Back to Journals » OncoTargets and Therapy » Volume 12
Midkine Is a Potential Urinary Biomarker for Non-Invasive Detection of Bladder Cancer with Microscopic Hematuria
Authors Lin H , Zhou Q, Wu W, Ma Y
Received 18 October 2019
Accepted for publication 12 December 2019
Published 3 January 2020 Volume 2019:12 Pages 11765—11775
DOI https://doi.org/10.2147/OTT.S235134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Hao Lin,1 Qingwen Zhou,1 Weichu Wu,1 Yulin Ma1,2
1Department of Urology, The Second Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong 515041, People’s Republic of China; 2Department of Urology, Hongsen Hospital of Harbin Medical University, Sanya, Hainan 572000, People’s Republic of China
Correspondence: Yulin Ma
Email [email protected]
Background: To determine the role of Midkine (MDK) in non-invasive detection of bladder cancer (Bca) and the relationship with Ki67.
Methods: Sixty-five Bca patients and 55 non-Bca patients or healthy volunteers were enrolled and voided urine samples were prospectively obtained on the first day of enrollment. Tissue samples were collected by surgery. MDK and Ki67 expressions were analyzed by immunohistochemistry and Western Blot (WB). Specificity and sensitivity of MDK mRNA testing in the detection of Bca were determined by Receiver Operating Characteristic curve (ROC). The relationship between MDK and Ki67 was also assessed.
Results: MDK was overexpressed in Bca tissues than that in the non-cancer tissues. The specificity and sensitivity for MDK mRNA testing in urine in the identification of Bca was 80% and 72.3%. MDK detected 85.7% of high-grade tumors, 87.5% of muscle-invasive tumors and 79.4% of tumors larger than 3 cm in patients without gross hematuria. Microscopic hematuria may even increase the detection rate of Bca by MDK testing. Furthermore, the correlation of MDK and Ki67 was found positive.
Conclusion: MDK was overexpressed in Bca tissues and positively correlated with Ki67. MDK might be a potential biomarker for the detection of Bca, especially for those without gross hematuria but with microscopic hematuria.
Keywords: Midkine, non-invasive detection, bladder cancer, biomarker, Ki67
Introduction
Bladder cancer (Bca) is one of the most common malignancies around the world. Due to its high risk of recurrence and progression, long-term surveillance is required. To date, cystoscopy screening with biopsies and void urinary cytology (VUC) are still the mainstays of confirmation of Bca. However, cystoscopy is an invasive and unpleasant measure for patients. Additionally, although the specificity of >93% is high for VUC, the sensitivity for VUC is dismal at 25–40%, especially for those with low-grade and low-stage tumors.1 Noninvasive detection and monitoring of Bca with cost-effective signatures are still big challenges. Although several commercially available tests involving urinary biomarkers are now available, none of these assays have reached widespread use owing to insufficient evidence for clinical benefits.
According to the American Urological Association (AUA) and American College of Physicians recommendations, patients with asymptomatic microscopic hematuria should undergo full clinical investigations including cystoscopy.2 Due to the lack of effective non-invasive tests for Bca suspicion patients with hematuria, results from these currently used tests may lead to an unnecessary work-up for diagnosis. Therefore, new markers with qualities of high sensitive and specificity in confirming Bca presenting with microscopic hematuria are urgently needed.
Midkine (MDK), a 13-kDa heparin-binding growth factor, was originally discovered in embryonal carcinoma cells and involved in the retinoic acid-induced differentiation.3 MDK has been proved to be overexpressed in various types of human cancers including Bca.4–9 Down-regulation of MDK expressions by oncolytic virus can achieve antitumor effect in vitro.10 In addition, elevated MDK levels in blood suggested it could be a biomarker for cancer patients.11–14
Recently, researchers have showed that, by combining with the other markers, MDK was able to detect non-muscle invasive bladder cancer (NMIBC) in high specificity and sensitivity.15,16 Furthermore, a combined model with the MDK gene test included was able to triage out patients with hematuria but with low probability of urothelial carcinoma.17 In the present study, we evaluated MDK as a singular bio-marker for detecting Bca, especially for those without gross hematuria. Furthermore, we also accessed the relationships between MDK and clinical characteristics of Bca and Ki67 expressions.
Materials and Methods
Samples
The current study included 65 Bca patients and 55 non-Bca patients and healthy volunteers from the Second Affiliated Hospital of Shantou University Medical College between 2014 and 2015. All the 65 Bca patients were confirmed by biopsies through cystoscopy. Furthermore, the Bca cohort mainly recruited patients without gross hematuria (54/65). Fifty-two of the 65 Bca patients were treated with trans-urethral resection of bladder tumor (TURBt) or radical cystectomy, while the rest of them denied any further intervention after diagnosis. The histological grade and stage were recorded according to the 2004 World Health Organization grading system and the seventh edition of the tumor-nodes-metastasis (TNM) classification system, respectively. All tumors were predominantly urothelial carcinoma. Among the 55 non-Bca subjects, 26 were healthy volunteers and 29 were patients with benign urological diseases including 12 with benign prostate hyperplasia (BPH) and 17 with urolithiasis. Fifty mL urine samples were prospectively collected on the first day of enrollment and kept frozen at −80°C until analyzed. This study was approved by the Medical Ethics Committee of The Second Affiliated Hospital of Shantou University Medical College and was carried out in accordance with the principles of good clinical practice and the Declaration of Helsinki. Written informed consent was obtained from each patient for surgery and research purposes.
Quantitative Polymerase Chain Reaction (Q-PCR)
The sample urine was thawed and total RNA was isolated using TRIZAL reagent (Invitrogen) according to manufacturer’s instructions. RNA concentration was quantified and integrity was checked with electrophoresis by observing the 28S and 18S RNA bands. Reverse transcription of RNA was carried out with the Prime Script TM RT Kit (TaKaRa). The sequences of primers are listed in Table 1. PCR was performed with Premix Ex Taq TM kit (TaKaRa) in a final reaction mixture of 25 µL containing 2 µL cDNA, 12.5 µL 2× Premix Ex Taq, 0.5 µL each 10 µM forward and reverse primer, 1 µL TaqMan probe and 8.5 µL ddH2O.
 |
Table 1 The Sequences of MDK and GAPDH Primers |
All samples were analyzed in duplicate and the values of sample copies were obtained after quantitative amplification and normalized to GAPDH using 2−ΔCTmethod, where ΔCT = CT(MDK) - CT(GAPDH). Each sample was measured in triplicate.
Immunohistochemistry (IHC)
The sample tissues were paraffin-embedded and cut into 4µM. Immunohistochemical staining was performed as previously described.18 Monoclonal mouse IgG anti-MDK antibody (1:100, Santa Cruz) and polyclonal rabbit IgG anti-Ki67 (1:100, Santa Cruz) were used in IHC staining.
IHC staining was reviewed and scored separately by two pathologists blinded to the clinical parameters. The staining intensity was graded (negative = 0; weak = 1; moderate = 2; strong = 3), and percentage of positive-staining cells was counted (<5% = 0; 6–25% = 1; 26–50% = 2; 51–75% = 3; >76% = 4). The total score was obtained by multiplying the intensity by the proportion (intensity score × proportion score). Positive was defined as staining index ≥4, whereas negative was defined as staining index <4. The cutoff of Ki67 labeling index was 15%.19 Those with the nuclear staining of tumor cells >15% was defined as positive, and those with the nuclear staining of tumor cells ≤15% was defined as negative.
Immunocytochemistry
Cells were plated onto slides and fixed with 4% paraformaldehyde. The primary antibodies MDK (Santa Cruz) and Ki67 (Santa Cruz) were used at 1:50 and 1:100 work solutions. The fluorescein mouse and rabbit IgG antibody (1:1500, Life technologies) were used as labels for immunofluorescence assay. After immune-labeling, cells were stained with DAPI (Sigma) and viewed with fluorescent microscopy (Nikon, Japan). Photograph was then taken and analyzed by Image J software.
Western Blot (WB)
Frozen tissues or cells were lysed by lysis buffer and protein concentrations were measured by BCA protein assay kit (Beyotime). Total protein was separated by SDS-PAGE using a 12% polyacrylamide gel and transferred onto a polyvinylidene fluoride membrane (Millipore). The membrane was blocked and incubated with the MDK antibody (1:500, Santa Cruz), followed by incubation with a secondary antibody (1:1500; Santa Cruz). Blots were detected with ECL detection reagent (Bio-Rad). The images were quantified by Alpha Ease FC (Alpha View).
Statistical Analysis
SPSS 17.0 for Windows software system (SPSS Inc., Chicago, IL) was used for the statistical analyses. The χ2 or Fisher’s exact tests were used to analyze the categorical data. Univariate ANOVA analysis and the least significant difference test or the Mann–Whitney U-test or the Kruskal–Wallis test were used to compare the means of more than two independent groups. The cut-off values for the urine MDK mRNA levels to predict the presence of Bca were determined using ROC curves. The Spearman test was used to investigate the correlation between 2 quantitative variables. Differences or correlations with P values <0.05 were considered statistically significant.
Results
MDK Was Overexpressed in Bladder Cancer
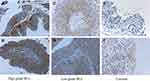
We first investigated the MDK expressions in Bca and non-cancer tissues by IHC (Figure 1). Our results showed that 86.5% (45/52) of Bca tissues stained positive for MDK, which was significantly higher than 26.7% (4/15) of non-cancer tissues positive for MDK (P<0.01, Table 2). However, the differences of MDK expressions in Bca patients with high grade or low grade, muscle invasive or non-muscle invasive, were not statistically significant (P=0.656 and P=0.578, respectively, Table 2).
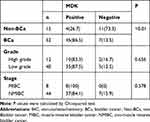 |
Table 2 IHC of MDK in BCa and Non-BCa Tissues |
Similar to the results of IHC, our WB (Figure 2A) also showed that MDK protein expressions in the high and low-grade Bca were significantly higher than that in the adjacent tissues (0.62±0.31 and 0.38±0.25 vs 0.03±0.01, all P<0.05). MDK expression of high-grade Bca was 1.63 fold higher than that of low-grade Bca, it was not statistically significant (P=0.099, Figure 2B).
MDK mRNA Could Be Used as a Biomarker for the Detection of Bca
Characteristics of Study Subjects
Table 3 presents the clinical and pathological characteristics of the subjects. The Bca set comprised 54 patients without gross hematuria and 11 with gross hematuria. The control group included 26 healthy volunteers and 29 patients with benign urological diseases. There were no significant differences in sex and age between the two groups (P>0.05). However, the different smoking habits showed statistically significant (P=0.027).
 |
Table 3 Clinical and Pathological Data of BCa and Non-BCa Cohorts |
Association of Urinary MDK mRNA Levels with Bca Presence
Figure 3 shows the urinary concentration of MDK mRNA of Bca and Non-cancer groups. The mean urinary level of MDK mRNA was significantly higher in the Bca subjects without gross hematuria than that in the controls (0.223(0.30) vs 0.050(0.08), P<0.01). However, there was no significant difference between the subjects of the Bca group with gross hematuria and those in the non-cancer group (0.116(0.29) vs 0.050(0.08), P=0.554). Additionally, the MDK mRNA expression in the Bca patients with gross hematuria did not show significant differences in comparison with those without gross hematuria (0.116(0.29) vs 0.223(0.30), P=0.283).
Based on our data, the appropriate cut-off value of urinary MDK mRNA for predicting the presence of Bca was 0.116. At this cut-off value, the sensitivity, specificity, positive predict value, negative predict value and the accuracy of the urinary MDK mRNA for identifying Bca patients were 72.3%, 80%, 81%, 71% and 75.8%, respectively. However, when subdivided into two groups of all the Bca patients, those with gross hematuria was shown with difficulty in identification of tumor by MDK mRNA level (AUC=0.65, P=0.15) while those without gross hematuria exhibited a slight improvement in diagnosis (AUC=0.785, P<0.01) This may imply that the MDK mRNA test for confirmation of Bca is superior in patients without gross hematuria.
Diagnostic Performance of MDK for Detecting Bca Patients Without Gross Hematuria
Therefore, we further analyzed the ROC curves generated using the Bca patients without gross hematuria and the control group (Figure 4). As shown in Table 4, at a specificity of 80%, MDK showed higher sensitivity for detecting high-grade, muscle-invasive and large volume (≥3cm) tumors than that for low-grade, non-muscle-invasive and small volume (<3cm) tumors. MDK detected 85.7% of high-grade tumors, 87.5% of muscle-invasive tumors and 79.4% of tumors larger than 3 cm. Interestingly, while microscopic hematuria had minor effect on AUC (0.796 vs 0.798) in Bca patients without gross hematuria, the accuracy rate (ACC) for those without microscopic hematuria was only 65.7%, which was 15.1% lower than that for those with microscopic hematuria (81.8%). Furthermore, Bca patients with infections but without gross hematuria also showed higher probability in the detection of MDK than those without infections and gross hematuria (under 80% specificity and 85% sensitivity, AUC 0.832 vs AUC 0.771).
 |
Table 4 Diagnostic Performance of MDK for Detecting Bca |
MDK Test for Triage Out Non-Bca Patients Without Gross Hematuria
To further determine the MDK test in non-Bca differentiation, we analyzed the ROC curves generated by 55 non-Bca patients and 54 Bca patients without gross hematuria (the positive control group). As shown in Table 4, we found that micro hematuria and infection had no effect on AUC (AUC ranges from 0.778 to 0.798). Furthermore, the MDK test for triage out non-Bca patients can reach a sensitivity of 75.9% for those with micro hematuria or infection, which is higher than 72.2% for those without micro hematuria or infection, under the specificity of 80%.
The Relationship Between MDK and Ki67 in Bca
Ki67 is a cell cycle and tumor growth marker. The mitogenicity of MDK may indicate its important role in tumor cell proliferation.20 Therefore, we try to determine the relationship between Ki67 and MDK in Bca. By immunofluorescent assay, we found that MDK mainly expressed in cytoplasm of Bca cell line 5637 (Figure 5). Interestingly, Ki67 staining, meanwhile, was found located in nucleus of those MDK-positive cells (Figure 5). By applying Ki67 IHC staining in Bca tissues (Figure 6), we analyzed the association of expressions of MDK and Ki67 by spearman test. Of the 52 specimens, 31(59.6%) and 21(40.4%) were classified as Ki67 positive and Ki67 negative, respectively. Moreover, we detected 30 Ki67 positive cases of the 45 MDK-positive group (66.7%) and 1 of the 7 MDK-negative group (14.3%). According to our research, we found that Ki67 labeling index was positively associated with MDK overexpression (r=0.364, P=0.008) in our Bca cohort.
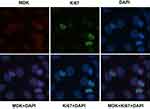 |
Figure 5 Immunofluorescence staining for MDK and Ki67 in 5637 cells. Scale bar: 100 μm (all ×400). |
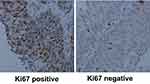 |
Figure 6 Positive (left) and negative (right) staining of Ki67 in Bca tissues. Ki67 was mainly expressed in nucleus of the tumor cells. Scale bar: 100 μm. |
Discussion
In the present study, we found that MDK was overexpressed in Bca tissues when compared with that in non-cancer tissues. This is consistent with the previous research by O’Brien et al9 Interestingly, although overexpression of MDK was found to be associated with the development of Bca and predicted a poor outcome in a short period follow-up, there was no significant difference in expressions between muscle invasive and non-muscle invasive tumors.9 Similarly, the MDK expression was not correlated with grade or stage in our study. Considering we only included 52 cases for analysis, bias should be taken into consideration. Therefore, the relationship between MDK and clinical grade and stage in Bca need to be further exploited in a larger cohort.
MDK has been identified as a promising cancer biomarker detected in serum in a variety of malignant tumors.12,13,21–23 In Bca, Andrew et al. found that the test of mRNA of MDK in urine was more sensitive in detecting high stage/high grade Bca than that of IGHBP5 and HOXA13.16 In combining with other genes, MDK test showed great improvement of Bca detection.15 Moreover, Kavalieris et al have proven that a combined genotypic-phenotypic model including the MDK gene testing in urine can triage out 80% of patients with microhematuria but without urothelial carcinoma.17 Clinically, although microscopic hematuria was only presented in 4% of patients with Bca, there was still plenty of patients with this manifestation were referred to further urological evaluation.24 Therefore, urine markers with high sensitivity and specificity in distinguishing between malignant and benign causes for patients with microhematuria are urgently needed. However, a multiplex test also means more time and money consuming. Thus, we tried to determine whether MDK could be used singly as a urine marker for Bca detection in patients without macrohematuria.
We found that MDK mRNA was upregulated in urine of Bca patients in compared with those in the control group. Similar to the previous studies,15,16 we also found that the higher urinary MDK mRNA level was associated with the increased risk of Bca presence and the more malignant phenotypes. Furthermore, we observed that while gross hematuria showed a negative effect on the diagnosis of Bca, microscopic hematuria may increase the sensitivity of detecting Bca by MDK testing. According to our research, MDK might be a potential urine marker in screening Bca patients without gross hematuria but with microscopic hematuria.
The presence of inflammatory cells may reduce the diagnostic accuracy of many Bca screening tests, including NMP22, BTA-Stat and even urine cytology.25,26 In our study, infection was not an obstacle for detecting Bca by MDK mRNA test, even though studies have shown that inflammation may affect the expression of MDK.27 According to different cut-off values, the sensitivity and specificity of NMP22 immunoassay could range from 47–100% and 60–90%.28 Our research suggested that MDK, in compared with NMP22, had an equivalent performance in Bca discrimination, especially for those without gross hematuria. However, further validations by multicenter clinical trials are needed.
MDK was involved in many biological processes including induction of chemotaxis, angiogenesis, and enhancement of fibrinolytic activity and inhibition of apoptosis. Mototsugu et al found that MDK overexpression was associated with angiogenic activity of Bca cells in vivo.29 Recently, researchers found that SP1 can promote the proliferation of glioma cells by MDK. However, the biological role of MDK in Bca has yet to be determined. The proliferation marker Ki67 has been correlated with poor prognosis in Bca.19,30 In our study, we found that MDK and Ki67 were co-occurrence in the same 5637 cells by immunofluorescence staining. We speculated that this may suggest a close relationship between MDK and Ki67. According to our IHC results, we found that Ki67 was positively associated with MDK overexpression. However, further explorations are expected to uncover the relationship between MDK and Ki67 in vitro.
Conclusions
Taken together, the present study confirmed that MDK was overexpressed in Bca tissues and positively correlated with Ki67; MDK might be a potential biomarker for the detection of Bca, especially for those without gross hematuria but with microscopic hematuria. These results presented here need to be further validated and explored to determine the role of MDK in Bca.
Abbreviations
MDK, midkine; Bca, bladder cancer; VUC, void urinary cytology; AUA, American Urological Association; NMIBC, non-muscle invasive bladder cancer; TURBt, trans-urethral resection of bladder tumor; BPH, benign prostate hyperplasia; WB, Western Blot; IHC, Immunohistochemistry; Q-PCR, quantitative polymerase chain reaction.
Ethics Approval and Consent to Participate
This study was approved by the Medical Ethics Committee of The Second Affiliated Hospital of Shantou University Medical College and was carried out in accordance with the principles of good clinical practice and the Declaration of Helsinki. Written informed consent was obtained from each patient for surgery and research purposes.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Present address for Qinwen Zhou is Zhongshan Chenxinghai Hospital, Affiliated Zhongshan Hospital of Guangdong Medical University; Present address for Weichu Wu is Shantou Central Hospital, Affiliated Shantou Hospital of Sun Yat-sen University. Hao Lin and Qingwen Zhou are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cajulis RS, Haines GK
2. Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196(4):1021–1029. doi:10.1016/j.juro.2016.06.049
3. Kadomatsu K, Tomomura M, Muramatsu T. cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem Biophys Res Commun. 1988;151(3):1312–1318. doi:10.1016/S0006-291X(88)80505-9
4. Ruan M, Ji T, Wu Z, Zhou J, Zhang C. Evaluation of expression of midkine in oral squamous cell carcinoma and its correlation with tumour angiogenesis. Int J Oral Maxillofac Surg. 2007;36(2):159–164. doi:10.1016/j.ijom.2006.09.004
5. Maeda S, Shinchi H, Kurahara H, et al. Clinical significance of midkine expression in pancreatic head carcinoma. Br J Cancer. 2007;97(3):405–411. doi:10.1038/sj.bjc.6603879
6. Moon HS, Park WI, Sung SH, Choi EA, Chung HW, Woo BH. Immunohistochemical and quantitative competitive PCR analyses of midkine and pleiotrophin expression in cervical cancer. Gynecol Oncol. 2003;88(3):289–297. doi:10.1016/S0090-8258(02)00070-7
7. Tanabe K, Matsumoto M, Ikematsu S, et al. Midkine and its clinical significance in endometrial carcinoma. Cancer Sci. 2008;99(6):1125–1130. doi:10.1111/cas.2008.99.issue-6
8. Tong Y, Mentlein R, Buhl R, et al. Overexpression of midkine contributes to anti-apoptotic effects in human meningiomas. J Neurochem. 2007;100(4):1097–1107. doi:10.1111/j.1471-4159.2006.04276.x
9. O’Brien T, Cranston D, Fuggle S, Bicknell R, Harris AL. The angiogenic factor midkine is expressed in bladder cancer, and overexpression correlates with a poor outcome in patients with invasive cancers. Cancer Res. 1996;56(11):2515–2518.
10. Terao S, Shirakawa T, Kubo S, et al. Midkine promoter-based conditionally replicative adenovirus for targeting midkine-expressing human bladder cancer model. Urology. 2007;70(5):1009–1013. doi:10.1016/j.urology.2007.07.003
11. Ibusuki M, Fujimori H, Yamamoto Y, et al. Midkine in plasma as a novel breast cancer marker. Cancer Sci. 2009;100(9):1735–1739. doi:10.1111/j.1349-7006.2009.01233.x
12. Rawnaq T, Kunkel M, Bachmann K, et al. Serum midkine correlates with tumor progression and imatinib response in gastrointestinal stromal tumors. Ann Surg Oncol. 2011;18(2):559–565. doi:10.1245/s10434-010-1191-0
13. Ma Z, Li H, Wang B, et al. Midkine mRNA level in peripheral blood mononuclear cells is a novel biomarker for primary non-small cell lung cancer: a prospective study. J Cancer Res Clin Oncol. 2013;139(4):557–562. doi:10.1007/s00432-012-1357-1
14. Zhu WW, Guo JJ, Guo L, et al. Evaluation of midkine as a diagnostic serum biomarker in hepatocellular carcinoma. Clin Cancer Res. 2013;19(14):3944–3954. doi:10.1158/1078-0432.CCR-12-3363
15. O’Sullivan P, Sharples K, Dalphin M, et al. A multigene urine test for the detection and stratification of bladder cancer in patients presenting with hematuria. J Urol. 2012;188(3):741–747. doi:10.1016/j.juro.2012.05.003
16. Holyoake A, O’Sullivan P, Pollock R, et al. Development of a multiplex RNA urine test for the detection and stratification of transitional cell carcinoma of the bladder. Clin Cancer Res. 2008;14(3):742–749. doi:10.1158/1078-0432.CCR-07-1672
17. Kavalieris L, O’Sullivan PJ, Suttie JM, et al. A segregation index combining phenotypic (clinical characteristics) and genotypic (gene expression) biomarkers from a urine sample to triage out patients presenting with hematuria who have a low probability of urothelial carcinoma. BMC Urol. 2015;15:23. doi:10.1186/s12894-015-0018-5
18. Nordin A, Wang W, Welen K, Damber JE. Midkine is associated with neuroendocrine differentiation in castration-resistant prostate cancer. Prostate. 2013;73(6):657–667. doi:10.1002/pros.22607
19. Long X, Zu X, Li Y, et al. Epidermal growth factor receptor and Ki-67 as predictive biomarkers identify patients who will be more sensitive to intravesical instillations for the prevention of bladder cancer recurrence after radical nephroureterectomy. PLoS One. 2016;11(11):e0166884. doi:10.1371/journal.pone.0166884
20. Xu C, Zhu S, Wu M, Han W, Yu Y. Functional receptors and intracellular signal pathways of midkine (MK) and pleiotrophin (PTN). Biol Pharm Bull. 2014;37(4):511–520. doi:10.1248/bpb.b13-00845
21. Lucas S, Reindl T, Henze G, Kurtz A, Sakuma S, Driever PH. Increased midkine serum levels in pediatric embryonal tumor patients. J Pediatr Hematol Oncol. 2009;31(10):713–717. doi:10.1097/MPH.0b013e3181b6db9f
22. Salaru DL, Albert C, Konigsmark U, et al. Serum levels for midkine, a heparin-binding growth factor, inversely correlate with angiotensin and endothelin receptor autoantibody titers in patients with macroangiopathy. Int Angiol. 2014;33(4):372–378.
23. Xia X, Lu JJ, Zhang SS, Su CH, Luo HH. Midkine is a serum and urinary biomarker for the detection and prognosis of non-small cell lung cancer. Oncotarget. 2016;7(52):87462–87472. doi:10.18632/oncotarget.13865
24. Buteau A, Seideman CA, Svatek RS, et al. What is evaluation of hematuria by primary care physicians? Use of electronic medical records to assess practice patterns with intermediate follow-up. Urol Oncol. 2014;32(2):128–134. doi:10.1016/j.urolonc.2012.07.001
25. Sarosdy MF, Kahn PR, Ziffer MD, et al. Use of a multitarget fluorescence in situ hybridization assay to diagnose bladder cancer in patients with hematuria. J Urol. 2006;176(1):44–47. doi:10.1016/S0022-5347(06)00576-3
26. van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol. 2005;47(6):736–748. doi:10.1016/j.eururo.2005.03.014
27. Yazihan N. Midkine in inflammatory and toxic conditions. Curr Drug Deliv. 2013;10(1):54–57. doi:10.2174/1567201811310010009
28. Xylinas E, Kluth LA, Rieken M, Karakiewicz PI, Lotan Y, Shariat SF. Urine markers for detection and surveillance of bladder cancer. Urol Oncol. 2014;32(3):222–229. doi:10.1016/j.urolonc.2013.06.001
29. Muramaki M, Miyake H, Hara I, Kamidono S. Introduction of midkine gene into human bladder cancer cells enhances their malignant phenotype but increases their sensitivity to antiangiogenic therapy. Clin Cancer Res. 2003;9(14):5152–5160.
30. Tian Y, Ma Z, Chen Z, et al. Clinicopathological and prognostic value of Ki-67 expression in bladder cancer: a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158891. doi:10.1371/journal.pone.0158891
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.