Back to Journals » International Journal of Nanomedicine » Volume 19
Microstructure Formation and Characterization of Long-Acting Injectable Microspheres: The Gateway to Fully Controlled Drug Release Pattern
Authors Wang M, Wang S, Zhang C, Ma M, Yan B, Hu X, Shao T , Piao Y, Jin L, Gao J
Received 2 November 2023
Accepted for publication 24 January 2024
Published 19 February 2024 Volume 2024:19 Pages 1571—1595
DOI https://doi.org/10.2147/IJN.S445269
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Mengdi Wang,1,* Shan Wang,1,* Changhao Zhang,2,* Ming Ma,1 Bohua Yan,1 Xinming Hu,2 Tianjiao Shao,2 Yan Piao,2 Lili Jin,2 Jing Gao1
1State Key Laboratory of Toxicology and Medical Countermeasures, Beijing Institute of Pharmacology and Toxicology, Beijing, 100850, People’s Republic of China; 2College of Pharmacy, Key Laboratory of Natural Medicines of the Changbai Mountain of Ministry of Education, Yanbian University, Yanji, Jilin, 133002, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jing Gao; Lili Jin, Email [email protected]; [email protected]
Abstract: Long-acting injectable microspheres have been on the market for more than three decades, but if calculated on the brand name, only 12 products have been approved by the FDA due to numerous challenges in achieving a fully controllable drug release pattern. Recently, more and more researches on the critical factors that determine the release kinetics of microspheres shifted from evaluating the typical physicochemical properties to exploring the microstructure. The microstructure of microspheres mainly includes the spatial distribution and the dispersed state of drug, PLGA and pores, which has been considered as one of the most important characteristics of microspheres, especially when comparative characterization of the microstructure (Q3) has been recommended by the FDA for the bioequivalence assessment. This review extracted the main variables affecting the microstructure formation from microsphere formulation compositions and preparation processes and highlighted the latest advances in microstructure characterization techniques. The further understanding of the microsphere microstructure has significant reference value for the development of long-acting injectable microspheres, particularly for the development of the generic microspheres.
Keywords: long-acting injectable microsphere, microstructure, PLGA, controlled drug release
Graphical Abstract:
Introduction
Long-acting injectable (LAI) microspheres have attracted considerable attention due to their unique advantages, such as reduced administration frequency, improved patient compliance, and avoidance of side effects, etc.1–3 Following the release of the first LAI microsphere product (Lupron Depot®) in 1989,4 the US Food and Drug Administration (FDA) has authorized 12 LAI microsphere products based on the poly(lactide-co-glycolide) (PLGA) polymer entirely, while no generic microspheres have been approved yet. To obtain FDA approval, a generic LAI microsphere must exhibit qualitative (Q1) and quantitative (Q2) similarity to its reference-listed drug (RLD).5,6 However, attaining Q1/Q2 similarity alone does not guarantee consistent drug release behavior with the RLD.7
The microstructure of microspheres mainly includes the spatial distribution and the dispersed state of drug, PLGA, and pores.8 Its complexity mainly derives from the multi-attribute formulation compositions and multi-step manufacturing processes.9–11 The properties and interactions of the drug, PLGA, and solvent, usually collectively dictate the impact of formulation composition on microstructure.12,13 Besides the formulation, the crucial manufacturing processes, such as emulsification, solidification, and drying, can generate LAI microspheres with distinct release performance.14,15 The significant impact of microstructure on LAI microsphere product release behavior has been emphasized in numerous studies.8,16–19 The FDA also recommended Q3, the similarity of microstructure, as part of the bioequivalence assessment. Many studies have also suggested that Q1/Q2/Q3 is necessary for bioequivalence.15,20–22
The numerous studies investigating the key factors impacting the microstructure and release behavior of LAI microspheres have primarily focused on formulation composition and preparation processes.23–25 However, these researches have not made a substantial contribution to the development of generic LAI microspheres. On the one hand, the microstructure has not received adequate consideration in the establishment of critical quality attributes (CQAs) of microspheres. On the other hand, the absence of high-resolution, reproducible, and efficient characterization techniques hinders the comprehension of the microstructure.26,27 Enhanced understanding will establish microstructure as a link between the formation and the release characteristics of microspheres.
Conventional techniques usually characterize the structural features of LAI microspheres by determining morphology, particle size and porosity. As shown in Figure 1, the resulted apparent data rather than detailed information at micro level always fail to reveal the interactions between formulation/manufacturing and release kinetics.10 Accurately characterizing the microstructure of LAI microspheres is important for assessing Q3 similarity but very challenging due to the micro size.15,28 The diameter of microspheres usually ranges from 10 to 100 μm, while the drug regions and micropore networks within the microspheres are as small as a few tens of nanometers, requiring techniques with higher resolution to characterize them.29
 |
Figure 1 Correlation between conventional structure/microstructure factors and the release of drugs from two compositionally equivalent LAI microspheres. |
Currently, more and more studies are emerging to focus on the characterization of the microstructure of LAI microspheres and to explore the relationship between microstructure and product performance.9,18,30,31 Significant advances in characterization techniques along with artificial intelligence (AI) based image analytics have been introduced into the microstructure study of LAI microspheres.29,30 The techniques utilized in the study of the microstructural characterization of LAI microspheres include focused ion beam scanning electron microscopy (FIB-SEM), energy dispersive spectroscopy (EDS), synchrotron radiation X-ray microcomputed tomography (SR-μCT), X-ray microscopy (XRM), confocal laser scanning microscopy (CLSM), confocal Raman microscopy (CRM), transition temperature microscopy (TTM) and time-of-flight secondary ion mass spectrometry (ToF-SIMS). These techniques offer comprehensive insights into the microstructure of LAI microspheres, provide a reliable method to compare Q3 equivalence, and establish a correlation between the formulation composition and preparation process with the final product’s performance, thus accelerating the development of LAI microsphere products.
In this review, we present a comprehensive overview of microsphere microstructure, and illustrate the effects of formulation composition (drug, PLGA and solvent) and preparation processes (emulsification, solidification and drying) on microstructure. We also summarize the latest characterization techniques employed for the microstructure of LAI microspheres, and describe the principles and applications of these techniques in detail. This review is of significant value for the development of long-acting injectable microspheres, particularly for the generic microspheres.
Microstructure of the Microsphere
Microstructure emphasizes the factual spatial relationships among the constituents, which contrasts with traditional properties of microspheres. LAI microspheres offer significant clinical benefits for sustained drug delivery after a single administration. As a sustained and controlled release skeleton-type preparation, the release process of microspheres is complex. The drugs diffuse directly from the surface partially or via the mass transfer channel mostly, making the formation and dynamic changes of the mass transfer channel pivotal to the evolutionary process of the microsphere structure. Given that different microspheres may have similar drug content, particle size and morphology, thus characterizing the structural distinctions at the micro level is crucial to understanding the product characteristics of microspheres, particularly drug release kinetics.
Distribution of the Drug in Microsphere
The location of the drug within the microsphere significantly influences the drug release rate. Drugs near the microsphere surface dissolve and diffuse once contact with the liquid medium, while internal drugs need more time to pass through mass transfer channels. If the loaded drug tends to distribute on the microsphere surface, the controlled release efficacy of the PLGA matrix will significantly decrease. The size of the drug distribution domain in microsphere also has noteworthy effects on the drug release. Aggregated drug dispersion in microspheres forms big pore instead of tiny one after drug release, which consequently creates a smaller surface area that does not accelerate PLGA degradation or drug release. In contrast, drugs dispersed uniformly in microspheres release faster.9
The distribution of drug is dependent on its compatibility with the PLGA. When utilizing the emulsification solvent evaporation method for microsphere preparation, hydrophilic drugs with poorly soluble in the PLGA solution are inclined to distribute on the microsphere surface due to their tendency to dissolve in water. Ma32 reported that the protein drug (or inner water phase) was easy to diffuse to particle surface due to coalescence between inner water and outer water phases, and then protein drug was easy to localize near the surface of the microsphere, leading to higher burst effect. However, if water is not involved in the microsphere preparation methods, the drug distribution trend may be reversed. Acharya et al33 discovered that the initial burst release of small-molecule drugs such as felodipine, risperidone, and progesterone is inversely related to their water solubility when using a hydrogel template method to prepare microspheres. The authors speculated that the lipophilic progesterone could migrate to the surface during the removal of dichloromethane (DCM), leading to an additional drug accumulation on the microsphere surface.
Compatibility issues between drugs and PLGA involve various interactions, such as adsorption of drugs by the PLGA, the formation of hydrogen bonds between active groups,34,35 and attacks on PLGA chains by drugs. In a study,9 palmitic acid molecules tended to aggregate into localized regions within the solidifying microsphere until saturation was reached because of the poor compatibility with PLGA. Then, palmitic acid molecules became insoluble in the PLGA leading to phase separation. Rotigotine was fully compatible with PLGA, and it was theorized that rotigotine should be uniformly dispersed in the PLGA. However, aggregations of rotigotine were observed which were believed to be caused by hydrogen bonding interaction with palmitic acid.
Drug distribution and uniformity of domain size can be enhanced through multiple steps in the preparation process. One approach is to increase the homogenization speed during emulsion formation, which produces microspheres with better drug distribution.16 Another method is to select suitable solvents or cosolvents to enhance hydrophobic drug solubility in the dispersed phase.36 Other proposed methods include selecting appropriate physical forms of active pharmaceutical ingredient (API) and accelerating the hardening rate of emulsion droplets into particles. Viewed from a microscopic angle, microsphere structure can reflect the domain size and distribution of drugs in the product.
Dispersion Form of the Drug
The characteristics of the encapsulated drug are largely responsible for the performance of microsphere products. The physical form of the drug, whether crystalline or amorphous, is a critical variable that affects the quality of microspheres. Amorphous compounds, with higher free energy than their crystalline counterparts, may result in increased solubility and dissolution rates.37,38 In the amorphous state, no energy is needed to disrupt the lattice structure, and drug molecules dissolve through intermolecular interaction with solvent molecules.39 The distribution and state of FITC-dextran in microspheres were studied by Cai et al using CLSM and differential scanning calorimetry (DSC).16 The findings indicated that below 10% drug loading, there were no interactions between PLGA and FITC-dextran, which was uniformly distributed within the microspheres and present in a molecular state. Zhang et al40 found that drug dispersion state and particle size (micronized, nanosized or dissolved drug) played an important role in the characteristics of microspheres, including encapsulation efficiency, drug stability, and in vitro release behavior. The order of drug release rate depended on the physical state of the encapsulated drug and was arranged in the order of amorphous > nanosized > micronized drug, and loading amorphous drug resulted in lower encapsulation efficiency.
US Patent 7,279,579 B2 states that naltrexone presents four forms in the microsphere and the proportion of these forms affects the drug release rate, while reducing the crystallinity of naltrexone can accelerate release. Meanwhile, Otte et al raised that the same batch of naltrexone microspheres had particle size-crystallinity dependence.22 The crystallinity increased with the microsphere size, and there were additional polymorphic forms of naltrexone at large sizes. Due to the large surface area and the faster solvent evaporation rate of small-sized microspheres, there was a lack of phase separation time, which was more likely to lead to an amorphous form of naltrexone. In addition, naltrexone in the benzyl alcohol solvate form appeared to be mostly devoid in the 53–106 μm fraction, but basically present in the size range of 106–150 μm. Note that 106–150 μm microspheres had the higher residual benzyl alcohol contents.
Moreover, a high drug concentration at a particular position in the microspheres could encourage partial or complete drug crystallization.17 Cai et al16 found that when drug loading is as high as 20%, the FITC-dextran distributed as much bigger drug strands, some of which crystallize. Pu et al12 discovered that progesterone in PLGA microspheres was in an amorphous form. However, the diffraction peak of progesterone began to emerge after 12 hours and the peak intensity gradually increased during the continuous release process. The results indicated that the amorphous form of the drug was thermodynamically unstable and had a tendency to undergo a phase transition.
Size and Distribution of the Pores
The pores of microspheres are critical to form mass transfer channels. The size and distribution of pores dominate the fluidity of the involved substances (such as drugs and short-chain acids) and the water uptake capacity of microspheres. In the case of post-drug loaded (porous) microspheres, the presence of pores is essential for free access of the drug as well as for adsorption.41
In particular, the pores are significantly related to the initial release of microspheres. With the rapid entry of water and certain high temperature conditions, the drug release is activated. At that time, the PLGA degradation is slow, which is not the main factor influencing the release. The drugs attached to the pore surface have higher free degree and faster dissolution rate to form initial release. In the later release phase, the drugs in the core of microspheres are also transported to the outside due to the connectivity between the pores.
There are a number of elements that influence the formation of pores in the matrix. From a drug point of view, the pore size of microspheres will be larger as the hydrophilic drug loading increases.42 From the polymer point of view, low molecular weight (Mw) PLGA microspheres will have a more porous structure than high Mw microspheres. Due to the short chain segment, the PLGA will be more hydrophilic. With the continuity increase of the oil phase, the solvent exchange process is more difficult to perform, resulting in the more porous microsphere structure.43 Different evaporation and PLGA precipitation rate will also lead to different porosity from the perspective of preparation process.44
Additionally, pore size change has been found to be bidirectional during the release process, including expansion and healing.45,46 When PLGA degradation occurs, the surface tension mediated by the hydrophobicity of PLGA drives the flow of PLGA segments to reduce the surface area so that the pores tend to heal.47
PLGA Density
As a biodegradable drug delivery system, the PLGA in microspheres can be used not only to encapsulate the drug and provide mechanical support but also to influence the micro-connectivity of microspheres due to its different physical forms (highly elastic state, glass state and rubber state) and PLGA properties.
The PLGA density is bound up with the entanglement of chain segment, which determines the difficulty of water infiltration and the formation of mass transfer channels. Therefore, the free movement space of the PLGA molecular chain is inversely related to the controlled release effect of PLGA on the drug. Wan et al48 found that an extended PLGA molecular conformational structure in an optimal solvent system caused a slow release rate during the initial release phase, which might be due to a higher density of the stable PLGA network formed during the spray drying process. In contrast, the more compact PLGA molecular conformation in a poor solvent system resulted in a significant initial burst release, which might be attributed to the weaker network formed in spray drying process.
In addition to the above-mentioned solvent selection, formulation composition and other process conditions also have a significant effect on the PLGA density in microspheres. The PLGA Mw and the dispersed phase concentration have a positive correlation with the microsphere density. Both the vacuum or low pressure in the curing process and the increase in the osmotic pressure of the external water phase in the emulsifying process will result in a more compact structure by compressing the internal space of microspheres.49
Microstructure describes the detailed material distribution as well as the material existence form in the spatial dimension, which are closely related with the designed release kinetics and other required attributes of PLGA microspheres. Knowing the factors contributed to the microstructure and performing systematic characterization and evaluation of the microstructure are essential for the development of related microsphere products.
Factors Contributing to the Microstructure of Microspheres
Due to the complexity of microsphere formulations, the challenge remains to produce products with desirable microstructures and release characteristics. The successful development of a qualified LAI microsphere product depends on the ideal combination of formulation composition and preparation process, where various factors profoundly affect the microsphere microstructure, which to some extent determines the complexity of the release mechanism. An effective combination of formulation composition, preparation process and microsphere microstructure facilitates the development, quality control and regulatory review of LAI microsphere products.8
Effects of Formulation Composition on the Microstructure
Drug
LAI microspheres have successfully delivered drug types ranging from hydrophilic (such as protein and peptide) to hydrophobic (such as naltrexone and risperidone) drugs.50–52 The type of encapsulated drug largely determines the encapsulation efficiency and release performance of microspheres. Hydrocortisone had a higher water solubility than dexamethasone, and therefore hydrocortisone was more partitioned in the external aqueous phase in the dissolved state, resulting in higher drug loss and lower encapsulation efficiency.40 Half of FDA-approved microsphere products encapsulate hydrophilic protein or peptide,53 which tend to distribute to the exterior of microspheres. Effective encapsulation and low burst release of hydrophilic drugs remain a challenge.
The drug solubility also determines the drug distribution within the microspheres. Compared to the hydrophilic rhodamine, relatively hydrophobic piroxicam was more homogeneously distributed in the microsphere matrix.54 For the PLGA microspheres, the immiscibility between PLGA and drug leads to phase separation, which will further lead to the emergence of drug-rich regions within the microspheres.55 Yang et al prepared nimodipine microspheres and bovine serum albumin (BSA) microspheres.42 Nimodipine, as a lipophilic and small molecular drug, presented great compatibility with PLGA, could be distributed uniformly in the amorphous state in the microsphere. While due to the incompatibility between BSA (a hydrophilic protein molecule) and PLGA, BSA tended to distribute near the microsphere surface and to accumulate on the cavities within the microsphere matrix.
The properties of the drug can also alter the microspheres release behavior by changing the microstructure during preparation. Weak bases can accelerate the release rate by catalyzing PLGA degradation.56 Meanwhile, alkaline drugs can neutralize the acidic environment generated by autocatalysis during microsphere degradation, which influences the formation of internal pores and eventually leads to the decrease of drug release rate.23 Drug solubility in the release medium is also a key variable affecting the microsphere microstructure during incubation. For hydrophilic drugs, the release rate increased with drug loading due to the pore production inside the microsphere with the drug consumption, thus providing a higher rate of drug diffusion. In contrast, the dissolution rate of hydrophobic drug is slower due to the obstruction of water influx, which reduces the release rate.57
PLGA
Microsphere carrier materials include natural and synthetic polymers. As the main excipient for the long-acting character in microspheres, polymer can encapsulate drugs to achieve stable and sustained drug release. Aliphatic polyesters are important members of the family of degradable polymers with excellent biocompatibility, biodegradability and bio-absorbability. Under in vivo or in vitro environments, the ester bonds within the material are cleaved by hydrolysis, allowing for gradual degradation. Aliphatic polyesters degrade under acidic, neutral and alkaline conditions, and the oligomers and monomers produced by hydrolysis are equally biocompatible. PLGA and polylactic acid are the most widely used polymeric materials, and all the approved LAI microspheres applied PLGA as polymer.
The PLGA properties have a critical influence on the microstructure and release behavior of microspheres, which are characterized by the parameters including Mw, glass transition temperature (Tg), terminal group, block ratio, etc.10,20,28 Mw is in a positive correlation with PLGA chain length and viscosity. PLGA with higher Mw will retard the distribution of drug from the interior to the surface during microsphere formation because of the denser coil formed by the longer chain, resulting in the relatively suppressed burst release.23 The PLGA with lower Mw are more hydrophilic via the short-chain segment and therefore experience longer solidification time, which results more drugs diffuse to the surface and more pores forming within the microspheres. Additionally, the microsphere prepared with higher Mw PLGA tends to be larger in size, which provides a relatively small surface area for drug diffusion.16 All the changes in microstructure brought from high Mw of PLGA are benefit for the sustained release of drugs.
Tg is an important physical property of amorphous polymers such as PLGA converted from glassy state to the rubbery state, the value of which depends on the mobility of the PLGA chain. In general, for the same type of PLGA, Tg increases with the Mw. At process temperature above Tg, the flexibility and plasticity of PLGA chains are enhanced, which facilitates the rearrangement into a dense matrix, resulting in less internal porosity and a smooth surface of microspheres. In contrast, porous microspheres will be produced when the preparation temperature is below the Tg.6,58
The terminal end groups of the PLGA also have a great influence on the microsphere microstructure. The acid-capped PLGA is more hydrophilic than the ester-capped one, which can improve the stability of the double emulsion droplet by reducing the interfacial tension, and can promote the connection between the inner and outer aqueous phases, resulting in higher porosity within the microspheres compared to the ester-capped PLGA.16 The hydrophilic nature of the acid-capped PLGA and the porous structure of microspheres promote water uptake, causing swelling and initial hydrolysis of the PLGA matrix,53 thereby causing higher burst release. The hydrophobicity of ester-capped PLGA also shows a longer degradation time than the acid-capped PLGA with a similar monomer ratio and molecular weight.59
Solvent
The PLGA used in LAI microspheres is usually hydrophobic to achieve sustained drug release, so a suitable solvent is required to prepare the PLGA and/or drug solution. Different solvents have different solvency capacities and evaporation rates, leading to different PLGA precipitation and solvent removal rates, ultimately resulting in different microsphere microstructure and release behavior. Selection of appropriate solvents/co-solvents is a proven method to achieve the desired release behavior.60,61
DCM is one of the most used solvents to prepare PLGA microspheres. PLGA is soluble in DCM, which causes the microspheres to remain in a semi-solid state for a long time during preparation, and the microspheres progressively shrink to form a dense PLGA matrix with fewer pores.62 Water-miscible solvents/co-solvents, such as ethyl acetate (EA) and dimethyl sulfoxide, lead to faster hardening of PLGA droplets, resulting in a more porous structure within the microspheres and more diffusion of the drug into the external aqueous phase.40
Shen et al63 prepared risperidone microspheres using O/W method and found that the solvent types (DCM or EA/BA) influenced the drug distribution and porosity, in which benzyl alcohol (BA) was added as a co-solvent to help dissolve risperidone. Microspheres prepared using DCM as a solvent had higher burst release and longer lag time, which were mainly due to two aspects. On the one hand, risperidone was well soluble in DCM, and a certain amount of risperidone accumulated on the microspheres surface when DCM was removed, thus increasing the burst release. On the other hand, since water was immiscible with DCM, it was hard for water to diffuse into the particles, resulting in fewer pores in the microspheres and extended lag time. The microspheres prepared by EA/BA had lower burst release and shorter lag time. A reasonable explanation was that the diffusion of risperidone along with BA to the surface was limited during microsphere precipitation, which was due to the fact that EA had a higher boiling point and evaporation rate than BA. The miscibility between EA and water also allowed water to penetrate into the interior of microspheres in the precipitation process. During the drying process, the EA will leave the pore structures that were once filled with water. The effect of solvent type on porosity was confirmed in another study of risperidone microspheres, where DCM and EA/BA were used as solvents to produce less porous and more porous microspheres, respectively.64
The boiling point of organic solvent also regulates the sphericity and surface smoothness of microspheres when evaporation is used to remove the solvent. Avachat et al65 found that DCM evaporated rapidly during the preparation of ropinirole hydrochloride microspheres due to its low boiling point (39.75 °C), which led to a rapid precipitation rate of the PLGA, and the prepared microspheres were non-spherical. The addition of methanol to DCM generated spherical microspheres with an uneven surface owing to the relatively high boiling point (64.7 °C). The higher boiling point of ethanol (78.3 °C) compared to other solvents (DCM, methanol, acetone, etc.) prevented the intermediate PLGA precipitation, and spherical microspheres with a smoother surface were prepared.
Co-solvents such as methanol, acetonitrile, acetone, and tetrahydrofuran are typically added during microsphere preparation to improve drug solubility. These co-solvents are usually water soluble and volatile, and their presence adds complexity to the oil-phase system. The co-solvent not only affects the encapsulation efficiency but also changes the microstructure of LAI microspheres. Le et al66 found that when DCM was used as the sole solvent, tiny pores were observed inside the lysozyme microspheres. When acetone was added as a co-solvent, the microspheres were non-porous. DCM was poorly miscible with water, which prolonged the mass transfer between the dispersed and continuous phases and caused a slow precipitation rate of the PLGA, in which water slowly penetrated into the microparticles and pores were formed inside the microspheres. The opposite was true with the addition of acetone.
PLGA concentration affects the particle size, morphology and porosity of the prepared microspheres, and these factors have a profound effect on the initial release stage. In a study of the effect for PLGA concentration on the release behavior of microspheres, Mao et al67 found that the burst release trend decreased with the increase of PLGA concentration, and this result was also confirmed in their other study.17 The effect of PLGA concentration could be explained in three ways. As the PLGA concentration increased, the microsphere size increased and had a smaller specific surface area. Secondly, the viscosity of the oil phase increased with the PLGA concentration, which limited the drug diffusion into the continuous phase, resulting in a more uniform distribution of the drug.54,67,68 In another words, the distribution of drug closed to the surface of microspheres was reduced. Finally, it was more evident that the lower concentration of PLGA lead to higher internal porosity significantly dominating the initial release of microspheres. Internal water droplets coagulated more readily in PLGA solutions with low concentration, and once the microspheres were dried, bigger pores and a less tortuous were formed internally.67 It was well known that the high porosity facilitated water penetration during incubation, which shortened the drug diffusion time and pathway, leading to higher burst release.
Effects of Process Parameters on the Microstructure
From the traditional techniques such as solvent evaporation method to the new techniques such as microfluidics, more and more strategies are used to obtain ideal microspheres.14,69 Due to the hydrophobic nature of the PLGA, various microsphere preparation methods share similar principles, and the microspheres production is subjected to the processes such as emulsification, solvent evaporation or microsphere solidification and drying. Solvent evaporation method is the earliest and most widely used manufacturing methods of LAI microspheres,5,7 either the single emulsion (oil-in-water, O/W) method for the hydrophobic drug or double emulsion (water-in-oil-in-water, W/O/W) method for the hydrophilic drug.70,71
Emulsification Process
For the drug, especially for the hydrophilic drug, emulsification is usually the first step to prepare microspheres. The size and stability of the emulsion directly affect the size, the size distribution and the microstructure of microspheres. Various emulsification homogenization methods have their own characteristics, but the homogenization rate is the key variable in common. High shear energy will break the oil phase into small emulsion droplets, which is benefit for the rapid hardening in the subsequent solidification process, and prevents the drug diffusion to the microsphere surface, thus obtaining a uniform microsphere. Berkland et al found that p-nitroaniline was uniformly distributed in the smallest microspheres, but there was a tendency for it to distribute on the surface as the particle size increased.54 The same phenomenon was observed in piroxicam-loaded microspheres. Piroxicam was mainly distributed on the surface of large (~112 μm) microspheres and was more uniformly distributed in 20 μm microspheres.
The emulsification homogenization rate does affect not only the drug distribution but also the PLGA density. In one study,16 ester-capped PLGA microspheres changed from “microcapsules” to a dense matrix structure with increasing homogenization speed. When the acid-capped PLGA was used to prepare microspheres, and as the homogenization speed decreased, the pores became larger and the drug aggregated into multiple drug domains. A more hydrophilic PLGA and large internal pores would allow more water to enter the microspheres, which accelerated the drug release during the second release phase.
Solidification Process
Microsphere solidification is a process of exposing emulsion droplets in the medium, with the organic solvent leaving from the gas–liquid interface, and the emulsion droplets finally granulate. It means that with the solvent volatilization, the viscosity of the droplet rapidly increases, and then the droplet solidifies to form a pore-participating drug-PLGA structure. The difference in the migration rates of the drug and PLGA molecule ultimately affects their location in the microsphere, and the drug will tend to stay with its preferred solvent. If its preferred solvent is the curing medium, the drug will easily diffuse out of the emulsion droplet, and the encapsulation efficiency is expected to be greatly reduced.
The environmental conditions such as temperature and vacuum level could also change the solidification process by solvent evaporation. High temperature and high vacuum level often lead to rapid solvent evaporation and rapid formation of the microsphere surface structure. Under these conditions, rough and porous shells and/or hollow structures are likely to be formed, such as the hollow or shrunken microspheres.55 On the contrary, a relatively slow solvent exchange leaves the microsphere in a semi-solid state longer time, which can lead to further shrinkage and the compact structure of the microsphere.55
Of note, stirring operation does not influence the solvent migration rate from the droplet interior to the surface, but only regulates its separation rate from the particle surface.72 Many variables have a negative impact on the solvent separation rate, including the strong affinity between solvent molecules and drugs and/or PLGA molecules, the high emulsion viscosity, etc.43,73 In most cases, we can reduce the interfacial tension between the organic phase and the aqueous phase by increasing the vacuum level or the surfactant concentration in the curing medium to shorten the curing time, reduce the drug loss, and obtain a smoother surface of microspheres.55,74
Wan et al75 found that in the solvent volatilization process of spray-dried celecoxib microspheres, different ratios of solvent composition (acetone and methanol) would result in different diffusion rates of drug and PLGA molecules, which controlled the surface properties of microspheres and the network structure of PLGA. Specifically, microspheres prepared with high methanol ratio (acetone: methanol = 69:31) had smaller particle size and denser internal structure than those prepared with high acetone ratio. In addition, a higher celecoxib release rate was observed with increasing acetone: methanol molar ratio.
Drying Process
Microspheres are usually stored in powder form to achieve long-term stability of the preparation and the convenience of product transport. At present, the main drying methods of microspheres are lyophilization and vacuum drying. The main difference between them largely lies in whether the temperature is set below the Tg of dry/wet PLGA microspheres or near the temperature. As the last step in the microsphere preparation process, the drying method also has a great impact on the microsphere microstructure.76,77
Lyophilization
Lyophilization involves the direct sublimation of ice crystals into vapor and includes three stages: sample freezing, primary drying (sublimation), and secondary drying (desorption), which may cause additional changes in the pore morphology of microspheres.78 During the freezing process of hardened microspheres, solvent crystals nucleate and grow. With the shape and size of ice crystals are fixed, drug and PLGA are excluded from the ice crystals, forming a continuous drug-PLGA-ice network. The vapors from the sublimation process of ice crystals escape to the outside, allowing more microchannels to be generated in the PLGA matrix from the location of the ice crystals to the microsphere surface. It was found that microspheres loaded with FITC-dextran showed higher burst release after lyophilization than those without lyophilization.79 In particular, the free water molecules in microspheres can be easily removed, but the sublimation rate of the bound water molecules is slow, which can exist 24 hours after freeze-drying.79 Therefore, the secondary drying process is to desorb the unfrozen water bound to the drug and/or PLGA. Additionally, for porous microsphere carriers, it is possible to have structural collapse after freeze-drying treatment. Freeze-dried products are also more hygroscopic.77
The rate of heat transfer and freezing are the most critical parameters affecting the pore structure.80 The lower the freezing temperature, the more microspheres tend to have the rapid formation of ice nuclei and growth of small ice crystals.78 Thus, the part of the sample closed to the freezing plate and air will crystallize rapidly and the heat transfer rate in the material center is slower, and then the number of ice crystals will decrease and the size of ice crystals will increase, which eventually leaves macropores. Here, in the process of freeze drying, we suggest that it is practical to reduce the sample thickness to some extent. In addition to ensuring the freeze-drying efficiency of the product, the consistency of the microstructure between microspheres can also be maintained.
Vacuum Drying
Vacuum drying evaporates water in materials by lowering the boiling point of water in a low-pressure environment. When the set temperature is close to the product Tg, microspheres are smoother and have the possibility to adhesion and aggregation. Wu et al investigated the effects of different drying methods (lyophilization and vacuum drying) on the morphology and structure of risperidone PLGA microspheres and conducted long-term and accelerated release studies.81 One batch of microspheres were precooled at −40 °C for 10 h and then were lyophilized for 24 h, while the other batch of microspheres was dried for 24 h at different temperatures (5 °C, 15 °C and 25 °C) and under different pressures (200 mmHg, 450 mmHg and 700 mmHg). They found that the surface of lyophilized microsphere was smooth with visible small pores, and the porosity was as high as 78.46%. In contrast, the vacuum dried microspheres had low porosity (52.45–61.88%) and obvious wrinkles and irregular particles on the surface. Wrinkles became less with pressure increasing and the surface of microspheres became smoother with temperature raising. Compared with vacuum drying microspheres, the lyophilized microspheres had larger particle size, higher solvent residues and lower drug loading (p>0.05). Meanwhile, the drug release rate and degradation rate of lyophilized microspheres were also relatively high. The drying method affected the microstructure change of microspheres during the transition process from wet microspheres to dry microspheres and ultimately affected the critical product performance and even release behavior.
Sterilization
The final preparation step of microspheres is terminal sterilization if the microsphere formulations were not prepared in an aseptic manufacturing manner. As a mature and commonly sterilization method, γ radiation sterilization will reduce the Mw of the PLGA, thereby affecting the drug release kinetics.82,83 Keles et al84 evaluated the γ irradiation on physical and chemical structures of PLGA microspheres. With the increase of gamma ray intensity, the surface roughness of microspheres decreased and the water absorption was higher. The Tg of microspheres dropped by 3.8 °C also indicated the Mw degradation after 100 kGy γ exposure. Barbosa et al82 examined the changes in critical quality attributes of dexamethasone microspheres after γ radiation sterilization at 25 kGy. There was no significant difference in microsphere morphology and particle size, whereas the release rate was accelerated due to the sterilization process and the release profiles had dissimilarity (f2=35.5).
In conclusion, the microstructure of microspheres is susceptible and is controlled by multiple variables. The comprehensive characterization of the microsphere microstructure and the establishment of correlations with formulation compositions and preparation processes are essential to guide product development.
Methods for Microstructural Characterization
The characterization of microsphere microstructure depends on the development of advanced characterization techniques. Traditional methods limited by resolution focus on the analysis of the bulk material rather than individual microsphere and are hardly to provide detailed information about the microsphere microstructure.82 Therefore, the establishment of characterization methods with high resolution is of great significance for the evaluation of microsphere microstructure. Several advanced microsphere characterization techniques are introduced in detail, and their main applications, advantages and limitations are summarized in Table 1.
 |
Table 1 The Techniques Utilized to Characterize the Microsphere Microstructure |
Focused Ion Beam Scanning Electron Microscopy
Focused ion beam scanning electron microscopy (FIB-SEM), as a powerful imaging tool, can be used to observe the internal microstructure of microspheres and supports 3D nano-tomography. The equipment can be simply understood as a coupling of a FIB and a SEM system (Figure 2). FIB has been used to prepare subsurface cross-sections of microspheres.97 The ion beam generated by the ion source is accelerated by the ion gun and acts on the sample surface, while the atoms on the surface are stripped by the ion beam with strong current to achieve the nano surface topography processing without disrupting microspheres in the next layer.29,85 Compared with mechanically-cut microspheres, FIB can provide higher accuracy and precision, thus obtaining a clean and artifact-free cross section for high-resolution SEM imaging.8,98 For subsequent SEM imaging, the beam emitted by the electron gun passes through a pair of objective lens and aperture to form an extremely narrow high-energy electron beam, which is further focused on the sample surface through objective lens. Various signals are excited through the interaction between the beam and the sample, and each of those signals could be collected, amplified and re-imaged by a specific detector to achieve the purpose of characterization of the material microstructure.26
In 2D FIB-SEM images, the internal microstructure of microspheres was classified into three material phases: API, PLGA and pores. These phases were identified by their characteristic grayscale values, drug distribution and pore morphology. The drug distribution could be confirmed by EDS, which will be discussed below. After repeated FIB milling and subsequent SEM imaging, hundreds of SEM images are collected from the same microsphere, so that the internal microstructure of microspheres can be visualized at nanometer resolution in 3D.29 Furthermore, microsphere reconstruction with AI-based image analytics combining with the 3D tomography allows the compute of quantitative information including volume fractions, spatial distribution homogeneity and particle/pore size distribution.8,29
 |
Figure 2 (a–c) Schematic overview of FIB-SEM imaging of PLGA microsphere samples: (a) cartoon representation of SEM imaging of the microsphere surface, (b) FIB milling and SEM imaging of the first cross section, and (c) FIB milling and SEM imaging of the 600th cross section. (d) SEM image of the microsphere surface prior to FIB milling and after stage rotation (inset showing top-down view of the same sphere) (e), the first cross section of the sphere and (f) the 600th cross section. Reprinted from Journal of Controlled Release, 349, Clark AG, Wang R, Qin Y, et al, Assessing microstructural critical quality attributes in PLGA microspheres by FIB-SEM analytics, 580–591, Copyright 2022,with permission from Elsevier.8 |
One of the most critical restrictions for this technique is the time-consuming nature. It takes several hours to obtain FIB-SEM images of a single microsphere and several days to analyze and compute images. Therefore, it is impractical to perform FIB imaging on a large number of microspheres in a batch, so the microspheres used for FIB-SEM imaging should be representative. The combination of multiple characterization techniques can be an effective way to compensate for this shortcoming. For imaging studies of inhomogeneous samples, the X-ray microscopy can capture a larger field of view (at low resolution) to target representative samples for further observation. The high-resolution FIB-SEM can capture more details of the target sample.85
Clark et al8 prepared eight microspheres with different microstructures by changing the formulation and processing procedure. 2D FIB-SEM imaging and 3D tomography microsphere reconstruction were conducted to fully quantify microstructures, which including API and pore fractions, size distribution and spatial distribution, and eventually used for the prediction of drug release. The results showed that the lack of porosity would cause a significant slowdown in the initial release and delay the hydrolytic degradation of the PLGA. The effect of process parameters on product quality can be robustly understood by imaging and quantifying the microstructure of microspheres.
Energy Dispersive Spectroscopy
Energy dispersive spectroscopy (EDS) as a semiquantitative method is usually used to identify and quantify elemental composition on the microsphere surface. In the EDS equipment, the atoms on the surface of the sample are excited to an excited state by the high-energy electrons, and emit X-rays of characteristic wavelengths as they return to the ground state. The characteristic wavelength and signal intensity can be used to determine the element composition and the sample content (Figure 3).
 |
Figure 3 (a) Solid-state energy-dispersion spectroscopy. From Fitzgerald R, Keil K, Heinrich KF. Solid-state energy-dispersion spectrometer for electron-microprobe x-ray analysis. Science. 1968;159:528–530. doi:10.1126/science.159.3814.528. Reprinted with permission from AAAS.99 (b) The S elemental mapping images of rotigotine microsphere. Reprinted from Journal of Controlled Release, 357, Xue Y, Xu L, Wang A, et al, Studying spatial drug distribution in golf ball-shaped microspheres to understand drug release, 196-209, Copyright 2023, with permission from Elsevier.9 |
The combination of EDS and SEM is advantageous to understand the distribution of various phases in the microspheres, which has been validated in several studies.29,100 The SEM cross-sectional image of microspheres showed grayscale contrast for different material phases. EDS can provide the element information of phases with different color to confirm the distribution of them in the microspheres.
Xue et al9 used broad-beam argon ion milling technology, which is similar to the working mechanism of FIB, to obtain the cross-sectional image of rotigotine microspheres, while EDS was used to obtain the element distribution to understand the formation mechanism of golf ball-shaped microspheres and the relationship between drug distribution and in vitro release behavior. They found that almost circular particles were uniformly distributed on the cross-section of rotigotine microspheres and the signal of S element from rotigotine molecules was also present in the PLGA matrix, indicating that rotigotine existed in microspheres in two forms, one as a phase-separated particle formed with palmitic acid and the other as a molecular dispersion. They demonstrated a direct relationship between drug distribution and in vitro release trend: aggregated rotigotines were released during the rapid-release phase, while rotigotine molecules were mainly released slowly in the later stage. Thus, the drug distribution helped to figure out the formation and release mechanisms of complex LAI microsphere products, which is crucial for the development of generic and innovative drugs.
Synchrotron Radiation X-Ray Microcomputed Tomography
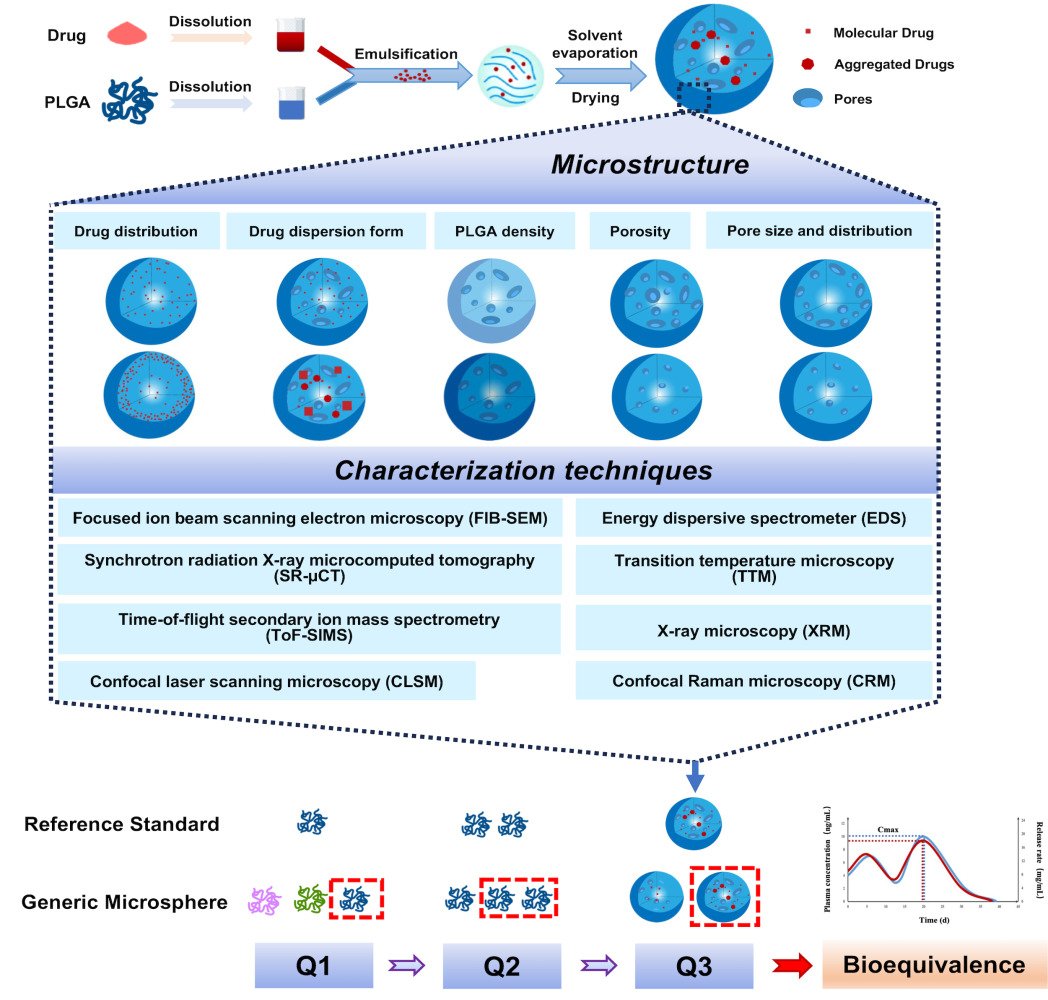
Synchrotron radiation X-ray microcomputed tomography (SR-µCT) as a novel technology has been used to non-destructively observe the internal microstructure of microspheres owing to its high resolution and contrast.88,101,102 Synchrotron radiation emits X-rays of high penetration power, high quality, high intensity and tunability which are transmitted to the sample, and then recorded by the detector as a 2D projection image. Different phases or components within the sample absorb X-rays to different extents to create a contrast. The sample is then rotated at an angle on the rotary stage, and another X-ray projection image is taken. The sample is rotated 180 degrees, and a series of X-ray projection images are captured103 (Figure 4). The projections are then used as input for the computerized tomographic reconstruction of the 3D sample.104
 |
Figure 4 (a) Schematic of synchrotron-based tomography setup with parallel beam configuration. SR-µCT images of acetaminophen microspheres at different sampling time (b–d) were images at 0 min; (e–g) were images at 5 min; (h–j) were images at 60 min; (b, e and h) were surface images of complete microspheres; (c, f and i) were images of microspheres partly split. Some protuberances of acetaminophen crystals were observed on the microspheres surface as the red arrows indicated in (c); (d, g and j) were images of acetaminophen crystals extracted from microspheres). Nothing could be seen in image j, because all the drug crystals had dissolved. Figure 4 (b-j) Reprinted from International Journal of Pharmaceutics, 499, Guo Z, Yin X, Liu C, et al, Microstructural investigation using synchrotron radiation X-ray microtomography reveals taste-masking mechanism of Acetaminophen microspheres, 47-57, Copyright 2016, with permission from Elsevier.89 Figure 4 (a) Reprinted from Vijayakumar J, Goudarzi NM, Eeckhaut G, Schrijnemakers K, Cnudde V, Boone MN. Characterization of Pharmaceutical Tablets by X-ray Tomography. Pharmaceuticals. 2023;16:1. https://creativecommons.org/licenses/by/4.0/.105 |
Guo et al89 investigated the fine architectures of acetaminophen microsphere at different times during incubation and quantitatively correlated the structural data with the release behavior. The results showed that the structure and morphology of microspheres were influenced by the shape and particle size of the drug, resulting in different drug release behaviors. Meanwhile, the drug content and cumulative release fraction of microsphere measured by SR-µCT was consistent with experimental data, demonstrating that the SR-µCT had advantages in distinguishing different phases and accurately calculating their content in complex microsphere systems. Additionally, the synchrotron radiation-based Fourier-transform infrared spectromicroscopy technique can provide the chemical distribution of constituents based on the functional group distribution in the microspheres, so Wang et al achieved non-destructive drug localization in exenatide microspheres.106
X-Ray Microscopy
X-ray microscopy (XRM) based on the same principle as SR-µCT is also a characterization technique in the X-ray microcomputed tomography family. For XRM, spatial variations in sample density will affect the intensity of the X-ray signal, allowing different phases of microspheres, such as PLGA, drug and pores, to be observed (Figure 5).21,30 As a high-throughput, and non-invasive technique, XRM has gained much attention in recent years and is increasingly being used for microstructural analysis of microspheres.
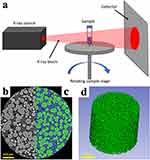 |
Figure 5 (a) Cartoon schematic demonstrating the principal of operation of XRM. (b) 2D slice of a reconstructed XRM image of a microsphere sample with (c) an example segmentation of the microspheres (green) from the surrounding air (blue). (d) 3D rendering of the imaged XRM volume showing all segmented microspheres. Reprinted from Journal of Controlled Release, 358, Clark AG, Wang R, Lomeo J, et al, Investigating structural attributes of drug encapsulated microspheres with quantitative X-ray imaging, 626-635, Copyright 2023, with permission from Elsevier.21 |
Clark et al21 prepared eight batches of minocycline microspheres by adjusting the preparation parameters. Thousands of microspheres from each batch were analyzed by XRM, and AI was used to determine inter- and intra-batch homogeneity. They found excellent homogeneity of microsphere microstructure within batches, but significant differences between batches corresponding to the changes in the preparation process.
However, due to resolution limitations, XRM cannot provide microstructural information at the nanometer scale, while high-resolution characterization techniques such as FIB-SEM are well suited to compensate for the deficiency of XRM. The combined application of multiple characterization techniques will provide richer microstructural information.
Confocal Laser Scanning Microscopy
Confocal laser scanning microscopy (CLSM) has been widely applied to detect the distribution of drug and pores within the PLGA matrix.66,107,108 In CLSM imaging, the light emitted from the laser source passes through a pinhole, which is reflected through the spectroscope to the objective mirror and then focuses on the sample. The specific focal plane of the sample is scanned point by point, line by line, and surface by surface. The fluorescence emitted by the excited fluorescent components in the sample is returned directly to the spectroscope through the incident optical path and is focused before passing through the detection pinhole.26,92 The focused light is collected by the photodiode to produce images (Figure 6).26 With different focus depths, images of different layers of the sample can be obtained, and high-resolution 3D images can be acquired by image reconstruction software.
 |
Figure 6 (a) Schematic diagram of the instrumental setup of CLSM. Reprinted from Advances in Colloid and Interface Science, 280, Falsafi SR, Rostamabadi H, Assadpour E, Jafari SM, Morphology and microstructural analysis of bioactive-loaded micro/nanocarriers via microscopy techniques, CLSM/SEM/TEM/AFM, 102166, Copyright 2020, with permission from Elsevier.26 (b) CLSM images of drug distribution in Ovalbumin (OVA) microsphere. OVA inside the microsphere was labeled with FITC, and OVA on the films on the surface of microspheres was labeled with Cy3. Polylysine film was labeled with Cy5. Reproduced from Di J, Wang J, Wang S, et al. Self-boosting vaccination based on pulsatile antigen release from core-shell microparticles. Small. 2023;19:e2207892. doi:10.1002/smll.202207892. © 2023 Wiley-VCH GmbH.109 |
Chen et al110 verified the release mechanism of FITC-BSA microspheres by detecting the changes in drug distribution on the microsphere surface during the release period using CLSM. The results showed that FITC-BSA loaded microspheres presented a typical triphasic release profile. Dissolution of the drug into the release medium at or near the surface of microspheres caused a burst release phase, after which the fluorescence almost disappeared, indicating that microspheres were in a lag phase. With the release rate increased, FITC-BSA diffused to the microsphere surface, and thus the fluorescence signal was observed again. The fluorescence gradually diminished in the later phase with the continuous release of FITC-BSA. In another study,111 CLSM was also used to investigate the release mechanism of leuprolide acetate microspheres. The fluorescent dye was added to the release medium and incubated with the microspheres. During the release process, the fluorescent dye diffused into aqueous pores inside the microspheres, which was monitored by CLSM. The CLSM images provided information on both the PLGA microstructure and the pore-interconnections inside microspheres. Hence, CLSM offers a powerful support for a more comprehensive study of the drug distribution and release mechanism of microspheres.
Garner et al18,19 used CLSM to track the three-dimensional structural changes of naltrexone microspheres after sequential semi-solvent vapor impact (SAVI) and further elucidated the factors that were critical to the microsphere microstructure (nucleophilic attack capacity and self-crystallizing capacity) and their effects on release behavior.
Confocal Raman Microscopy
Raman spectroscopy is based on the Raman effect, in which a laser is focused on a sample to produce scattered light that enters the spectrometer through the objective lens and is divided by the spectroscope into different wavelengths that reflect the properties of various chemical components, providing a wide range of physical/chemical and structural information (Figure 7).
 |
Figure 7 (a) Schematic layout of confocal Raman spectrometer. Reproduced with permission from Bergholt MS, Serio A, Albro MB. Raman Spectroscopy: guiding Light for the Extracellular Matrix. Front Bioeng Biotechnol. 2019;7:303. doi:10.3389/fbioe.2019.00303. https://creativecommons.org/licenses/by/4.0/.112 (b) A confocal Raman map indicating PLGA as green and lysozyme as red, showing large porous void spaces and protein adsorbed to the wall of the pore and small protein rich deposits measuring ~2 μm in diameter. (c) An interpolated 3D representation of the protein distribution within a 40×40×10 μm volume of a microsphere showing from 20 μm into the microsphere surface (top of image) to 30 μm (bottom of image) of the microsphere bulk. Reproduced with permission from Rafati A, Boussahel A, Shakesheff KM, et al. Chemical and spatial analysis of protein loaded PLGA microspheres for drug delivery applications. J Control Release. 2012;162(2):321-329. doi:10.1016/j.jconrel.2012.05.008.95 |
The combination of Raman spectroscopy with confocal microscopy allows non-destructive analysis in three spatial dimensions with high resolution.113 In recent studies, confocal Raman microscopy (CRM) has been mainly used to study drug distribution and the three-dimensional shape of microspheres. Sophocleous et al explored the interaction between acid-capped PLGA and leuprolide, and the distribution of leuprolide within the PLGA films was detected by CRM.13 In another study,95 CRM was applied to provide three-dimension spatial maps of the drug distribution on the surface and within the bulk of microspheres. The confocal Raman maps showed that the distribution of lysozyme was different in pores of different diameters, lysozyme only coated the inner surface of the larger pores. In contrast, lysozyme was widely distributed throughout the PLGA matrix of the smaller pores. The use of confocal Raman microscopy has facilitated the understanding of the microsphere microstructure in 3D.
Transition Temperature Microscopy
Transition temperature microscopy (TTM) is a novel nano-thermal and imaging technique for automated analysis of the local temperature transitions over a region of a sample surface. TTM expands the nano-thermal analysis (nano-TA) technique into an imaging or microscopy by assembling a series of nano-TA measurements into a map of the transition temperatures over the interest region.114 In TTM mode, the continuously heated probe is automatically repositioned on the sample and the nano-TA measurement is repeated. By observing the derivative of the displacement measurement, the transition temperature of each point or pixel in the scanned area is determined.115 These points or pixels are color-coded based on the measured transition temperature. Finally, a color-coded map across the sample surface is detected (Figure 8). Therefore, this method has the advantage to determine the distribution of the drug and PLGA across the surface and cross-section of individual microsphere in a systematic manner.
 |
Figure 8 The imaging principle of TTM. Reprinted from Advanced Drug Delivery Reviews, 64, Goh CF, Lane ME, Advanced structural characterisation of pharmaceuticals using nano-thermal analysis (nano-TA), 114077, Copyright 2022, with permission from Elsevier.114 (a) The workflow of TTM. (b) Establishing TTM map to visualize localized transition temperature. Each transition temperature is assigned a color code and they can be collectively visualized as a blended TTM map by combining each color pixel. The “i” “ii” “iii” labels in (b) referred to the test results obtained during the “i” “ii” “iii” process in (a). |
The critical limitation of TTM for microsphere microstructure characterization is that the thermal transition temperature of each individual phases within the microsphere need to be significantly different (>40 °C).42 If the thermal transition temperatures of the drug and the PLGA are similar, the TTM map will be composed of similar colors, which makes it difficult to distinguish the drug form the PLGA region. Therefore, TTM is not suitable for characterizing the microstructure of all microsphere preparations.
Yang et al42 applied TTM to investigate the drug microstructure and spatial distribution within BSA-loaded and nimodipine-loaded PLGA microspheres. The thermal transition temperatures of BSA and PLGA were significantly different, allowing that the regions of BSA and PLGA can be visually identified on the generated TTM map. The TTM map suggested that more BSA was distributed in the interior of microspheres rather than on the surface. Due to the similar thermal transition temperature between nimodipine and PLGA, the thermal transition temperature maps of nimodipine-loaded microspheres mainly showed a single color-block, and it was difficult to distinguish the regions associated with nimodipine and PLGA, and to characterize the drug distribution on the surface and section of microspheres.
Time-of-Flight Secondary Ion Mass Spectrometry
Time-of-flight secondary ion mass spectrometry (ToF-SIMS) as a powerful approach of surface imaging has been widely used to study the distribution of drug and excipient within pharmaceuticals. ToF-SIMS generates secondary ions from the sample surface by bombardment with a focused beam of high energy primary ions under ultra-high vacuum. These secondary ions are formed from the atoms and molecules of various species present on the sample surface.116 The charged secondary ions and other ions of the same polarity are accelerated to the same kinetic energy and then enter a time-of-flight analyzer to be separated according to the mass-to-charge ratio (m/z). For a fixed distance in the flight-tube, the ions with lower m/z values reach the detector earlier than the heavier ions with higher m/z values.117 The variation in time of flight is used to generate the mass spectra for each detector pixel (Figure 9). The mass spectra are then used to identify the analyte species on the sample surface. Thus, the image generated from each mass spectrum can be used to characterize the distribution of the drug, PLGA and other components on the microsphere surface.
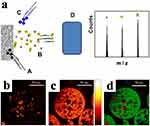 |
Figure 9 (a) Schematic diagram of ToF-SIMS system, including; primary ion beam (a), ejected secondary ions (b), electron flood gun for charge compensation (c) and mass analyzer (d). ToF-SIMS of a sectioned microsphere showing (b) lysozyme (CNO−), c) PLGA (C3H3O2−, C3H5O2−, C3H3O3−andC3H5O3−), and (d) an overlay showing lysozyme (red), PLGA (green) and PVA (C2H3O2− blue). Figure 9 (b-d) Reproduced with permission from Rafati A, Boussahel A, Shakesheff KM, et al. Chemical and spatial analysis of protein loaded PLGA microspheres for drug delivery applications. J Control Release. 2012;162(2):321-329. doi:10.1016/j.jconrel.2012.05.008.95 Figure 9 (a) Reprinted from International Journal of Pharmaceutics, 417/1-2, Barnes TJ, Kempson IM, Prestidge CA, Surface analysis for compositional, chemical and structural imaging in pharmaceutics with mass spectrometry: a ToF-SIMS perspective, 61-69, Copyright 2011, with permission from Elsevier.117 |
Due to its ability to provide high specific chemical information with fine depth resolution (~1 nm), several studies have used ToF-SIMS to investigate the distribution of drug and surfactant on the surface and cross-section of microspheres.25,118 Meeus et al96 have applied ToF-SIMS to analyze the drug distribution on the microsphere surface. They found that the drug content on the microsphere surface is a result of different microsphere preparation methods. The optimization of the surfactant concentration was very important in the preparation of microspheres by stabilizing the emulsion droplets to reduce the diameter of microspheres, reduce aggregation and improve encapsulation efficiency. Rafati et al95 used ToF-SIMS technology to conduct spatial imaging of the surfactant PVA and the drug adsorbed on the microsphere surface. They found that the PVA film was mostly circular or ring-shaped and dispersed on the surface of microspheres. The thickness of the PVA layer was also investigated by ToF-SIMS sputtering. It was shown that the ToF-SIMS is a powerful approach to map the distribution of the drug, PLGA and surfactant on the microsphere.
Overall, the microstructure is one of the key quality attributes of microsphere products. Its accurate and efficient characterization is essential to better understand the drug release mechanism and guide the development of microsphere products. Several characterization techniques have been used to observe the microstructure of microspheres, complemented by computational techniques to predict the release behavior of microspheres based on their microstructure. However, each technique has both advantages and limitations, and a reasonable selection of characterization means for product properties and characterization purposes is necessary. Meanwhile, in order to obtain comprehensive information about the microsphere microstructure, it is recommended that multiple characterization techniques be used in combination to accurately image and analyze the microsphere microstructure from multiple levels and perspectives.
For instance, identifying the interested region by XRM prior to high-resolution imaging using FIB-SEM saves time and labor; FIB-SEM combined with EDS analysis can provide more intuitive and richer information about the drug distribution within the microspheres; and the combined use of TOF-SIMS and CRM provides a powerful toolset for studying the spatial distribution of different components in microspheres. We also look forward to the emergence of new techniques that will allow the researchers to accomplish a faster and more comprehensive understanding of the microstructure between different formulations, different processes, and different batches of microspheres, as well as the changes in release performance that these differences bring.
Conclusion and Future Prospects
As a pivotal property of microspheres, the microstructure integrates information on many critical quality attributes, including drug loading, porosity, drug distribution, and so on. Therefore, a quantitative description based on the microstructure can provide data from multiple indicators simultaneously, simplifying the tedious and necessary characterization and providing accurate and quantifiable results.
The microstructural properties of the final microsphere product are the results of several variables from formulation composition and preparation process. The performance of the final product can be reflected by quantifying the microsphere microstructure. Inspired by the extraction and analysis of key information in the microstructure, in addition to obtaining essential attributes, such as drug loading and porosity, we also expect to disclose the drug release kinetics of microspheres.
As a sustained and controlled release preparation, the most prominent feature of microspheres is their long-term efficacy after administration, which can reduce the number of injections significantly and avoid the administration loss in patients. Therefore, the in vivo release characteristics of microspheres, both in the research and development stage and the regulatory stage on the market, are the most concerned issue. Nevertheless, the drug release environment in vivo is extremely complex, involving the multifaceted factors from the cell, tissue, organ and system level, and the routine in vitro release methods cannot replicate the real in vivo drug release trend. At this stage, the overall release characteristics cannot be accurately captured in advance.
On the one hand, the variables affecting the dissociation of drug molecules and the formation of mass transfer channels will influence the drug release kinetics, including the inherent properties (particle size, dispersion form, solubility and permeability) and uniformity of the drug, pore expansion and healing, configuration, conformation and degradation of the PLGA chains, etc. However, a thorough understanding of the phase distribution in the microspheres and the properties of PLGA is a prerequisite for designing and obtaining drug loaded microspheres with ideal release characteristics. At present, the change in residual drug content and porosity in microspheres during release can be obtained by continuous characterization of the microstructure. Therefore, a strong correlation exists between the drug release performance and the microsphere microstructure, and the quantitative description of the microstructure could provide us with the capacity to predict the drug release process.
Micro-characterization will vigorously promote the improvement of microsphere preparations, even though the current development of microspheres is still mainly relied on trial and error. Obviously, if the characterization results of the microsphere microstructure can provide enough information to indicate the drug release behavior, we can get out of the dilemma that is difficult to relate the microsphere release in vitro to the release in vivo, and directly explore the correlation between the microsphere microstructure and the drug release in vivo. With this pattern, repetitive work can be avoided, ranging from speeding up the design and selection of the formulation, minimizing the number of in vitro studies required for several months, reducing the need for animal studies for ethical reasons, effectively predicting the pharmacokinetic profiles, simplifying the procedures of quality control, etc., thus saving huge amounts of time and money in pre-formulation research and generic and innovative drug development and greatly accelerating the supervision process of microsphere products.
For marketed microspheres, many researchers have explored the drug distribution, drug dispersion forms, and pore formation in microspheres preliminarily by conventional means, including cryo-scanning electron microscopy, Raman spectroscopy, etc.52,119,120 They have used the results of microstructural changes during the release process to dissect the release mechanism, to understand drug burst release, to deepen insights into the relationship between in vitro release temperature and release rate, and so on. Garner et al characterized the microstructure of naltrexone microspheres (Vivitrol®) and the microstructure changes during release by SAVI and observed drug-containing core and cubic crystals of self-crystalized naltrexone.18 Wang et al characterized the surface drug distribution of octreotide acetate microspheres (Sandostatin LAR®) by CLSM.121 It has been recognized that microstructure is related to product performance closely. After accurately obtaining information on Q1 and Q2 and achieving the similarity of Q1 and Q2, precise microstructure analysis of microspheres with high technological barriers is the primary task in the development of generic drugs currently.
Although there are numerous variables that determine the microstructure and release process of microspheres, the complexity also provides more feasible strategies to optimize product performance and adjust drug release behavior. Meanwhile, real-time characterization of microsphere microstructures during the release process will be very interesting to further understand the details of drug release. It is also feasible to extend the microstructure results to formulation selection and monitoring based only on explicit formulation composition, processing parameters and target properties. Since some material attributes, such as the inherent solubility of the drug, the terminal group and the composition of the PLGA, cannot be acquired from the microstructure information of the final product, we need some prior knowledge to guide the research.
Moreover, some available tools for accurately characterizing the microsphere microstructure currently have a common limitation - the absence of general tendency. In the future, we hope that the characterization techniques can be executed based on a certain number of samples, which means that the microstructure is characterized in batches rather than the single or small part of the objects selected by human subjective consciousness. Quantitative or qualitative processing and analysis according to large numbers of samples will provide more robust, verifiable and universal outcomes.
We also expect that the characterization and control of the microstructure can be applied to the actual preparation and analysis of microspheres. The consistency and tiny differences of batch-to-batch products are investigated and distinguished by characterizing the microstructure to help confirm the product quality in real time. It is conceivable that predicting the product stability and carrying out quality control within the shelf life on account of microstructure results is viable to a certain extent. These valuable trials are expected to have a real positive influence on the advance of microsphere preparations.
How to characterize the microstructures of microsphere more accurately, quickly and deeply still require multipartite efforts and supports, including the inspiration and guidance of interdisciplinary knowledge. In the future, we can also deeply exploit the information that the microsphere microstructure can transmit and the application potential of the microstructure in the pharmaceutical industry based on the more creation and advance of characterization technologies.
Funding
This work was supported by the National Natural Science Foundation of China (82173788).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Andhariya JV, Burgess DJ. Recent advances in testing of microsphere drug delivery systems. Expert Opin Drug Deliv. 2016;13:593–608. doi:10.1517/17425247.2016.1134484
2. D’Souza S, Faraj JA, Giovagnoli S, DeLuca PP. In vitro-in vivo correlation from lactide-co-glycolide polymeric dosage forms. Prog Biomater. 2014;3:131–142. doi:10.1007/s40204-014-0029-4
3. Somayaji MR, Das D, Przekwas A. A new level A Type IVIVC for the rational design of clinical trials toward regulatory approval of generic polymeric long-acting injectables. Clin Pharmacokinet. 2016;55:1179–1190. doi:10.1007/s40262-016-0388-1
4. Zhong H, Chan G, Hu Y, Hu H, Ouyang D. A comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics. 2018;10:263. doi:10.3390/pharmaceutics10040263
5. O’Brien MN, Jiang W, Wang Y, Loffredo DM. Challenges and opportunities in the development of complex generic long-acting injectable drug products. J Control Release. 2021;336:144–158. doi:10.1016/j.jconrel.2021.06.017
6. Lim YW, Tan WS, Ho KL, et al. Challenges and Complications of Poly(lactic-co-glycolic acid)-based long-acting drug product development. Pharmaceutics. 2022;14:614. doi:10.3390/pharmaceutics14030614
7. Nkanga CI, Fisch A, Rad-Malekshahi M, et al. Clinically established biodegradable long acting injectables: an industry perspective. Adv Drug Deliv Rev. 2020;167:19–46. doi:10.1016/j.addr.2020.11.008
8. Clark AG, Wang R, Qin Y, et al. Assessing microstructural critical quality attributes in PLGA microspheres by FIB-SEM analytics. J Control Release. 2022;349:580–591. doi:10.1016/j.jconrel.2022.06.066
9. Xue Y, Xu L, Wang A, et al. Studying spatial drug distribution in golf ball-shaped microspheres to understand drug release. J Control Release. 2023;357:196–209. doi:10.1016/j.jconrel.2023.03.022
10. Hua Y, Su Y, Zhang H, et al. Poly(lactic-co-glycolic acid) microsphere production based on quality by design: a review. Drug Deliv. 2021;28:1342–1355. doi:10.1080/10717544.2021.1943056
11. Andhariya JV, Jog R, Shen J, et al. In vitro-in vivo correlation of parenteral PLGA microspheres: effect of variable burst release. J Control Release. 2019;314:25–37. doi:10.1016/j.jconrel.2019.10.014
12. Pu C, Wang Q, Zhang H, et al. In vitro-in vivo relationship of amorphous insoluble API (Progesterone) in PLGA microspheres. Pharm Res. 2017;34:2787–2797. doi:10.1007/s11095-017-2258-4
13. Sophocleous AM, Desai KG, Mazzara JM, et al. The nature of peptide interactions with acid end-group PLGAs and facile aqueous-based microencapsulation of therapeutic peptides. J Control Release. 2013;172:662–670. doi:10.1016/j.jconrel.2013.08.295
14. Wan B, Bao Q, Burgess D. Long-acting PLGA microspheres: advances in excipient and product analysis toward improved product understanding. Adv Drug Delivery Rev 2023;198:114857. doi:10.1016/j.addr.2023.114857
15. Muddineti OS, Omri A. Current trends in PLGA based long-acting injectable products: the industry perspective. Expert Opin Drug Deliv. 2022;19:559–576. doi:10.1080/17425247.2022.2075845
16. Cai C, Mao S, Germershaus O, et al. Influence of morphology and drug distribution on the release process of FITC-dextran-loaded microspheres prepared with different types of PLGA. J Microencapsul. 2009;26:334–345. doi:10.1080/02652040802354707
17. Mao S, Shi Y, Li L, Xu J, Schaper A, Kissel T. Effects of process and formulation parameters on characteristics and internal morphology of poly(d, l-lactide-co-glycolide) microspheres formed by the solvent evaporation method. Eur J Pharm Biopharm. 2008;68:214–223. doi:10.1016/j.ejpb.2007.06.008
18. Garner J, Skidmore S, Hadar J, et al. Scanning analysis of sequential semisolvent vapor impact to study naltrexone release from poly(lactide-co-glycolide) microparticles. Mol Pharm. 2022;19:4286–4298. doi:10.1021/acs.molpharmaceut.2c00595
19. Garner J, Skidmore S, Hadar J, et al. Surface analysis of sequential semi-solvent vapor impact (SAVI) for studying microstructural arrangements of poly(lactide-co-glycolide) microparticles. J Control Release. 2022;350:600–612. doi:10.1016/j.jconrel.2022.08.052
20. Park K, Skidmore S, Hadar J, et al. Injectable, long-acting PLGA formulations: analyzing PLGA and understanding microparticle formation. J Control Release. 2019;304:125–134. doi:10.1016/j.jconrel.2019.05.003
21. Clark AG, Wang R, Lomeo J, et al. Investigating structural attributes of drug encapsulated microspheres with quantitative X-ray imaging. J Control Release. 2023;358:626–635. doi:10.1016/j.jconrel.2023.05.019
22. Otte A, Turasan H, Park K. Implications of particle size on the respective solid-state properties of naltrexone in PLGA microparticles. Int J Pharm. 2022;626:122170. doi:10.1016/j.ijpharm.2022.122170
23. Mylonaki I, Allemann E, Delie F, Jordan O. Imaging the porous structure in the core of degrading PLGA microparticles: the effect of molecular weight. J Control Release. 2018;286:231–239. doi:10.1016/j.jconrel.2018.07.044
24. Wang J, Helder L, Shao J, Jansen JA, Yang M, Yang F. Encapsulation and release of doxycycline from electrospray-generated PLGA microspheres: effect of polymer end groups. Int J Pharm. 2019;564:1–9. doi:10.1016/j.ijpharm.2019.04.023
25. Meeus J, Lenaerts M, Scurr DJ, et al. The influence of spray-drying parameters on phase behavior, drug distribution, and in vitro release of injectable microspheres for sustained release. J Pharm Sci. 2015;104:1451–1460. doi:10.1002/jps.24361
26. Falsafi SR, Rostamabadi H, Assadpour E, Jafari SM. Morphology and microstructural analysis of bioactive-loaded micro/nanocarriers via microscopy techniques, CLSM/SEM/TEM/AFM. Adv Colloid Interface Sci. 2020;280:102166. doi:10.1016/j.cis.2020.102166
27. Wang X, Wang Y, Wei K, Zhao N, Zhang S, Chen J. Drug distribution within poly(ɛ-caprolactone) microspheres and in vitro release. J Mater Process Technol. 2009;209:348–354. doi:10.1016/j.jmatprotec.2008.02.004
28. Wan B, Andhariya JV, Bao Q, Wang Y, Zou Y, Burgess DJ. Effect of polymer source on in vitro drug release from PLGA microspheres. Int J Pharm. 2021;607:120907. doi:10.1016/j.ijpharm.2021.120907
29. Zhang S, Wu D, Zhou L. Characterization of controlled release microspheres using FIB-SEM and image-based release prediction. AAPS Pharm Sci Tech. 2020;21:194. doi:10.1208/s12249-020-01741-w
30. Yeoh T, Ma L, Badruddoza AZ, Shah J, Zhang S. Semisolid pharmaceutical product characterization using non-invasive X-ray microscopy and AI-based image analytics. AAPS J. 2022;24:46. doi:10.1208/s12248-022-00696-z
31. Koshari SHS, Shi X, Jiang L, et al. Design of PLGA-based drug delivery systems using a physically-based sustained release model. J Pharm Sci. 2022;111:345–357. doi:10.1016/j.xphs.2021.09.007
32. Ma G. Microencapsulation of protein drugs for drug delivery: strategy, preparation, and applications. J Control Release. 2014;193:324–340. doi:10.1016/j.jconrel.2014.09.003
33. Acharya G, Shin CS, Vedantham K, et al. A study of drug release from homogeneous PLGA microstructures. J Control Release. 2010;146:201–206. doi:10.1016/j.jconrel.2010.03.024
34. Kasperczyk J, Stoklosa K, Dobrzynski P, Stepien K, Kaczmarczyk B, Dzierzega-Lecznar A. Designing bioresorbable polyester matrices for controlled doxorubicin release in glioma therapy. Int J Pharm. 2009;382:124–129. doi:10.1016/j.ijpharm.2009.08.022
35. Jyothi NV, Prasanna PM, Sakarkar SN, Prabha KS, Ramaiah PS, Srawan GY. Microencapsulation techniques, factors influencing encapsulation efficiency. J Microencapsul. 2010;27:187–197. doi:10.3109/02652040903131301
36. Al-Maaieh A, Flanagan DR. Salt and cosolvent effects on ionic drug loading into microspheres using an O/W method. J Control Release. 2001;70:169–181. doi:10.1016/S0168-3659(00)00347-3
37. Xie T, Taylor LS. Dissolution performance of high drug loading celecoxib amorphous solid dispersions formulated with polymer combinations. Pharm Res. 2016;33:739–750. doi:10.1007/s11095-015-1823-y
38. Tian Y, Jones DS, Andrews GP. An investigation into the role of polymeric carriers on crystal growth within amorphous solid dispersion systems. Mol Pharm. 2015;12:1180–1192. doi:10.1021/mp500702s
39. Singh A, Van den Mooter G. Spray drying formulation of amorphous solid dispersions. Adv Drug Deliv Rev. 2016;100:27–50. doi:10.1016/j.addr.2015.12.010
40. Zhang C, Bodmeier R. A comparative study of PLGA microparticle properties loaded with micronized, nanosized or dissolved drug. Int J Pharm. 2022;628:122313. doi:10.1016/j.ijpharm.2022.122313
41. Giles MB, Hong JKY, Liu Y, et al. Efficient aqueous remote loading of peptides in poly (lactic-co-glycolic acid). Nat Commun. 2022;13:3282. doi:10.1038/s41467-022-30813-7
42. Yang F, Chen D, Guo ZF, et al. The application of novel nano-thermal and imaging techniques for monitoring drug microstructure and distribution within PLGA microspheres. Int J Pharm. 2017;522:34–49. doi:10.1016/j.ijpharm.2017.02.056
43. Martín-Sabroso C, Fraguas-Sánchez AI, Aparicio-Blanco J, Cano-Abad MF, Torres-Suárez AI. Critical attributes of formulation and of elaboration process of PLGA-protein microparticles. Int J Pharm. 2015;480:27–36. doi:10.1016/j.ijpharm.2015.01.008
44. Choi HS, Seo SA, Khang G, Rhee JM, Lee HB. Preparation and characterization of fentanyl-loaded PLGA microspheres: in vitro release profiles. Int J Pharm. 2002;234:195–203. doi:10.1016/S0378-5173(01)00968-1
45. Fredenberg S, Wahlgren M, Reslow M, Axelsson A. Pore formation and pore closure in poly(D, L-lactide-co-glycolide) films. J Control Release. 2011;150:142–149. doi:10.1016/j.jconrel.2010.11.020
46. Kang J, Schwendeman SP. Pore closing and opening in biodegradable polymers and their effect on the controlled release of proteins. Mol Pharm. 2007;4:104–118. doi:10.1021/mp060041n
47. Mazzara JM, Balagna MA, Thouless MD, Schwendeman SP. Healing kinetics of microneedle-formed pores in PLGA films. J Control Release. 2013;171:172–177. doi:10.1016/j.jconrel.2013.06.035
48. Wan F, Bohr XJ, Baldursdottir A, Maltesen GS, Bjerregaard JM. Impact of PLGA molecular behavior in the feed solution on the drug release kinetics of spray dried microparticles. Polymer. 2013;54:5920–5927. doi:10.1016/j.polymer.2013.08.044
49. Pistel KF, Kissel T. Effects of salt addition on the microencapsulation of proteins using W/O/W double emulsion technique. J Microencapsul. 2000;17:467–483. doi:10.1080/026520400405723
50. Yoo J, Won YY. Phenomenology of the initial burst release of drugs from PLGA Microparticles. ACS Biomater Sci Eng. 2020;6:6053–6062. doi:10.1021/acsbiomaterials.0c01228
51. Zhou J, Hirota K, Ackermann R, et al. Reverse Engineering the 1-Month Lupron Depot®. Aaps j. 2018;20:105. doi:10.1208/s12248-018-0253-2
52. Hua Y, Wang Z, Wang D, et al. Key Factor Study for Generic Long-Acting PLGA microspheres based on a reverse engineering of vivitrol(®). Molecules. 2021;26:1247. doi:10.3390/molecules26051247
53. Butreddy A, Gaddam RP, Kommineni N, Dudhipala N, Voshavar C. PLGA/PLA-based long-acting injectable depot microspheres in clinical use: production and characterization overview for protein/peptide delivery. Int J Mol Sci. 2021;22:8884. doi:10.3390/ijms22168884
54. Berkland C, Kipper MJ, Narasimhan B, Kim KK, Pack DW. Microsphere size, precipitation kinetics and drug distribution control drug release from biodegradable polyanhydride microspheres. J Control Release. 2004;94:129–141. doi:10.1016/j.jconrel.2003.09.011
55. Park K, Otte A, Sharifi F, et al. Formulation composition, manufacturing process, and characterization of poly(lactide-co-glycolide) microparticles. J Control Release. 2021;329:1150–1161. doi:10.1016/j.jconrel.2020.10.044
56. Quan P, Guo W, LinYang DC, Yang M, Yang M. Donepezil accelerates the release of PLGA microparticles via catalyzing the polymer degradation regardless of the end groups and molecular weights. Int J Pharm. 2023;632:122566. doi:10.1016/j.ijpharm.2022.122566
57. Chen W, Palazzo A, Hennink WE, Kok RJ. Effect of particle size on drug loading and release kinetics of gefitinib-loaded PLGA microspheres. Mol Pharm. 2017;14:459–467. doi:10.1021/acs.molpharmaceut.6b00896
58. Vay K, Frieß W, Scheler S. A detailed view of microparticle formation by in-process monitoring of the glass transition temperature. Eur J Pharm Biopharm 2012;81:399–408. doi:10.1016/j.ejpb.2012.02.019
59. Lanao RPF, Jonker AM, Wolke JGC, Jansen JA, van Hest JCM, Leeuwenburgh SCG. Physicochemical properties and applications of poly(lactic-co-glycolic acid) for use in bone regeneration. Tissue Eng Part B, Rev. 2013;19:380–390. doi:10.1089/ten.teb.2012.0443
60. Lagreca E, Onesto V, Di Natale C, La Manna S, Netti PA, Vecchione R. Recent advances in the formulation of PLGA microparticles for controlled drug delivery. Prog Biomater. 2020;9:153–174. doi:10.1007/s40204-020-00139-y
61. Choi J, Jang BN, Park BJ, Joung YK, Han DK. Effect of solvent on drug release and a spray-coated matrix of a sirolimus-eluting stent coated with poly(lactic-co-glycolic acid. Langmuir. 2014;30:10098–10106. doi:10.1021/la500452h
62. Muhaimin M, Chaerunisaa AY, Bodmeier R. Polymer type effect on PLGA-based microparticles preparation by solvent evaporation method with single emulsion system using focussed beam reflectance measurement. J Microencapsul. 2022;39:512–521. doi:10.1080/02652048.2022.2116120
63. Shen J, Choi S, Qu W, Wang Y, Burgess DJ. In vitro-in vivo correlation of parenteral risperidone polymeric microspheres. J Control Release. 2015;218:2–12. doi:10.1016/j.jconrel.2015.09.051
64. Andhariya JV, Shen J, Wang Y, Choi S, Burgess DJ. Effect of minor manufacturing changes on stability of compositionally equivalent PLGA microspheres. Int J Pharm. 2019;566:532–540. doi:10.1016/j.ijpharm.2019.06.014
65. Avachat AM, Bornare PN, Dash RR. Sustained release microspheres of ropinirole hydrochloride: effect of process parameters. Acta Pharm. 2011;61:363–376. doi:10.2478/v10007-011-0032-4
66. Le MQ, Violet F, Paniagua C, Garric X, Venier-Julienne MC. Penta-block copolymer microspheres: impact of polymer characteristics and process parameters on protein release. Int J Pharm. 2018;535:428–437. doi:10.1016/j.ijpharm.2017.11.033
67. Mao S, Xu J, Cai C, Germershaus O, Schaper A, Kissel T. Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. Int J Pharm. 2007;334:137–148. doi:10.1016/j.ijpharm.2006.10.036
68. Zhou J, Schutzman R, Shi N-Q, et al. Influence of encapsulation variables on formation of leuprolide-loaded PLGA microspheres. J Colloid Interface Sci. 2023;636:401–412. doi:10.1016/j.jcis.2022.11.122
69. Naskar S, Das SK, Sharma S, Kuotsu K. A review on designing poly (Lactic-co-glycolic Acid) nanoparticles as drug delivery systems. Pharm Nanotechnol. 2021;9:36–50. doi:10.2174/2211738508666201214103010
70. Ramazani F, Chen W, van Nostrum CF, et al. Strategies for encapsulation of small hydrophilic and amphiphilic drugs in PLGA microspheres: state-of-The-art and challenges. Int J Pharm. 2016;499:358–367. doi:10.1016/j.ijpharm.2016.01.020
71. Gaignaux A, Réeff J, Siepmann F, et al. Development and evaluation of sustained-release clonidine-loaded PLGA microparticles. Int J Pharm. 2012;437:20–28. doi:10.1016/j.ijpharm.2012.08.006
72. Ekanem EE, Nabavi SA, Vladisavljević GT, Gu S. Structured biodegradable polymeric microparticles for drug delivery produced using flow focusing glass microfluidic devices. ACS Appl Mater Interfaces. 2015;7:23132–23143. doi:10.1021/acsami.5b06943
73. Sarkar Das S, Lucas AD, Carlin AS, Zheng J, Patwardhan DV, Saylor DM. Controlled initial surge despite high drug fraction and high solubility. Pharm Dev Technol. 2017;22:35–44. doi:10.3109/10837450.2015.1135341
74. Li M, Rouaud O, Poncelet D. Microencapsulation by solvent evaporation: state of the art for process engineering approaches. Int J Pharm. 2008;363:26–39. doi:10.1016/j.ijpharm.2008.07.018
75. Wan F, Bohr A, Maltesen MJ, et al. Critical solvent properties affecting the particle formation process and characteristics of celecoxib-loaded plga microparticles via spray-drying. Pharm Res. 2013;30:1065–1076. doi:10.1007/s11095-012-0943-x
76. Frindy S, Primo A, Qaiss Ael K, et al. Insightful understanding of the role of clay topology on the stability of biomimetic hybrid chitosan-clay thin films and CO2-dried porous aerogel microspheres. Carbohydr Polym. 2016;146:353–361. doi:10.1016/j.carbpol.2016.03.077
77. Deshmukh R, Wagh P, Naik J. Solvent evaporation and spray drying technique for micro- and nanospheres/particles preparation: a review. Drying Technol 2016;34:1758–1772. doi:10.1080/07373937.2016.1232271
78. Qian L, Zhang H. Controlled freezing and freeze drying: a versatile route for porous and micro-/nano-structured materials. J Chem Technol Biotechnol. 2010;2010:1.
79. Kim TH, Park TG. Critical effect of freezing/freeze-drying on sustained release of FITC-dextran encapsulated within PLGA microspheres. Int J Pharm. 2004;271:207–214. doi:10.1016/j.ijpharm.2003.11.021
80. Ghaleh H, Abbasi F, Alizadeh M, Khoshfetrat AB. Mimicking the quasi-random assembly of protein fibers in the dermis by freeze-drying method. Mater Sci Eng C Mater Biol Appl. 2015;49:807–815. doi:10.1016/j.msec.2015.01.071
81. Wu Z, Zhao M, Zhang W, Yang Z, Xu S, Shang Q. Influence of drying processes on the structures, morphology and in vitro release profiles of risperidone-loaded PLGA microspheres. J Microencapsul. 2019;36:21–31. doi:10.1080/02652048.2019.1582723
82. Barbosa-Alfaro D, Andrés-Guerrero V, Fernandez-Bueno I, et al. Dexamethasone PLGA Microspheres for Sub-Tenon Administration: influence of Sterilization and Tolerance Studies. Pharmaceutics. 2021;13. doi:10.3390/pharmaceutics13020228
83. Selmin F, Puoci F, Parisi OI, Franzé S, Musazzi UM, Cilurzo F. Caffeic Acid-PLGA conjugate to design protein drug delivery systems stable to irradiation. J Funct Biomater. 2015;6:1–13. doi:10.3390/jfb6010001
84. Keles H, Naylor A, Clegg F, Sammon C. Investigation of factors influencing the hydrolytic degradation of single PLGA microparticles. Polym Degrad Stab. 2015;119:228–241. doi:10.1016/j.polymdegradstab.2015.04.025
85. Zhang S, Nagapudi K, Shen M, et al. Release mechanisms and practical percolation threshold for long-acting biodegradable implants: an image to simulation study. J Pharm Sci. 2022;111:1896–1910. doi:10.1016/j.xphs.2021.12.009
86. Liu Z, Li L, Zhang S, et al. Correlative image-based release prediction and 3D microstructure characterization for a long acting parenteral Implant. Pharm Res. 2021;38:1915–1929. doi:10.1007/s11095-021-03145-2
87. Serri C, Frigione M, Ruponen M, et al. Electron dispersive X-ray spectroscopy and degradation properties of hyaluronic acid decorated microparticles. Colloids Surf B Biointerfaces. 2019;181:896–901. doi:10.1016/j.colsurfb.2019.06.044
88. Wu L, Wang M, Singh V, et al. Three dimensional distribution of surfactant in microspheres revealed by synchrotron radiation X-ray microcomputed tomography. Asian J Pharm Sci. 2017;12:326–334. doi:10.1016/j.ajps.2017.02.001
89. Guo Z, Yin X, Liu C, et al. Microstructural investigation using synchrotron radiation X-ray microtomography reveals taste-masking mechanism of Acetaminophen microspheres. Int J Pharm. 2016;499:47–57. doi:10.1016/j.ijpharm.2015.12.045
90. Pivette P, Faivre V, Mancini L, et al. Controlled release of a highly hydrophilic API from lipid microspheres obtained by prilling: analysis of drug and water diffusion processes with X-ray-based methods. J Control Release. 2012;158:393–402. doi:10.1016/j.jconrel.2011.11.027
91. Zhu C, Peng T, Huang D, et al. Formation mechanism vitro and in vivo evaluation of dimpled exenatide loaded PLGA microparticles prepared by ultra-fine particle processing system, AAPS pharm. Sci Tech. 2019;20:64.
92. Pygall SR, Whetstone J, Timmins P, Melia CD. Pharmaceutical applications of confocal laser scanning microscopy: the physical characterisation of pharmaceutical systems. Adv Drug Deliv Rev. 2007;59:1434–1452. doi:10.1016/j.addr.2007.06.018
93. Butler HJ, Ashton L, Bird B, et al. Using Raman spectroscopy to characterize biological materials. Nat Protoc. 2016;11:664–687.
94. Moffat JG, Qi S, Craig DQ. Spatial characterization of hot melt extruded dispersion systems using thermal atomic force microscopy methods: the effects of processing parameters on phase separation. Pharm Res. 2014;31:1744–1752. doi:10.1007/s11095-013-1279-x
95. Rafati A, Boussahel A, Shakesheff KM, et al. Chemical and spatial analysis of protein loaded PLGA microspheres for drug delivery applications. J Control Release. 2012;162(2):321–329. doi:10.1016/j.jconrel.2012.05.008
96. Meeus J, Scurr DJ, Appeltans B, et al. Influence of formulation composition and process on the characteristics and in vitro release from PLGA-based sustained release injectables. Eur J Pharm Biopharm. 2015;90:22–29. doi:10.1016/j.ejpb.2014.11.009
97. Henry MD, Shearn MJ, Chhim B, Scherer A. Ga(+) beam lithography for nanoscale silicon reactive ion etching. Nanotechnology. 2010;21:245303. doi:10.1088/0957-4484/21/24/245303
98. Zhang Z, Ekanem EE, Nakajima M, Bolognesi G, Vladisavljević GT. Monodispersed sirolimus-loaded PLGA microspheres with a controlled degree of drug-polymer phase separation for drug-coated implantable medical devices and subcutaneous injection. ACS Appl Bio Mater. 2022;5:3766–3777. doi:10.1021/acsabm.2c00319
99. Fitzgerald R, Keil K, Heinrich KF. Solid-state energy-dispersion spectrometer for electron-microprobe x-ray analysis. Science. 1968;159:528–530. doi:10.1126/science.159.3814.528
100. Nagapudi K, Zhu A, Chang DP, et al. Microstructure, quality, and release performance characterization of long-acting polymer implant formulations with X-Ray microscopy and quantitative AI analytics. J Pharm Sci. 2021;110:3418–3430. doi:10.1016/j.xphs.2021.05.016
101. Yang S, Yin X, Wang C, et al. Release behaviour of single pellets and internal fine 3D structural features co-define the in vitro drug release profile. AAPS J. 2014;16:860–871. doi:10.1208/s12248-014-9611-x
102. Qin W, He Y, Guo Z, et al. Optimization of taste-masking on ibuprofen microspheres with selected structure features. Asian J Pharm Sci. 2019;14:174–182. doi:10.1016/j.ajps.2018.05.003
103. Yin X, Li H, Guo Z, et al. Quantification of swelling and erosion in the controlled release of a poorly water-soluble drug using synchrotron X-ray computed microtomography. AAPS J. 2013;15:1025–1034. doi:10.1208/s12248-013-9498-y
104. Tang FC, Wu ZB, Yang C, et al. Synchrotron X-Ray tomography for rechargeable battery research: fundamentals, setups and applications. Small Methods. 2021;5:1.
105. Vijayakumar J, Goudarzi NM, Eeckhaut G, Schrijnemakers K, Cnudde V, Boone MN. Characterization of Pharmaceutical Tablets by X-ray Tomography. Pharmaceuticals. 2023;16:1.
106. Wang M, Lu X, Yin X, et al. Synchrotron radiation-based Fourier-transform infrared spectromicroscopy for characterization of the protein/peptide distribution in single microspheres. Acta Pharm Sin B. 2015;5:270–276. doi:10.1016/j.apsb.2015.03.008
107. Zhai P, Chen XB, Schreyer DJ. PLGA/alginate composite microspheres for hydrophilic protein delivery. Mater Sci Eng C Mater Biol Appl. 2015;56:251–259. doi:10.1016/j.msec.2015.06.015
108. Sun F, Yu C, Liu X, et al. Butyl stearate prolongs the drug release period of isoperidone‑loaded poly (lactic‑co‑glycolic acid) microspheres: in vitro and in vivo investigation. Mol Med Rep. 2019;19:1595–1602. doi:10.3892/mmr.2018.9797
109. Di J, Wang J, Wang S, et al. Self-boosting vaccination based on pulsatile antigen release from core-shell microparticles. Small. 2023;19:e2207892. doi:10.1002/smll.202207892
110. Shi C, Feng S, Liu P, Liu X, Feng X, Fu D. A novel study on the mechanisms of drug release in PLGA-mPEG microspheres with fluorescent drug. J Biomater Sci Polym Ed. 2016;27:854–864. doi:10.1080/09205063.2016.1166727
111. Hirota K, Doty AC, Ackermann R, et al. Characterizing release mechanisms of leuprolide acetate-loaded PLGA microspheres for IVIVC development I: in vitro evaluation. J Control Release. 2016;244:302–313. doi:10.1016/j.jconrel.2016.08.023
112. Bergholt MS, Serio A, Albro MB. Raman Spectroscopy: guiding Light for the Extracellular Matrix. Front Bioeng Biotechnol. 2019;7:303. doi:10.3389/fbioe.2019.00303
113. Kroneková Z, Pelach M, Mazancová P, et al. Structural changes in alginate-based microspheres exposed to in vivo environment as revealed by confocal Raman microscopy. Sci Rep. 2018;8:1637. doi:10.1038/s41598-018-20022-y
114. Goh CF, Lane ME. Advanced structural characterisation of pharmaceuticals using nano-thermal analysis (nano-TA. Adv Drug Deliv Rev. 2022;180:114077. doi:10.1016/j.addr.2021.114077
115. Dai X, Moffat JG, Wood J, Reading M. Thermal scanning probe microscopy in the development of pharmaceuticals. Adv Drug Deliv Rev. 2012;64:449–460. doi:10.1016/j.addr.2011.07.008
116. Chan CM, Weng LT. Surface characterization of polymer blends by XPS and ToF-SIMS. Materials (Basel). 2016;9. doi:10.3390/ma9080655
117. Barnes TJ, Kempson IM, Prestidge CA. Surface analysis for compositional, chemical and structural imaging in pharmaceutics with mass spectrometry: a ToF-SIMS perspective. Int J Pharm. 2011;417:61–69. doi:10.1016/j.ijpharm.2011.01.043
118. Meeus J, Scurr DJ, Amssoms K, Davies MC, Roberts CJ, Van den Mooter G. Surface characteristics of spray-dried microspheres consisting of PLGA and PVP: relating the influence of heat and humidity to the thermal characteristics of these polymers. Mol Pharm. 2013;10:3213–3224. doi:10.1021/mp400263d
119. Schutzman R, Shi NQ, Olsen KF, et al. Mechanistic evaluation of the initial burst release of leuprolide from spray-dried PLGA microspheres. J Control Release. 2023;361:297–313.
120. Tipnis NP, Shen J, Jackson D, Leblanc D, Burgess DJ. Flow-through cell-based in vitro release method for triamcinolone acetonide poly (lactic-co-glycolic) acid microspheres. Int J Pharm. 2020;579:119130.
121. Wang T, Xue P, Wang A, et al. Pore change during degradation of octreotide acetate-loaded PLGA microspheres: the effect of polymer blends. Eur J Pharm Sci. 2019;138:104990.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
