Back to Journals » Journal of Pain Research » Volume 13
Migraine-Specific Quality-of-Life Questionnaire (MSQ) Version 2.1 Score Improvement in Japanese Patients with Episodic Migraine by Galcanezumab Treatment: Japan Phase 2 Study
Authors Shibata M, Nakamura T, Ozeki A , Ueda K , Nichols RM
Received 21 October 2020
Accepted for publication 16 December 2020
Published 31 December 2020 Volume 2020:13 Pages 3531—3538
DOI https://doi.org/10.2147/JPR.S287781
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Mamoru Shibata,1 Tomomi Nakamura,2 Akichika Ozeki,2 Kaname Ueda,2 Russell M Nichols3
1Department of Neurology, Tokyo Dental College Ichikawa General Hospital, Ichikawa, Japan; 2Medicines Development Unit Japan and Medical Affairs, Eli Lilly Japan K.K., Kobe, Japan; 3Global Medical Affairs, Eli Lilly and Company, Indianapolis, IN, USA
Correspondence: Tomomi Nakamura
Medicines Development Unit Japan and Medical Affairs, Eli Lilly Japan K.K., 5-1-28 Isogamidori, Chuo-Ku, Kobe 651-0086, Japan
Tel +81-78-242-9389
Email [email protected]
Purpose: Evaluate changes from baseline in health-related quality of life (QoL) in Japanese patients with episodic migraine receiving preventive treatment with galcanezumab (GMB).
Patients and Methods: Preventive treatments for migraine have been shown to improve QoL, but few clinical trials have examined QoL outcomes in Japanese patients. This phase 2, randomized, double-blind, placebo-controlled study was conducted at 40 centers in Japan. Patients aged 18– 65 years with episodic migraine (4– 14 monthly migraine headache days) received GMB 120 mg (n=115), 240 mg (n=114), or placebo (PBO, n=230) as monthly subcutaneous injections for 6 months. QoL was measured monthly using the Migraine-Specific Quality-of-Life Questionnaire (MSQ) version 2.1. Prespecified analyses were differences between GMB and PBO for change from baseline in all 3 domains of the MSQ and MSQ-Total, for each month and the average over Months 4– 6.
Results: Treatment with GMB significantly increased MSQ scores from baseline vs PBO. Average change ± SE from baseline across Months 4– 6 was 10.12± 0.72 (PBO), 17.13± 1.03 (GMB 120 mg; P< 0.001), and 15.91± 1.03 (GMB 240 mg; P< 0.001) for MSQ Role Function-Restrictive; 4.80± 0.65 (PBO), 9.64± 0.93 (GMB 120 mg; P< 0.001), and 8.35± 0.93 (GMB 240 mg; P< 0.05) for MSQ Role Function-Preventive (MSQ-RFP); 3.46± 0.77 (PBO), 10.04± 1.10 (GMB 120 mg; P< 0.001), and 7.73± 1.10 (GMB 240 mg; P< 0.05) for MSQ Emotional Function, and 7.14± 0.67 (PBO), 13.46± 0.95 (GMB 120 mg; P< 0.001), and 11.98± 0.95 (GMB 240 mg; P< 0.001) for MSQ-Total. Significantly greater improvement in scores in all MSQ domains and MSQ-Total was observed for both GMB doses vs PBO at Month 1 and was maintained for Months 1– 6 (excluding Month 5 for MSQ-RFP).
Conclusion: Preventive treatment with GMB 120 mg/240 mg improves MSQ scores in Japanese patients with episodic migraine. Improvements were seen within the first month and maintained for 6 months and are similar to those seen in global studies enrolling primarily Caucasian patients.
Clinical Trial Registration: ClinicalTrials.gov, NCT02959177 (registered November 7, 2016).
Keywords: MeSH: preventive therapy, quality of life
Introduction
Migraine is a chronic neurological disease that is estimated to affect approximately 1 billion people worldwide and is a common cause of disability, particularly in young adult to middle-aged women.1 People with migraine experience significant functional and quality-of-life (QoL) impacts as a result of the disease, such as reduced occupational functioning, reduced ability to perform household work, and limitations on family responsibilities and social and leisure activities.2,3 In Japan, migraine affects 6.0–8.4% of the population.4,5 More than 25% of Japanese patients with migraine experience more than 3 headache days per month.5 For those who seek care from a healthcare provider, 34–38% of Japanese patients experience reductions in productivity in both paid and unpaid activities.6
Preventive treatment benefits many people with migraine, in terms of not only reduction in frequency of migraine attacks,7,8 but also QoL improvements.9–11 The identification of calcitonin gene-related peptide (CGRP) and its role in the pathophysiology of migraine has led to the development of preventive treatment comprising monoclonal antibodies such as galcanezumab (GMB), as well as small molecules. Galcanezumab is a humanized IgG4 monoclonal antibody that binds CGRP and prevents its biological activity without binding the CGRP receptor. Treatment with GMB has been shown to significantly reduce monthly migraine headache days12,13 and improve patient-reported QoL measures.14,15 To date, detailed QoL studies associated with GMB trials have focused on Caucasian populations.14,15 It is currently unknown whether similar functional improvements would be seen in Japanese populations.
The objective of this prespecified analysis was to evaluate changes from baseline in health-related QoL, measured via the Migraine-Specific Quality-of-Life Questionnaire (MSQ) version 2.1, using data from a randomized, double-blind, placebo (PBO)-controlled clinical trial in Japanese patients treated with GMB (120 mg or 240 mg) or PBO.16
Patients and Methods
Study Design
This was a phase 2, randomized, double-blind, PBO-controlled study of GMB in Japanese patients with episodic migraine. The study was conducted at 40 sites in Japan from December 2016 to January 2019. The protocol was approved by local independent ethics review boards. The study was conducted in accordance with the Declaration of Helsinki, the International Council for Harmonisation (Guideline for Good Clinical Practice), and applicable laws and regulations. All patients provided written informed consent before participating in the study. The study was registered at www.clinicaltrials.gov (NCT02959177).
The study comprised 4 periods (Figure S1): a screening period, including full clinical assessment and washout of preventive treatments for migraine for ≥30 days; a baseline period to confirm patient eligibility and establish baseline number of migraine headache days using an electronic patient-reported outcome (ePRO) diary; a 6-month, double-blind treatment phase, in which patients completed the ePRO diary every day and QoL questionnaires using an electronic clinical outcome assessment tool every month; and a 4-month washout and follow-up phase. Patients who completed the treatment phase had the option to roll over to an open-label extension study (NCT02959190). Most patients (54%) moved to the extension study without completing the follow-up phase.
Study Population
Eligible patients were male or female, aged 18–65 years, with migraine with or without aura, had onset of migraine before age 50, and had the disease for at least 1 year before entering the study. Migraine frequency of eligible patients was 4–14 monthly migraine headache days with at least 2 attacks per month; monthly migraine headache days were defined as a calendar day on which a migraine headache or probable migraine headache occurred. Patients were not eligible if they were currently taking preventive treatments for migraine (although they had the option to stop other preventive treatments and go through a washout period of ≥30 days), had a higher monthly frequency of headache days (≥15 monthly headache days during the 3 months before the screening period), or had chronic migraine. Patients were also excluded if they had failed to respond to 3 or more adequately dosed preventive treatments for migraine from different classes. Additional reasons for exclusion are described in the primary analysis of the clinical trial.16
Treatment Protocol
Patients were randomized (2:1:1) to 1 of 3 treatment groups, receiving PBO, GMB 120 mg, or GMB 240 mg, respectively. Treatments were administered once monthly by subcutaneous injection. Patients randomized to GMB 120 mg received a loading dose of 240 mg at the first injection only (Month 0).
Health Outcome Measures
The impact of migraine on health-related QoL, a secondary objective of the clinical trial, was measured using the MSQ version 2.1 (distributed by Mapi Research Trust; https://eprovide.mapi-trust.org/instruments/migraine-specific-quality-of-life-questionnaire).17 The MSQ version 2.1 is a 14-item questionnaire that measures QoL impacts in 3 domains: Role Function-Restrictive (RFR), 7 items that measure the functional impact of migraine through limitations on daily social and work activities; Role Function-Preventive (RFP), 4 items that measure the impact of migraine through prevention of daily work and social activities; and Emotional Function (EF), 3 items that assess the emotional impact of migraine.17,18 Raw scores in each domain were computed as a sum of relevant item scores, whereas the raw total score was the sum of all item scores; these were rescaled from 0–100, with a higher score indicating better QoL.18 The MSQ version 2.1 has demonstrated construct validity17 and is a psychometrically valid tool for reliable measurement of QoL impacts on patients with migraine.18 In particular, the RFR domain is a valuable tool for assessing the functional impact of migraine in chronic and episodic migraine clinical trials.19 The MSQ was administered at baseline, monthly from Months 1–6, and at the end of the washout period (Month 10).
Statistical Analysis
Mean changes from baseline to each visit for total MSQ score (MSQ-Total) and individual domain scores were assessed using a restricted maximum likelihood–based mixed-model repeated measures (MMRM) technique and are reported as least squares (LS) means ± standard error (SE). The prespecified MMRM analysis included fixed categorical effects (treatment, month, treatment-by-month interaction, and baseline monthly migraine headache days category [<8, ≥8]) and continuous fixed covariates (baseline value and baseline-by-month interaction). No adjustments for multiplicity were made across the arms, time points, and analyses described in this article. Significance was based on a 2-sided alpha level of 0.05. The prespecified endpoint for comparison of GMB with PBO was estimated as the average across Months 4–6 (LS means) using PROC MIXED. This endpoint was selected because it was hypothesized that QoL improvements might not be apparent until after patients experienced a significant reduction in monthly migraine headache days, which would be more likely in the latter half of the study period. MSQ-RFR domain responders were defined as patients whose average change from baseline was ≥25 over Months 4–6; a change of ≥25 is equivalent to 9 points on the raw scale and is an appropriate threshold to interpret a benefit from treatment.19 These data were analyzed using a logistic regression model, which included treatment, baseline, and baseline monthly migraine headache days category (<8, ≥8).20 A post hoc MMRM analysis of the change from baseline in MSQ scores by baseline monthly migraine headache days (subgroups: <8 days/month vs ≥8 days/month) was performed for each group separately. This analysis included fixed categorical effects (treatment, month, and treatment-by-month interaction) and continuous fixed covariates (baseline value and baseline-by-month interaction). All statistical analyses were performed using SAS® version 9.4 (SAS Institute, Cary, NC, USA). All authors had access to the data during the preparation of the manuscript.
Results
Demographic and Baseline Clinical Characteristics
Patient demographics have been previously reported.16 Briefly, patient demographics and clinical characteristics were similar across all treatment groups, with the majority of patients being female (83.4%) and an average age of 44 years. Approximately 80% of patients were engaged in paid employment. Patients had a mean duration of migraine of >20 years and experienced 8.6–9.0 monthly migraine headache days. Baseline Migraine Disability Assessment (MIDAS) scores ranged from 13.7–15.8 (MIDAS Grade III). Baseline MSQ scores were not significantly different across the treatment groups (Table 1).
 |
Table 1 MSQ Scores at Baseline |
QoL Outcomes
Functional Outcome (MSQ-RFR Domain)
Treatment with GMB significantly increased MSQ-RFR scores from baseline compared with PBO, indicating an improvement in patient functioning (Figure 1). As previously reported, the mean change ± SE from baseline over Months 4–6 in MSQ-RFR was 17.13±1.03 for GMB 120 mg and 15.91±1.03 for GMB 240 mg, compared with only 10.12±0.72 for PBO (P<0.001 GMB vs PBO).16 The current analyses demonstrate that the effects of GMB treatment on patient functioning were observed within the first month. The change from baseline in MSQ-RFR scores was significantly higher for both doses of GMB than PBO at Month 1 and was maintained through Month 6 (Figure 1; P<0.05 GMB vs PBO at each month). At the end of the 4-month washout and follow-up phase (Month 10), the significant differences between GMB-treated groups and PBO had disappeared; however, MSQ-RFR had not returned to baseline levels (Figure 1). Change from baseline in MSQ-RFR was not affected by baseline monthly migraine headache day frequency (Table S1).
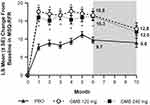 |
Figure 1 Monthly LS mean change in MSQ-RFR scores from baseline. Washout period denoted by gray shading. Patients had the option of entering an open-label extension period after Month 6. Number of patients at Month 10 reflects this option: n=98 for PBO, n=51 for GMB 120 mg, and n=51 for GMB 240 mg. *P<0.05 vs PBO (MMRM analysis). The average LS mean change from baseline over Months 4–6 in MSQ-RFR was 10.12±0.72 for PBO, 7.13±1.03 for GMB 120 mg, and 15.91±1.03 for GMB 240 mg.16 The MMRM model included the fixed categorical effects of treatment, month, and treatment-by-month interaction, the baseline number of monthly migraine headache days (<8, ≥8), and the continuous fixed covariates of baseline value and baseline-by-month interaction. Abbreviations: GMB, galcanezumab; LS, least squares; MMRM, mixed-model repeated measures; MSQ-RFR, Migraine-Specific Quality-of-Life Questionnaire Role Function-Restrictive domain; PBO, placebo; SE, standard error. |
MSQ-RFR domain responders were defined as those with an average change in RFR scores from baseline ≥25 for Months 4–6. GMB-treated patients had a higher responder rate than patients receiving PBO (Table 2; P=0.008 GMB 120 mg vs PBO, P<0.001 GMB 240 mg vs PBO).
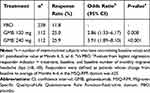 |
Table 2 MSQ-RFR Responders by Treatment Group |
Preventive and Emotional Outcomes (MSQ-RFP and MSQ-EF)
Other QoL measurements were also improved by GMB treatment. Patients receiving GMB showed significant increases from baseline in the other MSQ domain scores compared with PBO (Figure 2). Although changes from baseline in MSQ scores in the RFP and EF domains were smaller than those in RFR, they were statistically significant (P<0.001 vs baseline). In the RFP domain, the average change from the baseline score over Months 4–6 was 9.6 for GMB 120 mg and 8.4 for GMB 240 mg vs 4.8 for PBO (Figure 2A; P<0.05 GMB vs PBO). In the EF domain, the average increase from baseline over Months 4–6 was 10.0 and 7.7 for GMB 120 mg and 240 mg, respectively, compared with 3.5 for PBO (Figure 2B; P<0.05 GMB vs PBO). The changes from baseline in RFP and EF scores were significantly higher (P<0.05) for the GMB groups vs PBO in each month from Month 1 through Month 6 (Figure 2D and 2E), with the exception of Month 5 for MSQ-RFP scores (Figure 2D). At Month 10, both RFP and EF domain scores remained above baseline levels for all treatment groups (Figure 2D and 2E). The change from baseline at Month 10 in MSQ-EF was significantly higher for GMB 120 mg vs PBO (P<0.05; Figure 2E), but there was no difference between GMB-treated groups and PBO at Month 10 in the RFP domain (Figure 2D). Changes from baseline in MSQ-RFP and MSQ-EF were not affected by baseline monthly migraine headache day frequency (Table S1).
Overall QoL Outcome (MSQ-Total)
Overall, treatment with GMB resulted in improved QoL compared with PBO, as measured by changes in MSQ-Total. The increase from baseline in MSQ-Total was significantly higher for both doses of GMB vs PBO (Figure 2C and 2F; P<0.05). MSQ-Total scores increased over Months 4–6 by an average of 13.5 and 12.0 for the GMB 120 mg and GMB 240 mg groups, respectively, compared with 7.1 for the PBO group (Figure 2C; P<0.001 GMB vs PBO). The change from baseline in MSQ-Total scores was significantly higher (P<0.05) for the GMB groups vs PBO in each month from Month 1 through Month 6 (Figure 2F). There were no differences between GMB-treated groups and PBO at Month 10, although MSQ-Total in GMB-treated groups had not returned to baseline levels (Figure 2F). Change from baseline in MSQ-Total was not affected by baseline monthly migraine headache day frequency (Table S1).
Discussion
This is the first randomized controlled trial in Japanese patients with migraine receiving preventive treatment with GMB (120 mg or 240 mg) that demonstrates improvements from baseline in QoL measures (MSQ scores) compared with PBO. Improvement within 1 month after initiating treatment was observed across all MSQ domains and individual questions; this is clinically relevant as improved function in daily life is essential for patients with migraine. The findings of this study are consistent with those seen in Caucasian, Hispanic, and East Asian populations and support the consistency of benefit of GMB across different ethnic populations.
Evaluation of the consistency of efficacy, safety, and overall patient experience of new medications across different ethnic populations is important from both a scientific and a regulatory perspective. In the current study, the pattern of increased scores for MSQ-Total and all MSQ domains with GMB treatment in Japanese patients parallels the results seen in the large global EVOLVE-1 and EVOLVE-2 Phase 3 clinical trials.15 However, the observed changes from baseline in the current study were smaller than those seen in the global clinical trials. This can be explained in part by the Japanese population having higher baseline MSQ scores than the patients in either EVOLVE-1 or EVOLVE-2 (up to 25 points higher).15 A previous study in Japanese patients found that mean MSQ domain scores were 15–20 points higher than those of patients from the United States,21 and it has been suggested that the higher MSQ scores may reflect cultural differences around perception and coping with pain and discomfort.16 Despite this, the observed changes from baseline in the present study were significantly different from PBO in all 3 domains of the MSQ; these QoL findings are consistent with the efficacy and safety data for GMB in Japanese patients with episodic migraine.16 Notably, the current analyses showed higher odds ratios for MSQ-RFR responders compared with those seen in previous studies of GMB. For episodic migraine, an MSQ-RFR responder is a patient whose RFR score increases by ≥25 from baseline to Months 4–6 on average.19 In the current study, GMB-treated patients had a higher MSQ-RFR responder rate than patients receiving PBO (odds ratio 2.86 [95% CI: 1.33–6.17] and 3.91 [1.89–8.10] for GMB 120 mg and 240 mg, respectively). In the EVOLVE-1, EVOLVE-2, and REGAIN trials, MSQ-RFR responder odds ratios were 1.64–2.27 for patients receiving GMB 120 mg and 1.66–2.45 for those receiving GMB 240 mg.19 It appears that, despite on average starting from a higher MSQ-RFR baseline than Caucasian populations, more Japanese patients are able to achieve clinically significant improvements in this QoL domain when receiving GMB treatment compared with PBO. In addition to the EVOLVE-1 and EVOLVE-2 trials noted above, other clinical trials of GMB that assessed MSQ measures are not as directly comparable because of differences in trial durations and migraine diagnoses (chronic or episodic). However, these studies, conducted in the United States, Europe, and Central and South America, also show lower baseline scores and greater increases from baseline than in the present study.14,22,23
Insufficient efficacy of preventive medications can impact patient adherence and persistence with treatment.24 For Japanese patients with episodic migraine or chronic migraine who have experienced problems with medication, lack of efficacy was the most common issue.6 In the present study, improvements in MSQ scores were seen within 1 month of treatment with GMB. Mean monthly migraine headache days were also reduced within 1 month of GMB treatment.16 In a clinical setting, these early improvements in symptoms and health-related QoL might be expected to contribute to adherence and persistence with GMB treatment. This supposition is supported by the high compliance rate (100%) and continuation rate (95.9%)16 in the present study.
In the current study, MSQ was measured again 4 months after GMB treatment ceased. Although post-treatment MSQ scores decreased somewhat, they remained above baseline levels. However, no differences in most MSQ domains were observed between GMB-treated groups and PBO 4 months after ceasing GMB. The maintenance in the treatment gains observed with GMB on MSQ scores is clinically important, as it suggests that withdrawal of GMB is unlikely to lead to an immediate worsening in patient health-related QoL.
Positive effects on QoL outcomes have been reported in global trials for other CGRP monoclonal antibodies such as erenumab, eptinezumab, and fremanezumab.25–29 However, because of the differences in the time point evaluation, trial design, and patient-reported outcomes used, it is difficult to make direct comparisons between studies. Furthermore, these trials focused primarily on Caucasian populations and not on Japanese people with migraine. Similarly, although preventive medications such as antiepileptic drugs, beta-blockers, triptans, and antidepressants have demonstrated improvements in QoL in global trials, most studies have not used migraine-specific QoL instruments such as the MSQ.9,30 Two notable exceptions are onabotulinumtoxinA and topiramate, neither of which has received regulatory approval for the prevention of migraine in Japan. Both these medications have demonstrated statistically significant improvements in the MSQ. Depending on the study, these improvements were seen in all 3 domains or in the RFR and RFP domains only.10,11,30
The strengths of the present study include its randomized, controlled, double-blind design and the fact that its 6-month duration of treatment enabled more accurate measurement of changes in functioning over time. Additionally, the patient-reported outcome measure used (MSQ) is a migraine-specific tool and, unlike more general QoL scales, assesses aspects of daily life that are important to patients with migraine. Another strength of this study is the focus on Japanese patients, who have previously been understudied with regard to CGRP antibody therapies.
There are limitations to this research. The results reported were not the primary objective of the clinical trial, and no adjustments for multiplicity were performed. It is unclear whether these results can be extrapolated to patients with lower-frequency episodic migraine (all study participants had a monthly migraine headache frequency of 4–14 headache days per month). GMB treatment was examined as a monotherapy (although acute medications were allowed under certain circumstances). QoL outcomes for concurrent usage of GMB with other preventive medications are currently unknown.
Conclusion
This is the first study to demonstrate that both 120-mg and 240-mg doses of GMB were superior to PBO in improving patient functioning as measured by the MSQ version 2.1. This benefit in Japanese patients with episodic migraine was observed within the first month of treatment and was maintained for 6 months. The improvements in functioning observed in Japanese patients with migraine were consistent with findings from global studies, which have primarily examined Caucasian populations.
Abbreviations
CGRP, calcitonin gene-related peptide; EF, Emotional Function; ePRO, electronic patient-reported outcome; GMB, galcanezumab; LS, least squares; MIDAS, Migraine Disability Assessment; MMRM, mixed-model repeated measures; MSQ, Migraine-Specific Quality-of-Life Questionnaire; PBO, placebo; QoL, quality of life; RFP, Role Function-Preventive; RFR, Role Function-Restrictive; SE, standard error.
Data Sharing Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Ethics Approval and Consent to Participate
This study was reviewed and approved by appropriate institutional review boards and was conducted according to the Declaration of Helsinki. All participants gave written informed consent. The Institutional Review Boards at each of the 40 study sites are listed in Table S2.
Acknowledgments
This study was sponsored by Eli Lilly and Company, manufacturer of galcanezumab. Medical writing assistance was provided by Koa Webster, PhD, and Tania Dickson, PhD, CMPP, of ProScribe – Envision Pharma Group, and was funded by Eli Lilly. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3). Eli Lilly Japan K.K. was involved in the study design, data collection, data analysis, and preparation of the manuscript. The authors thank Atsushi Kuga, a former employee of Eli Lilly Japan K.K., for his substantial contributions to the conception and design of the study. The authors would also like to thank all study participants. The data in this paper were presented at the 62nd Annual Scientific Meeting of the American Headache Society as a poster presentation. The poster’s abstract was published in “Abstracts” (2020) Headache 60:114, doi:10.1111/head.13854https://doi.org/10.1111/head.13854
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Tomomi Nakamura, Akichika Ozeki, Kaname Ueda, and Russell M Nichols are employees of Eli Lilly. Mamoru Shibata has received payment from Eli Lilly and Otsuka for consultancies and has received honoraria from Amgen. The authors report no other conflicts of interest in this work.
References
1. Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954–976. doi:10.1016/S1474-4422(18)30322-3
2. Lipton RB, Bigal ME, Kolodner K, Stewart WF, Liberman JN, Steiner TJ. The family impact of migraine: population-based studies in the USA and UK. Cephalalgia. 2003;23(6):429–440. doi:10.1046/j.1468-2982.2003.00543.x
3. Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31(3):301–315. doi:10.1177/0333102410381145
4. Sakai F, Igarashi H. Prevalence of migraine in Japan: a nationwide survey. Cephalalgia. 1997;17(1):15–22. doi:10.1046/j.1468-2982.1997.1701015.x
5. Takeshima T, Ishizaki K, Fukuhara Y, et al. Population-based door-to-door survey of migraine in Japan: the Daisen study. Headache. 2004;44(1):8–19. doi:10.1111/j.1526-4610.2004.04004.x
6. Ueda K, Ye W, Lombard L, et al. Real-world treatment patterns and patient-reported outcomes in episodic and chronic migraine in Japan: analysis of data from the Adelphi migraine disease specific programme. J Headache Pain. 2019;20(1):68. doi:10.1186/s10194-019-1012-1
7. Agostoni EC, Barbanti P, Calabresi P, et al. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain. 2019;20(1):92. doi:10.1186/s10194-019-1038-4
8. Loder E, Rizzoli P. Pharmacologic prevention of migraine: a narrative review of the state of the art in 2018. Headache. 2018;58(Suppl 3):218–229. doi:10.1111/head.13375
9. Bordini CA, da Silva HM, Garbelini RP, Teixeira SO, Speciali JG. Effect of preventive treatment on health–related quality of life in episodic migraine. J Headache Pain. 2005;6(5):387–391. doi:10.1007/s10194-005-0233-7
10. Dodick DW, Silberstein S, Saper J, et al. The impact of topiramate on health-related quality of life indicators in chronic migraine. Headache. 2007;47(10):1398–1408. doi:10.1111/j.1526-4610.2007.00950.x
11. Lipton RB, Varon SF, Grosberg B, et al. OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine. Neurology. 2011;77(15):1465–1472. doi:10.1212/WNL.0b013e318232ab65
12. Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–1088. doi:10.1001/jamaneurol.2018.1212
13. Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim B-K, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–1454. doi:10.1177/0333102418779543
14. Ayer DW, Skljarevski V, Ford JH, Nyhuis AW, Lipton RB, Aurora SK. Measures of functioning in patients with episodic migraine: findings from a double-blind, randomized, placebo-controlled Phase 2b trial with galcanezumab. Headache. 2018;58(8):1225–1235. doi:10.1111/head.13383
15. Ford JH, Ayer DW, Zhang Q, et al. Two randomized migraine studies of galcanezumab: effects on patient functioning and disability. Neurology. 2019;93(5):e508–e517. doi:10.1212/WNL.0000000000007856
16. Sakai F, Ozeki A, Skljarevski V. Efficacy and safety of galcanezumab for prevention of migraine headache in Japanese patients with episodic migraine: a phase 2 randomized controlled clinical trial. Cephalalgia Rep. 2020;3:1–10.
17. Martin BC, Pathak DS, Sharfman MI, et al. Validity and reliability of the Migraine-Specific Quality of Life Questionnaire (MSQ Version 2.1). Headache. 2000;40(3):204–215. doi:10.1046/j.1526-4610.2000.00030.x
18. Rendas-Baum R, Bloudek LM, Maglinte GA, Varon SF. The psychometric properties of the Migraine-Specific Quality of Life Questionnaire version 2.1 (MSQ) in chronic migraine patients. Qual Life Res. 2013;22(5):1123–1133. doi:10.1007/s11136-012-0230-7
19. Speck RM, Shalhoub H, Wyrwich KW, et al. Psychometric validation of the Role Function Restrictive domain of the Migraine Specific Quality-of-Life Questionnaire Version 2.1 electronic patient-reported outcome in patients with episodic and chronic migraine. Headache. 2019;59(5):756–774. doi:10.1111/head.13497
20. Cole JC, Lin P, Rupnow MFT. Minimal important differences in the Migraine-Specific Quality of Life Questionnaire (MSQ) version 2.1. Cephalalgia. 2009;29(11):1180–1187. doi:10.1111/j.1468-2982.2009.01852.x
21. Sakai F, Fukuuchi Y, Iwata M, et al. Reliability and validity of the Japanese version of the migraine quality of life survey [in Japanese]. Neurological Therapeutics. 2004;21:449–458.
22. Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91(24):e2211–e2221. doi:10.1212/WNL.0000000000006640
23. Camporeale A, Kudrow D, Sides R, et al. A phase 3, long-term, open-label safety study of Galcanezumab in patients with migraine. BMC Neurol. 2018;18(1):188. doi:10.1186/s12883-018-1193-2
24. Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second International Burden of Migraine Study (IBMS-II). Headache. 2013;53(4):644–655. doi:10.1111/head.12055
25. Torres-Ferrus M, Alpuente A, Pozo-Rosich P. How much do calcitonin gene-related peptide monoclonal antibodies improve the quality of life in migraine? A patientʼs perspective. Curr Opin Neurol. 2019;32(3):395–404. doi:10.1097/WCO.0000000000000689
26. Reuter U. A review of monoclonal antibody therapies and other preventative treatments in migraine. Headache. 2018;58(Suppl 1):48–59. doi:10.1111/head.13302
27. Lipton RB, Gandhi SK, Fitzgerald T, et al.The impact of fremanezumab on migraine-specific health-related quality of life in episodic migraine (P4.115). Neurology. 2018;90(15 Supplement):
28. Lipton RB, Tepper SJ, Reuter U, et al. Erenumab in chronic migraine: patient-reported outcomes in a randomized double-blind study. Neurology. 2019;92(19):e2250–e2260. doi:10.1212/WNL.0000000000007452
29. Tepper SJ, Diener H-C, Ashina M, et al. Erenumab in chronic migraine with medication overuse: subgroup analysis of a randomized trial. Neurology. 2019;92(20):e2309–e2320. doi:10.1212/WNL.0000000000007497
30. Buse DC, Rupnow MFT, Lipton RB. Assessing and managing all aspects of migraine: migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Mayo Clin Proc. 2009;84(5):422–435. doi:10.1016/S0025-6196(11)60561-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

