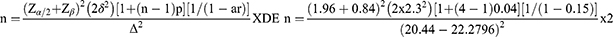Back to Journals » Patient Preference and Adherence » Volume 17
MICROS: Asthma Control App for School Adolescents in a Low Resource Setting - A Cluster Randomized Controlled Trial Protocol
Authors Katumba JD , Kirenga B, Muwagga Mugagga A, Kalyango JN, Nantanda R, Karamagi C
Received 30 September 2023
Accepted for publication 14 November 2023
Published 30 November 2023 Volume 2023:17 Pages 3125—3133
DOI https://doi.org/10.2147/PPA.S438549
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
James Davis Katumba,1 Bruce Kirenga,2 Anthony Muwagga Mugagga,3 Joan N Kalyango,1 Rebecca Nantanda,2 Charles Karamagi1
1Clinical Epidemiology Unit, College of Health Sciences, Makerere University, Kampala, Uganda; 2Lung Institute, College of Health Sciences, Makerere University, Kampala, Uganda; 3College of Education and External Studies, Makerere University, Kampala, Uganda
Correspondence: James Davis Katumba, Clinical Epidemiology Unit, College of Health Sciences, Makerere University, P O Box 7072, Kampala, Uganda, Tel +256 726119537, Email [email protected]; [email protected]
Introduction: Poor asthma control in adolescents is partly attributed to inadequate asthma education for self-management. This study is set to determine the effectiveness of the “KmAsthma” self-management app in improving the control of asthma among adolescents in a low-resource setting.
Methods: The two-arm 6-month cluster randomized controlled trial, will aim at enrolling 120 day scholars aged 12-19 years in secondary schools with a clinician’s diagnosis and self-reported uncontrolled asthma in Kampala City Uganda. The primary endpoint of asthma control will be measured as a change in mean Asthma Control Test (ACT) scores. Asthma quality of life, adherence to medications, and self-efficacy will also be assessed. The iMprovIng the ContROl of aSthma (MICROS) study will employ the “KmAsthma” app for self-management education. The intervention group will receive the app on their smartphones and training on its eight sections: the profile, asthma history, goals, inspirations, reminders, connect, information about asthma, and emergency support. Participants will navigate these sections to set asthma control goals, schedule medication reminders, log daily symptoms, and receive guidance for attacks. All participants will be encouraged to seek routine care. A study nurse will follow up with each participant via the phone six weeks post-intervention. The MICROS study was approved by the Makerere University School of Medicine Research and Ethics Committee and the Uganda National Council of Science and Technology. This protocol is registered on Clinicaltrials.gov (NCT05850806).
Conclusion: The MICROS study will provide comprehensive insights into how effective a mHealth intervention can be an aid for adolescents in a low-resource setting in managing their asthma. The findings of this study will contribute to filling the gap leading to unsatisfactory asthma control in adolescents.
Keywords: asthma control, uncontrolled asthma, smartphone app, adolescents, school, low-resource setting, adherence, self-efficacy
Introduction
Asthma remains a global health challenge, impacting an estimated 339 million individuals of all ages.1,2 In Africa, approximately 119.3 million people grapple with asthma.3 In Uganda, asthma prevalence varies from 6.8% to 20.43% with higher rates in urban areas (12.99%) compared to rural regions (8.86%).4 Despite advances in asthma care in countries like Finland and Brazil,5–7 adolescents in low-resource settings face distinct obstacles to controlling the condition. The burden of asthma among school-going adolescents in these settings is significant, with consequences extending beyond physical symptoms.1 Effective asthma management is possible,8 yet a substantial proportion (45–46%) of school children with asthma experience uncontrolled or partially controlled asthma.9,10
In low-resource settings, adolescents with asthma often encounter a multifaceted challenge.11 Limited access to healthcare services, medication, and asthma education, compounds the difficulties of managing this chronic condition. Consequently, adolescents with uncontrolled asthma experience frequent exacerbations12 leading to missed school days, compromised academic performance, and in some cases death.13,14
The intersection of adolescence, a phase marked by increased independence, and asthma management can be particularly challenging as adolescents may struggle with medication adherence and self-management.15–17
In recent years, mobile health (mHealth) interventions have emerged as promising tools to address healthcare disparities and improve disease management, even in resource–constrained environments.18 The ubiquity of smartphones and the ease of access they offer to health-related information and tools makes them an attractive avenue for supporting adolescents in managing their asthma.19 Health interventions delivered on smartphones can facilitate remote access to health education, and patient monitoring and positively motivate and influence attitudes towards change.20,21
This protocol outlines a cluster randomized controlled trial (RCT) that seeks to evaluate the effectiveness of the KmAsthma self-management app in improving the control of asthma among day scholars aged 12-19 years in secondary schools in Kampala City Uganda. A review22 found the KmAsthma app to have a high-quality rating with the potential to facilitate asthma self-management.23 We hypothesize that the use of this app will empower adolescents to take a more active role in their asthma management, ultimately leading to improved asthma control, enhanced medication adherence, self-efficacy, and a better quality of life.
The findings of this study are anticipated to bridge the gap in asthma care for adolescents in low-resource settings by leveraging the potential of mobile technology. The findings will not only contribute to the growing body of evidence on mHealth interventions for chronic diseases but also hold the potential to revolutionize asthma care for a vulnerable and underserved population. By addressing the unique challenges faced by adolescents with asthma in resource-constrained environments, we aspire to pave the way for a more equitable and effective approach to managing this chronic condition, ultimately enhancing the well-being and academic prospects of these adolescents.
Methods and Design
Study Design and Setting
The MICROS study will utilize a two-arm cluster-randomized, single-blinded, pragmatic parallel trial design to investigate how the KmAsthma smartphone app can enhance asthma control among day scholars in secondary schools in a resource-limited setting. Participants will be encouraged to continue attending their regular hospital appointments and follow prescribed medication guidance from their healthcare team. The intervention arm will download and receive training on the use of the app while the control arm will have no intervention. The primary endpoint is to enhance asthma control measured through the Asthma Control Test (ACT) scores24 during a 6-month self-management intervention. The study will select secondary schools in Kampala City and cluster randomization will be performed after collecting baseline data. The allocation ratio is 1:1, and 30 schools will participate. This study is designed as a single-blinded trial since schools and teachers cannot be blinded. Data collectors will not know group allocations and the statistician performing data analysis will use treatment numerical codes without knowing the groups. Assessments will occur at baseline, 3 months, and 6 months using the ACT. Ethical approval and permissions were obtained from Makerere University College of Health Sciences School of Medicine Research and Ethics Committee (SOMREC) under reference number Mak-SOMREC-2021-67, as well as by the Uganda National Council of Science and Technology under reference number HS2791ES. The study protocol is registered on ClinicalTrials.gov under reference number NCT05850806. Informed consent will be obtained from participants and their guardians, and the study will follow CONSORT25 and SPIRIT guidelines.26,27 All the study experiments will be performed in accordance with relevant guidelines and regulations (such as the Declaration of Helsinki).28,29 Figure 1 shows the study flow plan.
 |
Figure 1 Study flow plan of the cluster randomized controlled trial on the effectiveness of the self-management app. |
Study Participants
The selection criteria for secondary schools in Kampala City for the randomized controlled trial include being listed in the Ministry of Education and Sports Placement guide,30 enrolling students aged 12 and above, not participating in other asthma trials, and having a history of presenting candidates for at least five years between 2011–2016.28 A total of 30 schools will be sampled using cluster sampling, with participants drawn from various types of secondary schools in the city. Eligible participants will include day secondary school adolescents aged 12–19 years with a clinician’s diagnosis and self-reported uncontrolled asthma (ACT score < 19),24 owning and regularly using a smartphone, and providing assent and informed consent. Participants in another asthma trial and those with a severe illness preventing smartphone use will be excluded.
Recruitment, Screening, and Consent
Recruitment will occur in Uganda’s first term to account for school transfers. With permission from the Directorate of Education and Training of Kampala City, the research team will seek school approval and collaboration with the school nurse or health teacher. The study’s purpose will be explained to all students during a general assembly. Eligible students will complete a case detection survey using the adapted ISAAC questionnaire31 to identify asthma symptoms and their parents will receive letters offering the option to opt-out through passive consent. Students will also complete a survey about their asthma history, and those scoring 19 or lower on the Asthma Control Test24 will qualify for enrollment. Eligible students will be enrolled and randomized in the first three months of Uganda’s secondary school calendar.
Trial Size Estimates
The study posits that among day scholar secondary school adolescents aged 12–19 years with uncontrolled asthma in Kampala City, Uganda, utilizing the KmAsthma self-management app for six months on their mobile phones, compared to having no mHealth app, will lead to a 9% increase in individual total Asthma Control Test scores from baseline.
To calculate the sample size, we employ a formula comparing means32 and refer to a similar study33 involving early adolescents (ages 12–15) with an Asthma Control Test score of 20.44 (±2.30). Considering the 9% increase in the ACT score, the sample size is determined using the formula:
With a two-sided significance level (α) of 0.05, (Zα/2=1.96), 80% power (Zβ=0.84), a treatment effect (Δ) of 9% increase in the ACT score, a variance in the control group δ2 of 5.29, an estimated intra-cluster correlation (p) of 0.04 (due to differences in socio-economic backgrounds), a cluster size (n) of 4 students with uncontrolled asthma per school, an assumed 15% attrition rate (ar) and loss to follow-up, and a design effect (DE) of 2, the required sample size is 120 students (60 per arm). To distribute the sample proportionally, publicly available data,34,35 is used. However, since a cluster size of four is needed, fifteen schools will be selected per arm.
Randomization, Concealment, and Blinding
In this study, cluster randomization will be employed at the school level to prevent treatment “contamination” between intervention and control participants.36 While schools are randomized as clusters to minimize contamination, the primary unit of inference will remain the individual, enabling the assessment of differences between comparable individuals in the intervention and control groups. Secondary schools in Kampala City, representing diverse socio-economic backgrounds, will be categorized into five types based on publicly available data.34 Schools will then be randomly allocated to either the intervention or control arm in a 1:1 ratio, using a computer-generated random sequence with a predetermined seed value of 400, considering 30 schools (clusters) with block sizes of even number 6. An independent statistician from Makerere University will ensure that the allocation remains concealed from the study team in sequentially numbered, opaque, sealed envelopes. Researchers delivering the intervention, school staff, parents, and students will not be completely masked to group allocation. The study will be conducted as a single-blinded trial, with data collectors unaware of group assignments. However, full blinding may not be guaranteed if disclosed by school staff or students. The statistician analyzing the data will remain blinded to the study group by using treatment numerical codes exclusively.
The KmAsthma Self-Management Smartphone App Intervention
The KmAsthma self-management smartphone app, previously described in detail,37 offers asthma tracking, action plans, education, and goal setting. It covers various aspects of asthma management and is available in English, the official language in Ugandan schools. Participants in the intervention group will receive a download link for the KmAsthma app on their Android or iPhone and undergo training. The training will encompass all app sections, including profile, asthma history, goal setting, reminders, external resources, and emergency support. Participants will be encouraged to set asthma control goals, log daily symptoms, and contact healthcare providers if necessary. A follow-up call will be conducted six weeks post-intervention by a study team nurse interventionist.
Control Arm
Participants in the control group will not receive the KmAsthma self-management smartphone app during the intervention period. They will only take part in baseline, end-line, and follow-up assessments. All participants will be encouraged to receive usual asthma care from their healthcare provider(s). However, at the study’s conclusion,38 all participants, including those in the control group, will be provided with links to access the KmAsthma app, which is freely available and easy to download.
Measures
Participant Characteristics
A researcher-designed questionnaire will be used to collect data on the participant characteristics (age, sex, region of origin, use of controller medicines before study, Basal Metabolic Index, and use of asthma-related mobile apps on phone before the study). Data on asthma history will also be collected, summarizing the age at asthma diagnosis, the frequency of asthma attacks in the previous year, the use of steroid prescriptions in the past year, and any prior occurrences of asthma-related emergency department visits, hospitalizations, or intensive care admissions.
Primary Outcome measures
The study will assess outcomes at baseline, 3 months, and 6 months based on patient-centered priorities. The primary outcome is the mean asthma control score, measured using the validated five-item Asthma Control Test (ACT).24 The ACT comprises five questions, each rated on a scale from 1 to 5, with a total score range of 5 to 25. An ACT score of 19 or higher indicates well-controlled asthma.
Secondary Outcome Measures
Adherence
Medication adherence will be gauged using the 10-item Medication Adherence Report Scale for Asthma (MARS).39 MARS employs a scoring system ranging from 0 to 5, where higher mean scores (4.5 or above) signify strong adherence.
Asthma Quality of Life
The quality of life assessment will utilize the validated 32-item Asthma Quality of Life Questionnaire with Standardized Activities (AQLQ-S).40 AQLQ-S employs a scale from 1 (lowest) to 7 (highest), indicating improved quality of life with higher scores.
Self-Efficacy
Self-efficacy for asthma management will be assessed through the validated 27-item Adolescent Asthma Self-Efficacy Questionnaire (AASEQ).41 AASEQ scores range from 0 to 100, with the total mean score (0–100), reflecting higher self-efficacy levels. Subscale scores will be computed by summing and dividing by the respective subscale item count.
Data Collection and Management
Data collection will be done using self-administered paper-based questionnaires or via telephone in both arms at baseline (after recruitment), at 3 and 6 months. Completed questionnaires will be checked for completeness, coded, and entered using Kobotoolbox, and a copy frozen for future reference while the other copy will be imported into STATA version 14 for analysis.
Loss to Follow-Up
In case any trial participant becomes unreachable during the study, attempts to reestablish contact will be made through the school nurse, the teacher overseeing students’ health, and the principal investigator. If these efforts prove unsuccessful, the research team will document and note the reasons for the loss to follow-up. This data will then be summarized and included in the study’s report, alongside the count of participants who could not be reached for follow-up.
Data and Safety Monitoring
An independent Data and Safety Monitoring Board (DSMB) with a detailed charter will oversee participant safety, data quality, and trial continuation. All adverse events will be documented and assessed for seriousness and intervention-relatedness. Serious, unexpected events will be reported to SOMREC within five working days. Any protocol modifications will be reported promptly to investigators, SOMREC, participants, trial registries, and regulators within five working days.
Statistical Analysis
Data distribution and missing data proportions will be assessed using descriptive statistics. Multiple imputations will address missing data.42 Baseline characteristics will be summarized by the study arm. Continuous variables will be presented as means (±Standard Deviations) or medians (interquartile range), while categorical variables will be reported as frequencies and percentages. To detect imbalances of clinical importance between the study arms, standard methods for comparisons of proportions (chi-square tests) and means (paired t-tests) will be used. The primary analysis will assess how ACT scores change over 6 months using repeated measures ANCOVA, accounting for baseline scores and study groups. Proportions of participants with >9% ACT score improvement and those with uncontrolled asthma will be reported. The intention-to-treat principle will guide the analysis. The significance level will be α=0.05. For secondary outcomes, adherence (MARS), quality of life (AQLQ (S)), and self-efficacy (AASEQ), scores will be analyzed similarly, with secondary analyses assessing improvement in proportions. Odds ratios and 95% confidence intervals will be presented. In case interim analyses are requested by the DSMB, O’Brien-Fleming alpha-spending functions will be used.43 Decisions regarding study termination or adaptation will be guided by the predefined criteria and conducted confidentially by an independent statistician. A CONSORT flow diagram44 will illustrate eligibility assessment, dropout rates, randomization, withdrawals, and loss-to-follow-up. These comprehensive analyses will provide insights into asthma control, adherence, quality of life, and self-efficacy outcomes on the use of the KmAsthma app among study participants.
The randomized controlled trial will follow the schedule indicated in Table 1.
 |
Table 1 Schedule of Procedures During the Study Period of the Randomized Controlled Trial |
Quality Control
To enhance data accuracy, research assistants will receive training, participants will be briefed on the study’s importance and accuracy, and user-friendly validated tools will be used, checked daily by the principal investigator. Data backups will be stored in multiple locations to prevent loss. Tools not validated in Uganda will undergo validity and reliability assessments through statistical analyses such as the STATA validscale command.
Dissemination of Results
The study’s findings will be disseminated through various channels, including submitting the report to Makerere University School of Graduate Studies and sharing results at local, national, and international conferences. Additionally, the research will be communicated to the Ugandan Ministry of Education and Sports and the Ministry of Health. The results will also be published in both local and international peer-reviewed journals with broad and specialized readership.
Study Limitations
The study’s findings will be specific to adolescents with uncontrolled asthma in the urban area of Kampala City, Uganda, and may not apply to the entire country. Data collected through questionnaires may be affected by recall bias, especially for past events and medication usage. Students without smartphone access will be excluded from the trial. Single-blinding and a universally accessible mobile app may lead to potential contamination or confounding as teachers, students, and data collectors interact and become aware of the study’s details. Another limitation of this study is the absence of spirometry for assessing asthma control, stemming from limited access to equipment and trained personnel, especially in resource-constrained regions like Uganda. The complexity of spirometry interpretation, requiring specialized knowledge, hinders its widespread use for asthma control assessment, particularly in pediatric patients.45,46 Additionally, concerns about Covid-19 safety in the school community further impeded spirometry use.
Data Sharing Statement
Statistical codes and the dataset will be available from the corresponding author on request.
Ethics Approval and Consent to Participate
This study obtained ethical approval from Makerere University (Mak-SOMREC-2021-67) and the Uganda National Council for Science and Technology (HS2791ES). Administrative clearance was also secured from Kampala City (DPHE/KCCA/1301), and additional permissions will be sought from participating schools. The study protocol is registered on ClinicalTrials.gov and will be fully disclosed in a published report. Participants 18 and older will provide written consent, while children aged 12-17 will require written assent and parental/guardian consent. Children newly diagnosed with asthma or with uncontrolled asthma will receive counseling from the study clinician and referrals to Mulago National Referral Hospital Chest Clinic for further management. Research records will be stored securely, with data coded for confidentiality.
Acknowledgments
The authors extend their sincere thanks to the developers of the app at the University of Sydney, the Woolcock Institute of Medical Research and The University of Melbourne; Florence Nanteza and Gerald Jjuuko at Makerere University for the thorough review of the methods of the randomized controlled trial.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. GAN. The Global Asthma Report 2018; 2018. Available from: www.globalasthmareport.org.
2. Teresa T, Sanja S, Ginette M, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12(1):204. doi:10.1186/1471-2458-12-204
3. Adeloye D, Chan KY, Rudan I, Campbell H. An estimate of asthma prevalence in Africa: a systematic analysis. Croat Med J. 2013;54(6):519–531. doi:10.3325/cmj.2013.54.519
4. Kirenga BJ, de Jong C, Katagira W, et al. Prevalence and factors associated with asthma among adolescents and adults in Uganda: a general population based survey. BMC Public Health. 2019;19(1):227. doi:10.1186/s12889-019-6562-2
5. Haahtela T, Tuomisto LE, Pietinalho A, et al. A 10 year asthma programme in Finland: major change for the better. Thorax. 2006;61(8):663–670. doi:10.1136/thx.2005.055699
6. Kupczyk M, Haahtela T, Cruz A, Kuna P. Reduction of asthma burden is possible through National Asthma Plans. Allergy. 2010;65(4):415–419. doi:10.1111/j.1398-9995.2009.02265.x
7. van Asperen P. Deaths from childhood asthma, 2004–2013: what lessons can we learn? Med J Aust. 2015;202(3):125–126. doi:10.5694/mja14.01645
8. de Benedictis D, Bush A. Asthma in adolescence: is there any news? Pediatr Pulmonol. 2017;52(1):129–138. doi:10.1002/ppul.23498
9. Mpairwe H, Tumwesige P, Namutebi M, et al. Asthma control and management among schoolchildren in urban Uganda: results from a cross-sectional study. Wellcome Open Res. 2019;4(168):168. doi:10.12688/wellcomeopenres.15460.1
10. Mosler G, Oyenuga V, Addo-Yobo E, et al. Achieving Control of Asthma in Children in Africa (ACACIA): protocol of an observational study of children’s lung health in six sub-Saharan African countries. BMJ open. 2020;10(3):e035885. doi:10.1136/bmjopen-2019-035885
11. Newacheck PW, Prevalence HN. impact, and trends in childhood disability due to asthma. Arch Pediatr Adolesc Med. 2000;154(3):287–293. doi:10.1001/archpedi.154.3.287
12. Bousquet J, Mantzouranis E, Cruz AA, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126(5):926–938. doi:10.1016/j.jaci.2010.07.019
13. Basheti IA, Reddel HK, Armour CL, Bosnic-Anticevich SZ. Improved asthma outcomes with a simple inhaler technique intervention by community pharmacists. J Allergy Clin Immunol. 2007;119(6):1537. doi:10.1016/j.jaci.2007.02.037
14. Bitimwine H. Prevalence of Asthma and Characteristics of Primary School Children with Asthma in Kampala District. Makerere University; 2011.
15. Fiese BH, Everhart RS. Medical adherence and childhood chronic illness: family daily management skills and emotional climate as emerging contributors. Curr Opin Pediatr. 2006;18(5):551–557. doi:10.1097/01.mop.0000245357.68207.9b
16. Gillaspy SR, Hoff AL, Mullins LL, Van Pelt JC, Chaney JM. Psychological distress in high-risk youth with asthma. J Pediatr Psychol. 2002;27(4):363–371. doi:10.1093/jpepsy/27.4.363
17. Surís J-C, Parera N, Puig C. Chronic illness and emotional distress in adolescence. J Adolescent Health. 1996;19(2):153–156. doi:10.1016/1054-139X(95)00231-G
18. Perry TT, Marshall A, Berlinski A, et al. Smartphone-based vs paper-based asthma action plans for adolescents. Ann Allergy Asthma Immunol. 2017;118(3):298–303. doi:10.1016/j.anai.2016.11.028
19. WHO. mHealth: New Horizons for Health Through Mobile Technologies. mHealth: New Horizons for Health Through Mobile Technologies; 2011.
20. Jácome C, Guedes R, Almeida R. mINSPIRERS—Feasibility of a mobile application to measure and improve adherence to inhaled controller medications among adolescents and adults with persistent asthma. Rev Port Imunoalergol. 2018;26:47–61.
21. McLean S, Chandler D, Nurmatov U, et al. Telehealthcare for asthma: a Cochrane review. Cmaj. 2011;183(11):E733–E742. doi:10.1503/cmaj.101146
22. Ramsey RR, Caromody JK, Voorhees SE, et al. A systematic evaluation of asthma management apps examining behavior change techniques. J Allergy Clin Immunol Pract. 2019;7(8):2583–2591. doi:10.1016/j.jaip.2019.03.041
23. Dennison L, Morrison L, Conway G, Yardley L. Opportunities and challenges for smartphone applications in supporting health behavior change: qualitative study. J Med Internet Res. 2013;15(4):e86. doi:10.2196/jmir.2583
24. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi:10.1016/j.jaci.2003.09.008
25. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732. doi:10.7326/0003-4819-152-11-201006010-00232
26. Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207. doi:10.7326/0003-4819-158-3-201302050-00583
27. Moher D, Chan AW. SPIRIT (standard protocol items: recommendations for interventional trials). Guidelines Rep Health Res. 2014;56–67.
28. Zion D, Gillam L, Loff B. The Declaration of Helsinki, CIOMS and the ethics of research on vulnerable populations. Nat Med. 2000;6(6):615–617. doi:10.1038/76174
29. WMA. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81(3):14–18.
30. MoES. Placement Information Guide for Primary Seven Leavers Department of Guidance & Counselling; 2017.
31. Asher M, Keil U, Anderson H, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur respir j. 1995;8(3):483–491. doi:10.1183/09031936.95.08030483
32. Röhrig B, du Prel J-B, Wachtlin D, Kwiecien R, Blettner M. Sample size calculation in clinical trials: part 13 of a series on evaluation of scientific publications. Deutsches Ärzteblatt Int. 2010;107:31–32.
33. Fedele DA, McConville A, Thomas JG, et al. Applying Interactive Mobile health to Asthma Care in Teens (AIM2ACT): development and design of a randomized controlled trial. Contemp Clin Trials. 2018;64:230–237. doi:10.1016/j.cct.2017.09.007
34. Namubiru L, Ssenabulya B. Data from: UNEB Results by school. Kampala. 2017;2017:56.
35. MoES. Placement information guide for primary seven leavers. Dep Guidance Counselling. 2017.
36. Donner A, Klar N. Design and analysis of cluster randomization trials in health research. Int J Med. 2000.
37. Davis S, Peters D, Calvo R, Sawyer S, Foster J, Smith L. “Kiss myAsthma”: using a participatory design approach to develop a self-management app with young people with asthma. J Asthma. 2018;55(9):1018–1027. doi:10.1080/02770903.2017.1388391
38. Singh H, Rao SV, Kakkar AK, Singh J, Manohar HD. Posttrial access to medical interventions: intricacies, challenges, and solutions. Int J Appl Basic Med Res. 2019;9(1):3. doi:10.4103/ijabmr.IJABMR_218_18
39. Cohen JL, Mann DM, Wisnivesky JP, et al. Assessing the validity of self-reported medication adherence among inner-city asthmatic adults: the Medication Adherence Report Scale for Asthma. Ann Allergy Asthma Immunol. 2009;103(4):325–331. doi:10.1016/S1081-1206(10)60532-7
40. Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest. 1999;115(5):1265–1270. doi:10.1378/chest.115.5.1265
41. Holley S, Knibb R, Latter S, et al. Development and validation of the adolescent asthma self-efficacy questionnaire (AASEQ). Eur respir j. 2019;54(1):1801375. doi:10.1183/13993003.01375-2018
42. Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338.
43. Chakraborty A. Interim analysis in clinical Trials-A simulation based approach. Int J Pharm Eng. 2017;5(4):741–758.
44. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11(1):1–8. doi:10.1186/1745-6215-11-32
45. Banasiak NC. Spirometry in primary care for children with asthma. Pediatr Nurs. 2014;40(4):195–199.
46. Dombkowski KJ, Hassan F, Wasilevich EA, Clark SJ. Spirometry use among pediatric primary care physicians. Pediatrics. 2010;126(4):682–687. doi:10.1542/peds.2010-0362
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.