:10.4103/tjo.tjo_18_20
2. Wiersinga WM. Advances in treatment of active, moderate-to-severe Graves’ ophthalmopathy. Lancet Diabetes Endocrinol. 2017;5:134–142. doi:10.1016/S2213-8587(16)30046-8
3. Şahlı E, Gündüz K. Thyroid-associated ophthalmopathy. Turk J Ophthalmol. 2017;47:94–105. doi:10.4274/tjo.80688
4. Hodgson NM, Rajaii F. Current understanding of the progression and management of thyroid associated orbitopathy: a systematic review. Ophthalmol Ther. 2020;9:21–33. doi:10.1007/s40123-019-00226-9
5. Krause G, Eckstein A, Schülein R. Modulating TSH receptor signaling for therapeutic benefit. Eur Thyroid J. 2020;9:66–77. doi:10.1159/000511871
6. Łacheta D, Miśkiewicz P, Głuszko A, et al. Immunological aspects of Graves’ ophthalmopathy. Biomed Res Int. 2019;2019:7453260. doi:10.1155/2019/7453260
7. Xia N, Ye X, Hu X, et al. Simultaneous induction of Graves’ hyperthyroidism and Graves’ ophthalmopathy by TSHR genetic immunization in BALB/c mice. PLoS One. 2017;12:e0174260. doi:10.1371/journal.pone.0174260
8. Eckstein AK, Plicht M, Lax H, et al. Thyrotropin receptor autoantibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91:3464–3470. doi:10.1210/jc.2005-2813
9. Krieger CC, Neumann S, Gershengorn MC. TSH/IGF1 receptor crosstalk: mechanism and clinical implications. Pharmacol Ther. 2020;209:107502. doi:10.1016/j.pharmthera.2020.107502
10. Krieger CC, Neumann S, Gershengorn MC. Is there evidence for igf1r-stimulating abs in Graves’ orbitopathy pathogenesis? Int J Mol Sci. 2020;21(18):21.
11. Smith TJ, Janssen J. Insulin-like growth factor-I receptor and thyroid-associated ophthalmopathy. Endocr Rev. 2019;40:236–267. doi:10.1210/er.2018-00066
12. Smith TJ, Tsai CC, Shih MJ, et al. Unique attributes of orbital fibroblasts and global alterations in IGF-1 receptor signaling could explain thyroid-associated ophthalmopathy. Thyroid. 2008;18:983–988. doi:10.1089/thy.2007.0404
13. Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181:4397–4405. doi:10.4049/jimmunol.181.6.4397
14. Chen H, Mester T, Raychaudhuri N, et al. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J Clin Endocrinol Metab. 2014;99:E1635–E1640. doi:10.1210/jc.2014-1580
15. Paik JS, Kim SE, Kim JH, Lee JY, Yang SW, Lee SB. Insulin-like growth factor-1 enhances the expression of functional TSH receptor in orbital fibroblasts from thyroid-associated ophthalmopathy. Immunobiology. 2020;225:151902. doi:10.1016/j.imbio.2019.151902
16. Jha S, Ting JP. Inflammasome-associated nucleotide-binding domain, leucine-rich repeat proteins and inflammatory diseases. J Immunol. 2009;183:7623–7629. doi:10.4049/jimmunol.0902425
17. Lu A, Wu H. Structural mechanisms of inflammasome assembly. FEBS J. 2015;282:435–444. doi:10.1111/febs.13133
18. Luo D, Liu F, Zhang J, et al. Functional crosstalk between long non-coding RNAs and the NLRP3 inflammasome in the regulation of diseases. Mol Immunol. 2021;131:191–200. doi:10.1016/j.molimm.2020.12.038
19. Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328.
20. Chen B, Tsui S, Smith TJ. IL-1 beta induces IL-6 expression in human orbital fibroblasts: identification of an anatomic-site specific phenotypic attribute relevant to thyroid-associated ophthalmopathy. J Immunol. 2005;175:1310–1319. doi:10.4049/jimmunol.175.2.1310
21. Dik WA, Virakul S, van Steensel L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves’ ophthalmopathy. Exp Eye Res. 2016;142:83–91. doi:10.1016/j.exer.2015.02.007
22. Pawlowski P, Poplawska I, Mysliwiec J, et al. Search of reference biomarkers reflecting orbital tissue remodeling in the course of Graves’ orbitopathy. Folia Histochemica Et Cytobiologica. 2020;58:37–45. doi:10.5603/FHC.a2020.0003
23. He Y, Franchi L, Núñez G. TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol. 2013;190:334–339. doi:10.4049/jimmunol.1202737
24. Kaback LA, Smith TJ. Expression of hyaluronan synthase messenger ribonucleic acids and their induction by interleukin-1beta in human orbital fibroblasts: potential insight into the molecular pathogenesis of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 1999;84:4079–4084. doi:10.1210/jcem.84.11.6111
25. Khong JJ, McNab AA, Ebeling PR, Craig JE, Selva D. Pathogenesis of thyroid eye disease: review and update on molecular mechanisms. Br J Ophthalmol. 2016;100:142–150. doi:10.1136/bjophthalmol-2015-307399
26. Zhang L, Masetti G, Colucci G, et al. Combining micro-RNA and protein sequencing to detect robust biomarkers for Graves’ disease and orbitopathy. Sci Rep. 2018;8:8386. doi:10.1038/s41598-018-26700-1
27. Hu ZJ, He JF, Li KJ, Chen J, Xie XR. Decreased microRNA-146a in CD4+T cells promote ocular inflammation in thyroid-associated ophthalmopathy by targeting NUMB. Eur Rev Med Pharmacol Sci. 2017;21:1803–1809.
28. Thiel J, Alter C, Luppus S, et al. MicroRNA-183 and microRNA-96 are associated with autoimmune responses by regulating T cell activation. J Autoimmun. 2019;96:94–103. doi:10.1016/j.jaut.2018.08.010
29. Woeller CF, Roztocil E, Hammond C, Feldon SE. TSHR signaling stimulates proliferation through PI3K/Akt and induction of mir-146a and mir-155 in thyroid eye disease orbital fibroblasts. Invest Ophthalmol Vis Sci. 2019;60:4336–4345. doi:10.1167/iovs.19-27865
30. Yang Z, Wang J, Pan Z, Zhang Y. miR-143-3p regulates cell proliferation and apoptosis by targeting IGF1R and IGFBP5 and regulating the Ras/p38 MAPK signaling pathway in rheumatoid arthritis. Exp Ther Med. 2018;15:3781–3790. doi:10.3892/etm.2018.5907
31. Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Euro J Endocrinol. 2021;185:G43–g67. doi:10.1530/EJE-21-0479
32. Wang J, Xiao Y, Zhang H. Role of miR-146a in the regulation of inflammation in an in vitro model of Graves’ orbitopathy. Invest Ophthalmol Vis Sci. 2016;57:6795. doi:10.1167/iovs.16-20559
33. Han SY, Choi SH, Shin JS, Lee EJ, Han SH, Yoon JS. High-mobility group box 1 is associated with the inflammatory pathogenesis of Graves’ orbitopathy. Thyroid. 2019;29:868–878. doi:10.1089/thy.2018.0285
34. Li H, Min J, Chen Y, Li H, Zhang Y. Polydatin attenuates orbital oxidative stress in Graves’ orbitopathy through the NRF2 pathway. Chem Biol Interact. 2020;315:108894. doi:10.1016/j.cbi.2019.108894
35. Zhao SX, Tsui S, Cheung A, Douglas RS, Smith TJ, Banga JP. Orbital fibrosis in a mouse model of Graves’ disease induced by genetic immunization of thyrotropin receptor cDNA. J Endocrinol. 2011;210:369–377. doi:10.1530/JOE-11-0162
36. Coulter I, Frewin S, Krassas GE, Perros P. Psychological implications of Graves’ orbitopathy. Euro J Endocrinol. 2007;157:127–131. doi:10.1530/EJE-07-0205
37. Wiersinga WM. Autoimmunity in Graves’ ophthalmopathy: the result of an unfortunate marriage between TSH receptors and IGF-1 receptors? J Clin Endocrinol Metab. 2011;96:2386–2394. doi:10.1210/jc.2011-0307
38. Wei Y, Li N, Zhao L, et al. MicroRNAs and autoimmune-mediated eye diseases. Front Cell Dev Biol. 2020;8:818. doi:10.3389/fcell.2020.00818
39. Mohyi M, Smith TJ. IGF1 receptor and thyroid-associated ophthalmopathy. J Mol Endocrinol. 2018;61:T29–t43. doi:10.1530/JME-17-0276
40. Smith TJ. Teprotumumab as a novel therapy for thyroid-associated ophthalmopathy. Front Endocrinol. 2020;11:610337. doi:10.3389/fendo.2020.610337
41. Ali F, Chorsiya A, Anjum V, Ali A. Teprotumumab (Tepezza): from the discovery and development of medicines to USFDA approval for active thyroid eye disease (TED) treatment. Int Ophthalmol. 2021;41:1549–1561. doi:10.1007/s10792-021-01706-3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.