Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
MicroRNA-579-3p Exerts Neuroprotective Effects Against Ischemic Stroke via Anti-Inflammation and Anti-Apoptosis
Authors Jia J, Cui Y, Tan Z, Ma W, Jiang Y
Received 1 December 2019
Accepted for publication 30 March 2020
Published 12 May 2020 Volume 2020:16 Pages 1229—1238
DOI https://doi.org/10.2147/NDT.S240698
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
This paper has been retracted
Jiaoying Jia, Yan Cui, Zhigang Tan, Wenjia Ma, Yugang Jiang
Department of Neurosurgery, The Second Xiangya Hospital of Central South University, Changsha City, Hunan Province 410011, People’s Republic of China
Correspondence: Yugang Jiang
Department of Neurosurgery, The Second Xiangya Hospital of Central South University, Changsha City, Hunan Province 410011, People’s Republic of China
Tel +8673185295880
Email [email protected]
Background/Aims: Multiple studies have found that microRNAs (miRNAs) are involved in the development of cerebral ischemia. MiR-579-3p can inhibit inflammatory responses and apoptosis, leading to ischemia/reperfusion (I/R) damage. However, the mechanism of how miR-579-3p actions in brain I/R injury remains unclear. This study aimed to investigate the mechanism of the role of miR-579-3p in brain I/R injury.
Methods: A rat model of cerebral ischemia–reperfusion injury was established by suture method. The effects of miR-579-3p on cerebral infarction size, brain water content, and neurological symptoms were evaluated. Flow cytometry was used to detect apoptosis. ELISA was used to detect the level of inflammatory factors. Western blot was used to detect the expression of P65, NCOA1, Bcl-2 and Bax. The relationship between miR-579-3p and NCOA1 was analyzed by bioinformatics analysis and luciferase assay.
Results: Overexpression of miR-579-3p reduced infarct volume, brain water content and neurological deficits. Overexpression of miR-579-3p inhibited the expression level of the inflammatory cytokines, such as TNF-α, IL-6, COX-2 and iNOS, and increased the expression level of IL-10. MiR-579-3p overexpression inhibited NF-кB activity by reducing NRIP1. In addition, miR-579-3p could reduce the apoptotic rate of cortical neurons. Overexpression of miR-579-3p inhibited the activity of caspase-3, increased the expression level of anti-apoptotic gene Bcl-2 in neurons, and decreased the expression level of apoptotic gene Bax.
Conclusion: miR-579-3p can be used to treat brain I/R injury, and its neuroprotective effect may be ascribed to the reduction of inflammation and apoptosis.
Keywords: ischemia/reperfusion, miR-579-3p, inflammation, apoptosis
Introduction
The central nervous system is composed of neurons and glial cells.1,2 Microglia, which acts as a resident macrophage of the central nervous system, accounts for 5–15% of the total number of cells in the brain.3,4 Ischemic stroke is the first disease in the world leading to long-term disability, with the second approximate death rate. Brain stroke poses a great threat to human health and life, causing great suffering to patients.5,6 Therefore, it is an urgent task to fully understand the severity of cerebral apoplexy, improve the treatment and prevent the level of cerebral apoplexy, reduce the morbidity, disability and mortality of cerebral apoplexy. The ischemia–reperfusion (I/R) injury refers to the tissue damage progressively worsened when the recovery of blood perfusion to the tissue after a certain period of ischemia. Although research on the pathogenesis of stroke has never stopped, there is still no good drug available for treatment of I/R injury. Therefore, more potential therapeutic effects need to be studied.
Cerebral ischemia/reperfusion (I/R) can activate various programmed cell death.7,8 Apoptosis is considered to be a major factor in ischemic brain injury.9,10 Inflammatory response is present in cerebral ischemia–reperfusion injury. Another important mechanism, which leads to toxic enzyme activation, free radical overload, etc., causes a series of tissue lesions.11, Therefore, it is speculated that in the treatment of cerebral I/R injury, intervention anti-apoptosis and anti-inflammatory may be a potentially effective measure.
MicroRNAs (miRNAs) regulate cell proliferation, differentiation, growth, metabolism and apoptosis.12 MiRNA plays a key role in the cardiovascular diseases.13 Researchers are concerned that the expression of miRNA alters the development of cardiovascular diseases.14 Some changes in miRNA make people realize that miRNA can be used as a biological target in the development, diagnosis, treatment and prognosis of cardiovascular diseases.15,16 At the same time, miRNA is involved in the mechanism of cerebral I/R injury.17,18 MiR-579-3p has a low expression level in a variety of tumors. Low expression of miR-579-3p is closely related to the occurrence of tumors.19 However, the mechanism of miR-579-3p in brain I/R injury has not been studied. This study focuses on the relationship between miR-579-3p and inflammatory response and apoptosis during cerebral I/R injury, and would provide a basis for diagnosis and treatment of clinical ischemic cerebrovascular diseases.
Materials and Methods
Animal
Male Sprague-Dawley rats (10–12 weeks), weighing 260–320 g, were obtained from Sparford Biology Co., Ltd., Beijing, China. The experiment was approved by the Animal Care and Use Committee of The Second Xiangya Hospital of Central South University. Rats were randomly divided into sham group, I/R group, I/R + control mimic (control mimic), and I/R + miR-579-3p mimic group (miR-579-3p mimic). Twenty-four rats were in each group. All experiments were performed in accordance with The Second Xiangya Hospital of Central South University Animal Experimental Guide and approved by The Second Xiangya Hospital of Central South University Animal Experimental Ethics Committee.
In vivo Gene Transfer and Animal Model of Focal Cerebral Ischemia and Reperfusion (I/R)
The miR-579-3p mimic/control was purchased from RiBoBio (Shanghai, China). Three days before the middle cerebral artery occlusion/reperfusion (MCAO/R), the rat brain was injected with miR-579-3p mimic/control. The miR-579-3p mimic/control was injected into the right ventricle of the rat (2.0 mm posterior atrium, 1.5 mm posterior abdomen, 1.8). In MCAO/R, rats were subcutaneously anesthetized with sodium pentobarbital (30 mg/kg). The middle cerebral artery occlusion (MCAO) rats were anesthetized for 2 h. The right internal carotid artery and the carotid artery were separated. Nylon filaments were inserted from the external carotid artery (ECA) into the internal carotid artery (ICA) until reaching the middle cerebral artery (MCA), which led to occlusion of the middle cerebral artery. The sham group and the I/R group were given an equal dose of normal saline by intraperitoneal injection.
Determination of Infarct Volume
Rats were decapitated 24 h after cerebral ischemia–reperfusion injury. The whole brain was taken out, the left and right brains were separated. Then, the right brain was taken, the cerebellum, low brainstem and olfactory bulb were removed, and the wet weight was weighed immediately. The right forebrain was then sectioned along the coronal plane and the sections were placed in TTC solution. The brain slices were fixed in 10% formaldehyde. The percentage of infarction was calculated according to the reference.20
Neurological Examination
The ZeaLonga scoring method was used to evaluate the neurological impairment. The specific criteria were as follows. There were no symptoms of neurological damage in 0 points. 1 point was that the forepaw on the opposite side of the I/R brain could not be fully extended. 2 points was that the forepaw rotated to the opposite side of the I/R brain during walking. 3 points was that the forepaw tilted to the opposite side of the I/R brain during walking. 4 points was that the forepaw could not be fully extended.
Determination of Brain Water Content
After 24 h of cerebral I/R injury, the rats were decapitated, the olfactory bulb, the cerebellum and the lower brainstem were removed. The wet mass of the brain was weighed. After baking, the dry mass was weighed. Brain water content (%) = (wet mass – dry mass)/wet mass × 100%.
Primary Cortical Neuron Culture
Rat primary cortical neurons were obtained from SD rats as described in the reference.21 The cortex of SD rats was collected and dissected, brain tissue was minced. The lysis was then stopped using DMEM/F12 medium with 10% FBS. The cell suspension was centrifuged. The cell density was adjusted to 1 × 106/mL and coated with 10 mg/L poly-L-lysine. After 72 h, arabinosylcytosine was added to the cell culture. After 24 hours, the normal medium was replaced.
Simulation of I/R in vitro and Gene Transfer
Oxygen glucose deprivation/reperfusion (OGD/P) experiments were performed as literature.22 Cortical neurons were exposed to glucose-free aCSF solution. Then, it was incubated for 2 hours in an incubator of 5% CO2 and 95% N2 (OGD). Then, glucose-free aCSF supplemented with 5.6 mmol/L glucose was added, and the cells were further cultured in an incubator of 5% CO2 and 95% O2 for 12 hours. Cortical neurons were infected with adenovirus or miR-34c-5p for 6 h. The cells were subjected to OGD/P for 72 hours after adenovirus treatment.
Total RNA Extraction and Quantitative Real-Time PCR
Total RNA from primary cultured neurons was extracted using TRIzol reagent (Xinhua, Haerbin, China). qRT-PCR was performed using a ViiATM 7 real-time PCR system (Life Technologies, Grand Island, NY). The expression levels of miR-579-3p and P65 were calculated by the 2−ΔΔCT method. The P65 expression level was normalized to β-actin, while the miR-579-3p level was normalized to U6. qRT-PCR method was performed with reference.23 The primer sequence is shown in Table 1.
 |
Table 1 Sequences of Primers Used in qRT-PCR |
Western Blot
Total protein was isolated using RIPA lysis buffer (Yaji, Shanghai, China). Protein concentration was quantified by the BCA Protein Assay Kit. Then, it was incubated with anti-p65 (1:500), Bcl-2 (1:500), Bax (1:500), COX-2 (1:500), iNOS (1:500), NRIP1 (1:500) and β-actin antibody (1:2000) (Huaan, Hangzhou, China) overnight. Then, anti-rabbit secondary antibody (1:1000, Amyjet, Wuhan, China) was added to incubate for 1 h. Western blot analysis was performed with reference.24
ELISA
The expression of proinflammatory cytokines IL-6, TNF-α and IL-10 in cortical neurons was measured by an ELISA kit (Puxin, Shanghai, China).
Flow Cytometry for Detection of Apoptotic Cells
After OGD/P treatment, cells were plated at a density of 5 x 105 cells per well, and cells were harvested and counted when cells were grown to logarithmic growth phase. After centrifugation of the cells, cells were suspended by adding 195 μL of Annexin V-FITC binding solution. Then, the cells were incubated for 10–20 min, then placed in an ice bath.
Caspase Activity Assay
Caspase 3 activity was measured by the caspase 3/7 assay (Promega). After 24 hours of OGD/P, cortical neurons were cultured in 96-well plates and the cells were incubated with Caspase-Glo reagent. Absorbance value 560 nm was determined on a TECAN GenioPro plate reader.
Statistical Methods
The monitoring data were analyzed by SPSS19.0 statistical software. The results of data analysis were shown as mean ± standard deviation (mean ±SD). Multigroup data analysis was founded on one-way ANOVA. LSD test was intended for subsequent analysis. P < 0.05 indicated the difference was significant.
Result
Cerebral I/R Induced Down-Regulation of miR-579-3p Expression
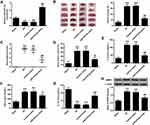
As shown in Figure 1A, miR-579-3p expression levels were significantly down-regulated in the I/R group and the control mimic group after 24 h of reperfusion (P < 0.01). After transfection miR-579-3p mimic into the brain for 3 days, the expression level of miR-579-3p was significantly raised in the miR-579-3p mimic group contrasted with the I/R group (P < 0.01). The infarct volume of the brain was first assessed using TTC staining. As shown in Figure 1B, the area of cerebral infarction was significantly raised in the I/R group contrasted with the sham group (P < 0.01). Contrasted with the I/R group, the cerebral infarction volume of the rats in the miR-579-3p mimic group was significantly reduced (P < 0.01). To be more precise, as shown in Figure 1C and D, contrasted with the sham group, the neurological score and brain water content were significantly raised in the I/R group (P <0.01). Contrasted with the I/R group, the neurological score and brain water content in the miR-579-3p mimic group were significantly reduced (P <0.01).And as shown in Figure 1E–G, contrasted with the Sham group, the level of IL-6 and TNF-α was significantly raised and IL-10 was significantly reduced in the I/R group (P<0.01). Contrasted with the I/R group, the level of IL-6 and TNF-α was significantly reduced and IL-10 was significantly raised in the miR-579-3p mimic group (P <0.01).And contrasted with the sham group, the protein level of NRIP1 was significantly raised in the I/R group (P <0.01). Contrasted with the I/R group, the level of NRIP1 was significantly reduced in the miR-579-3p mimic group (P <0.01, Figure 1H).
miR-579-3p Suppressed Inflammatory Cytokine Expression in OGD/R-Treated Primary Cortical Neurons
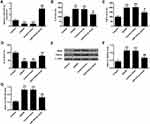
As shown in Figure 2A, miR-579-3p expression levels were significantly reduced in the OGD/R group and the control mimic group contrasted with the control group (P < 0.01). In the miR-579-3p mimic-transduced neurons, the expression level of miR-579-3p was significantly raised in the miR-579-3p mimic group contrasted with the OGD/R group (P < 0.01). Furthermore, the control mimic had no significant effect on the expression level of miR-579-3p contrasted with the OGD/R group. As shown in Figure 2B–D, the levels of IL-6 and TNF-α were significantly raised and the level of IL-10 was reduced in the OGD/R group and the control mimic group contrasted with the control group (P < 0.01). In the miR-579-3p mimic-transduced neurons, the levels of IL-6 and TNF-α were significantly reduced and the level of IL-10 was raised in the miR-579-3p mimic group contrasted with the OGD/R group (P<0.01). Furthermore, the control mimic had no significant effect on the level of IL-6, TNF-α and IL-10 contrasted with the OGD/R group. As shown in Figure 2E–G, the protein levels of iNOS and COX-2 were significantly raised in the OGD/R group and the control mimic group contrasted with the control group (P < 0.01). In the miR-579-3p mimic-transduced neurons, the protein levels of iNOS and COX-2 were significantly reduced in the miR-579-3p mimic group contrasted with the OGD/R group (P < 0.01). Furthermore, the control mimic had no significant effect on the level of iNOS and COX-2 contrasted with the OGD/R group.
The Effects of miR-579-3p on Neuronal Apoptosis
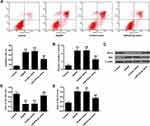
As shown in Figure 3A, the apoptosis rate of the primary cortical neurons in the OGD/R group and the control mimic group was significantly raised contrasted with the control group (P <0.01). MiR-579-3p mimic was significantly reduced apoptosis rate contrasted with OGD/R group (P <0.01).
These results indicated that miR-579-3p mimic inhibited the apoptosis of OGD/R-treated cortical neurons. The mechanism of miR-579-3p inhibiting apoptosis was further analyzed. The results are shown in Figure 3B–E, contrasted with the control group, the activity of caspase-3 and the protein expression level of Bax in primary cortical neurons were significantly raised (P < 0.01) in the OGD/R group and the control mimic group, and the protein expression level of Bcl-2 was significantly reduced (P <0.01). Contrasted with OGD/R group, caspase-3 activity and Bax protein expression levels were significantly reduced in primary cortical neurons of miR-579-3p mimic group (P <0.01), while Bcl-2 protein expression level was significantly raised (P <0.01) (Figure 4E). In summary, miR-579-3p exerted a biological role in I/R injury through anti-apoptotic and anti-inflammatory activity.
The Effects of miR-579-3p on NF-кB Activity
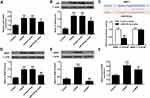
As shown in Figure 4A and B, there was a significantly increased nuclear the translocation of p65 subunit in the primary cortical neurons of the OGD/R group and the control mimic group contrasted with the control group, while miR-579-3p mimic attenuated the p65 subunit translocation (P <0.05).
Next, whether miR-579-3p inhibited the activity of NF-кB was analyzed by inhibiting the expression of NRIP1. As shown in Figure 4C, bioinformatics analysis indicated that the NRIP1 was predicted to be the target gene of miR-579-3p. Luciferase activity was significantly reduced in cells transfected with miR-579-3p mimic and NRIP1-WT (P < 0.05), but luciferase activity of NRIP1-MUT did not change significantly. These results indicated that miR-579-3p was capable of targeting NRIP1. In addition, as shown in Figure 4D, the expression level of NRIP1 was significantly raised in the OGD/R group and the control mimic group contrasted with the control group (P < 0.01), while the expression level of NRIP1 in the cortical neurons of the miR-579-3p mimic group was significantly reduced (P < 0.01), indicating that miR-579-3p could regulate the expression level of NRIP1. In addition, as shown in Figure 4E and F, si-NRIP1 could significantly inhibit the expression levels of NRIP1 and P65 (P<0.05). These results indicated that miR-579-3p activated NF-кB by modulating NRIP1.
Discussion
Cerebrovascular disease is one of the most serious problems in the world.25,26 The mortality of stroke is still rising. It develops rapidly in increasingly younger, low-income populations.27 The regional gender differences are obvious. Stroke refers to an acute cerebrovascular disease that causes sudden onset of local neurological damage.28,29 It can be divided into ischemia according to its unique nature. The onset and repair of ischemic stroke is complex and variable dynamic processes, which is affected by many physiological and pathological factors, such as inflammation, angiogenesis, ischemia–reperfusion injury, etc.30 However, no clinically effective drugs have been found to treat ischemic stroke. Studying the pathogenesis and repair mechanisms of ischemic stroke can provide new ideas for the development of new drugs and bring new hope to patients with clinical stroke.
At present, in the treatment of ischemic stroke, thrombolysis, neuroprotection and other measures cannot achieve a good therapeutic effect. Researchers have found that during the treatment and rescue of patients with ischemic stroke, ischemia is not the main cause of brain tissue damage, but excessive free radicals attack a large number of normal cells when the blood supply is restored, causing ischemia/reperfusion (I/R) injury.31 The brain injury after cerebral ischemia is caused by a series of events in the process of cerebral ischemia treatment.32 Abnormalities occurred in the early stage may lead to rapid necrosis of brain cells and eventually cerebral infarction. Thrombolysis and other endovascular treatment of cerebral ischemia have brought new hope to stroke patients.33 At the same time, brain tissue damage caused by ischemia–reperfusion has attracted more researchers’ attention. At present, people are still vague about the I/R injury. And the reperfusion injury caused by cerebral I/R, including inflammation, apoptosis and so on, plays a key role in the process of I/R injury.34
MicroRNA (miRNA) is involved in almost all known biological regulatory processes, such as cell differentiation, apoptosis, etc., and its effect is very potent.35,36 It can be highly expressed as a biological marker in the human circulating blood system.37 According to the latest research, miRNA is closely related to the occurrence of ischemic cerebrovascular diseases.38 Researchers have constructed a MCAO model. The results show that 17 kinds of miRNAs (miR-148b, miR-27a, miR-29, miR-137, etc.) are down-regulated, while 7 kinds of miRNAs (miR-497, miR-215, miR-324-3p, etc.) are significantly up-regulated.39 MiR-579-3p is a recently discovered miRNA, and it has been found abnormally expressed in various diseases.19 This study found that the expression level of miR-579-3p was down-regulated in the I/R group and the OGD/R group, and the expression level of miR-579-3p was significantly increased after transfection of miR-579-3p mimic into brain and cortical neurons. The miR-579-3p mimic group was able to reduce neurological score, infarct volume and brain water content. It was further confirmed that promoting miR-579-3p expression could partially alleviate cerebral ischemia–reperfusion injury and cell necrosis.
Inflammatory response is another key role in cerebral I/R injury, which leads to the activation of toxic enzymes, free radical overload, etc., resulting in a series of tissue lesions.40,41 Myocardial structure changes due to activation and release of inflammatory cells and regulation of the activity of inflammatory cells can effectively prevent heart diseases. Studies have shown that inflammatory factors are greatly increased by the inflammatory response that is exacerbated under stress conditions.42 Studies have shown that inflammatory mediators such as IL-6, TNF-α, iNOS, COX-2 and IL-10 play a major role in I/R injury.43 This study found that IL-6, TNF-α, iNOS, COX-2 expression levels were raised and IL-10 expression level was decreased in primary cortical neurons in the OGD/R-treated group. Overexpression of miR-579-3p in cortical neurons inhibited IL-6, TNF-α, iNOS and COX-2 expression levels and increased IL-10 expression. This indicated that miR-579-3p overexpression activated endogenous anti-inflammatory factors and could partially alleviate cardiomyocytes’ inflammation.
Apoptosis is also an important mechanism accompanying cerebral I/R injury.44 In the occurrence of cerebral ischemia, neuronal death around the ischemic central zone is mainly apoptosis.45 Caspase family member proteins play a leading role in both mitochondria-dependent and non-mitochondria-dependent pathways, and caspase-3 is one of the important indicators for detecting apoptosis.46 The Bcl-2 family is divided into inhibitory proteins and pro-apoptotic proteins, which also play an important regulatory role in the process of apoptosis. When the cells are in a state of cerebral ischemia and other stress, the expression of Bcl-2 is decreased, while pro-apoptotic protein Bax expression is increased.47 This study also confirmed this, the apoptotic rate of primary cortical neurons was increased. The overexpression of miR-579-3p could inhibit the rate of apoptosis. This study found that the expression levels of caspase-3 and Bax were up-regulated and Bcl-2 was down-regulated in the OGD/R-treated group. Overexpression of miR-579-3p could down-regulate caspase-3 and Bax, and up-regulate protein expression level of Bcl-2. These results suggested that the protective effect of miR-579-3p on cerebral I/R injury was related to anti-apoptotic activity.
NF-κB belongs to the Rel family of proteins, and a homologous or heterodimer composed of P65 is the main active form of NF-κB.48 Phosphorylation of the P65 subunit plays a key role in activating the transcription of the target gene. NF-κB plays a part in cerebral I/R injury. After hypoxia/reoxygenation stimulation, NF-κB is activated and its DNA binding activity is multiplied.49 NF-κB is activated in ischemia–reperfusion injury. The NF-κB regulates the expression of cytokines (TNF-α, iNOS, IL-1β, etc.), which affects the inflammatory cascade, amplifies the inflammatory effect, and ultimately leads to aggravation of brain damage.50 Inhibition of NF-κB activation can significantly reduce the cerebral ischemia damage.51 This study found that there was a significant translocation of the p65 subunit in primary cortical neurons in the OGD/R group, while miR-579-3p mimic attenuated the translocation of the p65 subunit. NRIP1 was a target gene of miR-579-3p. MiR-579-3pcould regulate the expression level of NRIP1. In addition, si-NRIP1 could inhibit the expression level of P65. These results indicated that miR-579-3p activated NF-кB by modulating the NF-кB coactivator NRIP1. In future research, we would try to use the miR-579-3p specific inhibitor in further studies to validate the understanding of the results.
Conclusion
miR-579-3p can partially alleviate brain I/R damage by inhibiting inflammation and apoptosis. It is suggested that miR-579-3p is a protective factor for I/R rat cerebral infarction, which may provide an experimental basis for the relief of myocardial ischemia–reperfusion injury.
This work was supported by Natural Science Foundation of Hunan Province Grant No: 2019JJ50894 and Xiangya Clinical Big Data Project of Central South University Grant No:2013063.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Komori T. The 2016 WHO classification of tumours of the central nervous system: the major points of revision. Neurol Med Chir (Tokyo). 2017;57(7):301–311. doi:10.2176/nmc.ra.2017-0010
2. Wohlfert EA, Blader IJ, Wilson EH. Brains and brawn: toxoplasma infections of the central nervous system and skeletal muscle. Trends Parasitol. 2017;33(7):519–531. doi:10.1016/j.pt.2017.04.001
3. Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23(9):1018–1027. doi:10.1038/nm.4397
4. Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi:10.1016/j.neuron.2012.03.026
5. Furie KL, Kasner SE, Adams RJ. Guidelines for the prevention of stroke in patient with stroke or transient ischemic attack. Stroke. 2011;42:1–50. doi:10.1161/STR.0b013e3181f7d043
6. Lopes RD, Alexander JH, Al-Khatib SM, et al. Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J. 2010;159(3):0–339.
7. Park CW, Lee TK, Cho JH, et al. Rufinamide pretreatment attenuates ischemia-reperfusion injury in the gerbil hippocampus. Neurol Res. 2017;39(11):1.
8. Jang S, Lewis TS, Powers C, et al. Elucidating mitochondrial electron transport chain supercomplexes in the heart during ischemia-reperfusion. Antioxid Redox Signal. 2017;27(1):57–69.
9. Gottlieb RA, Engler RL. Apoptosis in myocardial ischemia-reperfusion. Ann N Y Acad Sci. 2010;874(1):412–426.
10. Lin M, Li L, Li L, Pokhrel G, Zhu T. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC Complement Altern Med. 2014;14(1):19. doi:10.1186/1472-6882-14-19
11. Kamat P, Juon B, Jossen B, Gajanayake T, Vögelin E. Assessment of endothelium and inflammatory response at the onset of reperfusion injury in hand surgery. J Inflamm. 2012;9(1):18. doi:10.1186/1476-9255-9-18
12. Michael JV, Wurtzel JGT, Mao GF, Rao AK, Goldfinger LE. Platelet microparticles infiltrating solid tumors transfer miRNAs that suppress tumor growth. Blood. 2017;130(5):
13. Buie JN, Goodwin AJ, Cook JA, Halushka PV, Fan H. The role of miRNAs in cardiovascular disease risk factors. Atherosclerosis. 2016;254:271–281.
14. Ebrahimi R. Circulating exosomal miRNAs in cardiovascular disease? Pathogenesis: new emerging hopes. J Cell Physiol. 2019;234(12):21796–21809.
15. Dangwal S, Thum T. microRNA therapeutics in cardiovascular disease models. Annu Rev Pharmacol Toxicol. 2014;54(1):185–203. doi:10.1146/annurev-pharmtox-011613-135957
16. Zhu R, Xue J, Mengnan AN; Cardiology DO. MiRNAs in cardiovascular disease. Medl Recapitulate. 2018
17. Li HW, Wang JF, Yu G. Role of microRNAs in renal ischemia-reperfusion injury: an update. Acad J Second Mil Med Univ. 2014;33(12):1365–1370. doi:10.3724/SP.J.1008.2013.01365
18. Makhdoumi P, Roohbakhsh A, Karimi G. MicroRNAs regulate mitochondrial apoptotic pathway in myocardial ischemia-reperfusion-injury. Biomed Pharmacother. 2016;84:1635–1644.
19. Fattore L, Mancini R, Acunzo M, Romano G, Ciliberto G. miR-579-3p controls melanoma progression and resistance to target therapy. Proc Natl Acad Sci U S A. 2016;113(34):201607753.
20. Kroencke TJ, Scheurig C, Poellinger A, Gronewold M, Hamm B. Uterine artery embolization for leiomyomas: percentage of infarction predicts clinical outcome. Radiology. 2010;255(3):834–841. doi:10.1148/radiol.10090977
21. Meima L, Kljavin IJ, Moran P, Shih A, Winslow JW, Caras IW. AL-1-induced growth cone collapse of rat cortical neurons is correlated with REK7 expression and rearrangement of the actin cytoskeleton. Eur J Neurosci. 2010;9(1):177–188.
22. Liu Y, Wang H, Yang M, et al. Cistanche deserticola polysaccharides protects PC12 cells against OGD/RP-induced injury. Biomed Pharmacother. 2018;99:671–680. doi:10.1016/j.biopha.2018.01.114
23. Kong Q, Yuan J, Gao L, et al. Identification of Suitable Reference Genes for Gene Expression Normalization in qRT-PCR Analysis in Watermelon. PLoS One. 2014;9.
24. Sanchez-Campillo M, Bini L, Comanducci M, et al. Identification of immunoreactive proteins of Chlamydia trachomatis by Western blot analysis of a two-dimensional electrophoresis map with patient sera. Electrophoresis. 2015;20(11):2269–2279.
25. Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67(5):564–569.
26. Evans NR, Tarkin JM, Buscombe JR, Markus HS, Jhf R, Warburton EA. PET imaging of the neurovascular interface in cerebrovascular disease. Nat Rev Neurol. 2017;13(11).
27. Schreiner SJ, Kirchner T, Narkhede A, Wyss M, Unschuld PG. Brain amyloid-burden and cerebrovascular disease are synergistically associated with neurometabolism in cognitively unimpaired older adults. Neurobiol Aging. 2017;63:152. doi:10.1016/j.neurobiolaging.2017.12.004
28. Gotru SK, Chen W, Kraft P, et al. TRPM7 kinase controls calcium responses in arterial thrombosis and stroke in mice. Arterioscler Thromb Vasc Biol. 2018;38:344–352.
29. Patel PA, Zhao X, Fonarow GC, et al. Novel oral anticoagulant use among patients with atrial fibrillation hospitalized with ischemic stroke or transient ischemic attack. Circulation. 2018;8(4):2191–2201.
30. Hao JZ, Mao S. Unsteady aerodynamic force mechanisms of a hoverfly hovering with a short stroke-amplitude. Phys Fluids. 2017;29(8):081901.
31. Chen Z, Ding T, Ma CG. Dexmedetomidine (DEX) protects against hepatic ischemia/reperfusion (I/R) injury by suppressing inflammation and oxidative stress in NLRC5 deficient mice. Biochem Biophys Res Commun. 2017;493(2):1143.
32. Minami K, Uehara H, Bae S, Reder J, Tullius SG. The polymer pro-drug APP-103 mitigates I/R injury and improves graft function in a pre-clinical renal transplant model. Transplantation. 2018;102:S705–S706. doi:10.1097/01.tp.0000543669.54963.ab
33. Sahna E, Acet A, Ozer MK, Olmez E. Myocardial ischemia-reperfusion in rats: reduction of infarct size by either supplemental physiological or pharmacological doses of melatonin. J Pineal Res. 2010;33(4):234–238.
34. He J, Li H, Li G, Yang L. Hyperoside protects against cerebral ischemia-reperfusion injury by alleviating oxidative stress, inflammation and apoptosis in rats. Biotechnol Biotechnol Equip. 2019;33(1):798–806. doi:10.1080/13102818.2019.1620633
35. Chen X, Liu P; Pathophysiology DO. Expression of LncRNA HCG11 and mi R-590-3p in squamous carcinoma of cervix and their relationship with prognosis. Cancer Res Prev Treat. 2018;45(03):148–153
36. Zeng Y, Wang KX, Xu H, Hong Y. Integrative miRNA analysis identifies hsa‐miR‐3154, hsa‐miR‐7‐3, and hsa‐miR‐600 as potential prognostic biomarker for cervical cancer. J Cell Biochem. 2018;119:1558–1566. doi:10.1002/jcb.26315
37. Leidinger P, Backes C, Rheinheimer S, Keller A, Meese E. Towards clinical applications of blood-borne miRNA signatures: the influence of the anticoagulant EDTA on miRNA abundance. PLoS One. 2015;10(11):e0143321. doi:10.1371/journal.pone.0143321
38. He W, Chen S, Chen X, Li S, Chen W. Bioinformatic analysis of potential microRNAs in ischemic stroke. J Stroke Cerebrovasc Dis. 2016;S1052305716001658.
39. Altintas O, Ozgen Altintas M, Kumas M, Asil T. Neuroprotective effect of ischemic preconditioning via modulating the expression of cerebral miRNAs against transient cerebral ischemia in diabetic rats. Neurol Res. 1–9.
40. Jung HS, Joo J-D, Kim D-W, et al. Effect of milrinone on the inflammatory response and NF-kB activation in renal ischemia-reperfusion injury in mice. Korean J Anesthesiol. 2014;66(2):136. doi:10.4097/kjae.2014.66.2.136
41. Zheng Y, Lu M, Ma L, Zhang S, Qiu M, Ma X. Osthole ameliorates renal ischemia-reperfusion injury by inhibiting inflammatory response. Urol Int. 2013;91(3):350–356. doi:10.1159/000347191
42. Thaunat O. [Sterile inflammatory response to ischemia-reperfusion injury: immediate and long term consequences on graft function]. Bull Acad Natl Med. 2011;195(4–5):847.
43. Yu H, Wu M, Zhao P, Huang Y, Wang W, Yin W. Neuroprotective effects of viral overexpression of microRNA‐22 in rat and cell models of cerebral ischemia‐reperfusion injury. J Cell Biochem. 2015;116:233–241. doi:10.1002/jcb.24960
44. Zhang Z, Tong N, Gong Y, Qiu Q, Wu X. Valproate protects the retina from endoplasmic reticulum stress-induced apoptosis after ischemia-reperfusion injury. Neurosci Lett. 2011;504(2):88–92. doi:10.1016/j.neulet.2011.09.003
45. Jing H, Liu L, Jia Y, Yao H, Ma F. Overexpression of the long non-coding RNA Oprm1 alleviates apoptosis from cerebral ischemia-reperfusion injury through the Oprm1/miR-155/GATA3 axis. Artif Cells. 2019;47(1):2431–2439.
46. Liu B, Fan Z. The monoclonal antibody 225 activates caspase-8 and induces apoptosis through a tumor necrosis factor receptor family-independent pathway. Oncogene. 2001;20(28):3726–3734. doi:10.1038/sj.onc.1204490
47. Liu Y, Zuo H, Wang Y, Tian L, Pei X. Ethanol promotes apoptosis in rat ovarian granulosa cells via the Bcl-2 family dependent intrinsic apoptotic pathway. Cell Mol Biol (Noisy-Le-Grand). 2018;64(1):118. doi:10.14715/cmb/2018.64.1.21
48. Velaei K, Samadi N, Soltani S, Barazvan B, Rad JS. NFκBP65 transcription factor modulates resistance to doxorubicin through ABC transporters in breast cancer. Breast Cancer. 2016;24(4):1–10.
49. Shi CX, Ding YB, Jin F, et al. Effects of sevoflurane post-conditioning in cerebral ischemia-reperfusion injury via TLR4/NF-κB pathway in rats. Eur Rev Med Pharmacol Sci. 2018;22(6):1770.
50. Zhou L, Zhang HX, Liu LG, Huang H, Xuan LI, Yang M. Effect of scalp-acupuncture on plasma and cerebral TNF-α and IL-1β contents in acute cerebral ischemia/reperfusion injury rats. Acupunct Res. 2008;33:173–178.
51. Khan SI, Malhotra RK, Rani N, Sahu AK, Bhatia J Febuxostat modulates MAPK/NF- κ Bp65/TNF- α signaling in cardiac ischemia-reperfusion injury. Oxid Med Cell Longev. 2017;2017:1–13.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.