Back to Journals » OncoTargets and Therapy » Volume 11
MicroRNA-194 regulates cell viability and apoptosis by targeting CDH2 in prostatic cancer
Authors Gao S, Zhao Z, Wu R, Wu L, Tian X, Zhang Z
Received 23 March 2018
Accepted for publication 26 May 2018
Published 14 August 2018 Volume 2018:11 Pages 4837—4844
DOI https://doi.org/10.2147/OTT.S169101
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Samir Farghaly
Song Gao,1 Zhiying Zhao,2 Rong Wu,1 Lina Wu,1 Xin Tian,1 Zhenyong Zhang1
1The Second Department of Clinical Oncology, Shengjing Hospital, China Medical University, Shenyang 110022, China; 2Department of computer science and engineering, Northeastern University, Shenyang 110004, China
Introduction: Prostate cancer (PCa) is one of the most common malignancies in men. However, a lack of understanding of the mechanism underlying PCa metastasis has strongly limited the effectiveness of therapy for this disease. Thus, investigating the mechanism of PCa may help improve the prognosis of PCa patients. The goal of this study was to investigate the role of microRNA-194 (miR-194) in PCa.
Materials and methods: The expression of miR-194 and cadherin 2 (CDH2) at the transcriptional level was measured by quantitative real-time polymerase chain reaction (qRT-PCR). The MTT assay cell apoptosis assay and Western blotting were used to determine the role of miR-194 and CDH2 in the PC3 human PCa cell line. The dual luciferase reporter assay system was performed to clarify the relationship between miR-194 and CDH2. qRT-PCR results showed that miR-194 was downregulated and CDH2 was upregulated in PC3 cells.
Results: Transfection with miR-194 mimics decreased cell viability and increased the rate of apoptosis compared with the control group of PC3 cells. Bioinformatics and the luciferase reporter assay indicated that CDH2 was a target of miR-194, and Western blot analysis suggested that CDH2 was negatively regulated by miR-194. Further studies revealed that the downregulation of CDH2 suppressed cell viability and promoted the apoptosis of PC3 cells and that miR-194 directly targeted CDH2 in PC3 cells. Finally, the in vivo experiments showed that miR-194 mimics suppressed tumor growth and induced apoptosis in a greater proportion of cells by decreasing the expression of CDH2 compared with the control group.
Conclusion: The results of this study showed that miR-194 targeted CDH2 to regulate PCa cell survival in vitro and suppress tumor growth in vivo. These findings suggest that miR-194 may be a useful therapeutic target in PCa.
Keywords: prostatic cancer, microRNA-194, CDH2, cell viability, apoptosis
Introduction
Prostate cancer (PCa) is one of the most common malignancies in men and is a heterogeneous spectrum of disease ranging from indolent to lethal malignancy.1 Patients with advanced or metastatic PCa have poor prognosis and suffer from metastasis-related symptoms.2 Currently, effective treatment options for castration-resistant PCa are quite limited.3 Thus, there is an urgent need to identify more efficient, novel biomarkers that can be used to confirm the malignant potential of PCa and to predict patient clinical outcome, evaluate the risk of recurrence, and select optimized therapeutic strategies.4 Furthermore, investigating the mechanism underlying PCa will improve current therapeutic modalities and increase the survival of PCa patients.
MicroRNAs (miRNAs) are a group of small non-coding RNAs (20–22 nucleotides) that post-transcriptionally regulate gene expression.5 It is well known that some miRNAs are abnormally expressed in different types of tumors, which function as oncogenes or tumor suppressors,2 although studies have indicated that miRNAs may be involved in PCa progression and metastasis.6–9 However, their precise roles in this disease have not been fully elucidated.
Cadherin-2 (CDH2), also known as N-cadherin, is a member of the cadherin family that regulates many biological processes.10 It is upregulated in various cancers, including bladder, colorectal, and lung and gastric cancers.10–12 However, little research has been conducted to determine whether CDH2 is correlated with PCa.
In this study, we investigated the mechanism of the miR-194/CDH2 axis in regulating the pathological mechanisms in PCa cells. We found that miR-194 targeted CDH2 to regulate PCa cell survival in vitro and suppress tumor growth in vivo. The results of this study provide a better understanding of PCa pathogenesis, which indicate that miR-194 serve as a therapeutic target in PCa.
Materials and methods
Cell culture
Human PCa cell line (PC3) and normal prostate epithelial cell lines (RWPE-1) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cells were maintained in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) at 37°C and 5% CO2.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cells and tissues with TRIzol® reagent (Invitrogen, CA, USA) according to manufacturer’s instructions. RNA was reverse transcribed into cDNA using PrimeScript RT reagent kit (TaKaRa, Dalian, China). qRT-PCR was performed with SYBR Premix Ex Taq (TaKaRa). GAPDH was considered as an internal normalized reference. The relative level was calculated by relative quantification (2−ΔΔCt) method. The primers used were as follows: miR-194: forward, 5′-ATGGACCTGGGGCCAGCGAAG-3′ and reverse, 5′-TCTGGCCTGGGAGCGTCG-3′; GAPDH: forward, 5′-TGGTATCGTGGAAGGACTC-3′ and reverse 5′-AGTAGAGGCAGGGATGATG-3′; CDH2: forward, 5′-GTCAGCAGAAGTTGAAGAAATAGTG-3′ and reverse 5′-GCAAGTTGATTGGAGGGATG-3′.
Transfection
Cells were cultured for 24 h and seeded into 96-well plate for transfection. miR-194 mimic (20 nmol/L) and negative control (NC) (20 nmol/L) were purchased from Genscript (Shanghai, China) and were used according to manufacturer’s protocol. CDH2 small interfering RNA (siRNA) (20 nmol/L) and NC (20 nmol/L) were synthesized by GenePharma. Then they were transfected into cells using Lipofectamine™ 2000 reagent. After 48 h incubation, transfected cells were collected and purified.
MTT assay
MTT assay was performed for cell viability detection. Different groups of cells were seeded into 96-well plate. Then, MTT solution (20 μL, 5 mg/mL in phosphate buffered saline [PBS]) was added to each wells and incubated at 37°C for 4 h. Then the absorbance at 492 nm was measured and the proliferation efficiency was examined. The experiments were repeated three times independently.
Cell apoptosis assay
At 48 h post-transfection, the cells were collected and washed in ice-cold PBS. Annexin V-FITC/propidium iodide (PI) apoptosis detection kit (Multisciences, Shanghai, China) was used to assess cell apoptosis according to the manufacturer’s instructions. The cells were incubated with 5 μL of Annexin V-FIFC for labeling for 15 min. Then 10 μL of PI was added into each specimen. Cell apoptosis was analyzed in a flow cytometer (BD Biosciences). All experiments were performed in triplicate independently.
Western blot
The cells and tissue were lysed with RIPA buffer. The protein concentration was determined using a BCA Protein Assay kit (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s instructions. Protein lysates were separated by 10% SDS-PAGE gel and then transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Membranes were blocked and incubated with CDH2 antibody (1:2,000, ab18203; Abcam, Cambridge, UK) or GAPDH antibody (1:5,000, sc-365062; Santa Cruz) at 4°C overnight, respectively. Then membranes were washed in tris buffered saline Tween (TBST) and incubated with horseradish peroxidase-conjugated secondary antibodies (1:5,000, ab197527; Abcam) for 1 h at room temperature. ECL Western blotting substrate (Pierce) was used for visualizing and detection.
Luciferase reporter assay
For target gene assays, a wild-type (WT) 3′-UTR fragment of CDH2 containing the putative miR-194 binding sequence was inserted into a pmirGlO Dual-luciferase miRNA Target Expression Vector (Promega, Madison, WI, USA), while mutant (MUT) 3′-UTR was also cloned into the vector to generate CDH2-mutated-type contained mutated binding site. Cells at 60% confluence were co-transfected with CDH2-WT or CDH2-MUT and miR-194 mimics (20 nmol/L) using Lipofectamine™ 2000. After 48 h, dual Luciferase reporter assay system (Promega) was used to evaluate the Luciferase activity following manufacturer’s instructions.
Animal models
Male BALB/c nude mice were purchased from the experimental animal center of China Medical University. All animal studies were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals made by the Ministry of Science and Technology of China. All the procedures involved in rats were approved by the Ethics Committee of China Medical University. Subcutaneously, mice were injected with 2×106 PC3 cells transfected with or without miR-194 mimic, and healthy BALB/c mice were chosen as control group. Each group had 6 mice. The tumors’ volume was measured every 5 days after injection. Tumor volume was calculated according to the following formula: tumor volumes (mm3) = length × width × height. After 30 days post-injection, tumors were collected.
TUNEL assay
For detecting cell apoptosis in tumor tissues, TUNEL assay was performed using the TUNEL Apoptosis Assay Kit (Beyotime Institute of Biotechnology, Jiangsu, China) according to the manufacturer’s instructions. Tumors tissues were fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin, and cut into 4-μm-thick sections. Tumor sections were incubated in 3% H2O2 and then the sections were added in the TUNEL reaction mixture. The sections were subsequently rinsed and visualized using diaminobenzidine. Hematoxylin was used for counter-staining. The numbers of TUNEL-positive cells of 5 random fields were counted, and then the cell apoptosis rate was calculated as the percent of TUNEL-positive cells.
Statistical analysis
Experimental data were conducted using GraphPad Prism 4 software. The data were presented as mean ± SD. Student’s t-test and one-way ANOVA were used for statistical analyses. Differences were considered statistically significant at *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Results
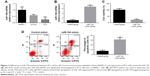
MiR-194 mimic suppresses PC3 cells survival
The expression level of miR-194 in PC3 cells was detected by qRT-PCR. The results showed that the relative expression of miR-194 was significantly downregulated in PC3 cells compared with the normal prostate epithelial cell line RWPE-1 (Figure 1A). Transfection with miR-194 mimics in PC3 cells markedly increased the expression of miR-194 compared with control cells (Figure 1B) and decreased cell viability (Figure 1C). The results of the cell apoptosis assay showed that the increased expression of miR-194 increased the apoptosis rate compared with control PC3 cells (Figure 1D). These results demonstrated that miR-194 mimics decreased cell viability and increased the apoptosis rate of PC3 cells.
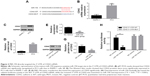
MiR-194 directly targets CDH2 in PC3 cells
To investigate the molecular mechanism by which miR-194 regulates cells survival, putative miR-194 targets were predicted through bioinformatics analysis, and CDH2 was shown to be a potential target of miR-194 (Figure 2A). We evaluated the mRNA and protein expression of CDH2 in PC3 cells by qRT-PCR and Western blotting, respectively, and found that CDH2 expression in PC3 cells was much higher than that in RWPE-1 cells (Figure 2B–D). Transfection of miR-194 mimics significantly decreased the expression of CDH2 (Figure 2E–G). In addition, the luciferase reporter assay showed that co-transfection of miR-194 mimics and WT CDH2 in PC3 cells led to a significant decrease in luciferase activity (Figure 2H). However, co-transfection of miR-194 mimic and MUT CDH2 did not lead to changes in luciferase activity compared to cells transfected with MUT CDH2 alone. These results confirmed that miR-194 targeted CDH2 and negatively regulated its expression.
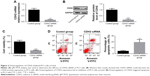
Downregulation of CDH2 suppresses PC3 cells survival
After understanding the relationship between miR-194 and CDH2, we explored the regulatory role of CDH2 in PC3 cells. To this end, PC3 cells were transfected with CDH2 siRNA to decrease its expression. Then qRT-PCR and Western blotting were conducted to determine transfection efficiency at the RNA and protein level (Figure 3A and B). CDH2 downregulation suppressed cell viability while promoting the apoptosis of PC3 cells (Figure 3C and D). These results showed that the downregulation of CDH2 suppressed PC3 cell survival.
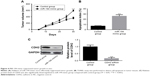
MiR-194 mimic suppressed tumor growth in vivo
A mouse model of PCa was used to explore the effects of miR-194 on tumor growth in vivo. Mice were injected with PC3 cells or miR-194 mimics transfected PC3 cells to form prostatic tumors. The tumor volume was significantly decreased by miR-194 mimic treatment compared with the control group (Figure 4A). The addition of miR-194 mimic more effectively suppressed tumor growth. Furthermore, our results also showed that miR-194 mimics induced apoptosis in a greater proportion of cells compared with the control group (Figure 4B). The relative protein expression of CDH2 was also significantly downregulated in the miR-194 mimic group compared with the control group (Figure 4C). Taken together, these data showed that miR-194 mimic suppressed tumor growth by decreasing CDH2 expression in vivo.
Discussion
PCa is a well-known epithelial malignant tumor characterized by frequent metastasis, and its growth and progression rely on the activation of androgen receptor.13–15 However, a lack of understanding of the mechanism underlying PCa has strongly limited the effectiveness of therapy for this disease. Thus, understanding the molecular mechanism of PCa may help to improve the prognosis of PCa patients. The main aim of this study was to evaluate the effects of miRNA on the survival of PCa cells to gain a better understanding of the molecular mechanisms of PCa. To this end, we examined the expression of miR-194 in PC3 and normal prostate epithelial cells and found that miR-194 was dramatically downregulated in PC3 cells. In addition, we found that miR-194 modulated cell survival and tumor growth by targeting CDH2 (Figure 5).
Many studies have revealed the effect of miRNAs on tumor progression including regulating proliferation, metastasis, and chemoresistance of tumor cells.16–19 In a previous study, the author analyzed the miRNA profile in hemolysis-free blood plasma of patients with PCa and found that the metastatic PCa was associated with increased levels of hsa-miR-22-3p, hsa-miR-663a, and hsa-miR-4674 compared with non-metastatic PCa.20 Previous studies have found that the level of hsa-miR-619-5p was elevated in patients with extracapsular spreading of the tumor, increasing significantly from stage pT2 to stage pT4.21 The results of a previous study indicated that miR-194 could downregulate the expression of oncogenic matrix metalloproteinase (MMP) 2 and MMP9 by targeting bone morphogenetic protein 1, suggesting a potential novel mechanistic target by which phenethyl isothiocyanate suppresses PCa cell invasion.22 Furthermore, overexpression of miR-194 in PCa cells could inhibit cell migration and invasion and induce multinucleated cells, and lentivirus-mediated stable expression of miR-194 in PCa cells reduced cell colony formation and decrease their tumorigenic ability.23 miR-194 also promoteed invasive capacity of PCa by inhibiting suppressor of cytokine signaling 2.24 Furthermore, miR-203 was highly upregulated in breast cancer tissues and estrogen receptor (ER)-positive breast cancer cell lines, and anti-miR-203 decreased mammosphere formation and the expression of stem cell markers in the MCF-7 and ZR-75-1 breast cancer cells. Therefore, anti-miR-203 may be a potential therapeutic strategy in ER-positive breast cancer.25 Here, our present study explored the interaction between miR-194 and PCa cells survival. MiR-194 was shown to be downregulated in paclitaxel (PTX)-resistant ovarian cancer cell lines and its overexpression attenuated PTX resistance in these cells.26 In accordance with previous study, our data suggested that the expression of miR-194 was significantly decreased in PC3 cells. In addition, miR-194 targeted CDH2 to regulate PC3 cells survival in vitro and suppressed tumor growth in vivo.
Our study also revealed that CDH2 is a potential target of miR-194. CDH2 is a member of the cadherin family, which regulates many cellular processes including apoptosis, angiogenesis, and chemoresistance.27 In addition, it is reported that CDH2 plays a significant part in the epithelial-mesenchymal transition (EMT).28 In a previous study, miR-194 was shown to inhibit tumor growth and osteosarcoma metastasis by downregulating CDH2 and insulin-like growth factor 1 receptor; thus, miR-194 may be a promising therapeutic agent for osteosarcoma.29 Furthermore, a recent study also showed that miR-194 was significantly reduced in osteosarcoma compared with normal bone tissue and could inhibit the malignant behavior of osteosarcoma by downregulating CDH2 expression.27 Our study showed similar results in that CDH2 was upregulated in PC3 cells compared with the normal prostate epithelial cell line. In addition, we observed that CDH2 expression was negatively regulated by miR-194. To further confirm our results, the luciferase reporter assay was conducted to explore the interaction between CDH2 and miR-194 and the results showed that miR-194 directly targeted CDH2 in PC3 cells. Then, we further investigated the effects of CDH2 on survival of PC3 cells and found that downregulation of CDH2 inhibited the cell survival of PC3 cells. Transfection of miR-194 mimics suppressed tumor growth even more effectively, and the protein level of CDH2 was also significantly downregulated in miR-194 mimics group compared with control group. Taken together, the results of this study showed that miR-194 mimics suppressed PCa cells survival and tumor growth by negatively regulating the expression of CDH2 in PCa cells. However, in the current study, clinical validation was lacking and further studies are required to determine whether miR-194 and CDH2 can serve as novel therapeutic targets in PCa.
Conclusion
In summary, our present study found that miR-194 targeted CDH2 to regulate PC3 cells survival in vitro and suppressed tumor growth in vivo. The newly found miR-194/CDH2 link provides new lights in the potential mechanism of PCa. As miRNA-based therapy is currently in clinical trial, our results indicate that miR-149 may be a novel candidate for PCa treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
Truong H, Gomella LG, Thakur ML, Trabulsi EJ. VPAC1-targeted PET/CT scan: improved molecular imaging for the diagnosis of prostate cancer using a novel cell surface antigen. World J Urol. 2018;36(5):719–726. | ||
Feng Q, He P, Wang Y. MicroRNA-223-3p regulates cell chemo-sensitivity by targeting FOXO3 in prostatic cancer. Gene. 2018;658:152–158. | ||
Pant MK, Abughaban A, Aragon-Ching JB. Advances in systemic therapies for metastatic castration-resistant prostate cancer. Future Oncol. 2014;10(14):2213–2226. | ||
Zhang Y, Jiang F, He H, et al. Identification of a novel microRNA-mRNA regulatory biomodule in human prostate cancer. Cell Death Dis. 2018;9(3):301. | ||
Shi Z, Zhou H, Lu L, et al. The roles of microRNAs in spinal cord injury. Int J Neurosci. 2017;127(12):1104–1115. | ||
Hao P, Kang B, Yao G, Hao W, Ma F. MicroRNA-211 suppresses prostate cancer proliferation by targeting SPARC. Oncol Lett. 2018;15(4):4323–4329. | ||
Hu B, Jin X, Wang J. MicroRNA-212 targets mitogen-activated protein kinase 1 to inhibit proliferation and invasion of prostate cancer cells. Oncol Res. 2018. | ||
Xu Y, Qin S, An T, Tang Y, Huang Y, Zheng L. MiR-145 detection in urinary extracellular vesicles increase diagnostic efficiency of prostate cancer based on hydrostatic filtration dialysis method. Prostate. 2017;77(10):1167–1175. | ||
Chi Y, Ding F, Zhang W, Du L. microRNA-503 suppresses the migration, proliferation and colony formation of prostate cancer cells by targeting tumor protein D52 like 2. Exp Ther Med. 2018;15(1):473–478. | ||
Ma T, Zhao Y, Wei K, et al. MicroRNA-124 functions as a tumor suppressor by regulating CDH2 and epithelial-mesenchymal transition in non-small cell lung cancer. Cell Physiol Bioch. 2016;38(4):1563–1574. | ||
van der Horst G, Bos L, van der Mark M, et al. Targeting of alpha-v integrins reduces malignancy of bladder carcinoma. PLoS One. 2014;9(9):e108464. | ||
Markou A, Lazaridou M, Paraskevopoulos P, et al. Multiplex gene expression profiling of in vivo isolated circulating tumor cells in high-risk prostate cancer patients. Clin Chem. 2018;64(2):297–306. | ||
Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11(13):4653–4657. | ||
Erdmann K, Kaulke K, Rieger C, Salomo K, Wirth MP, Fuessel S. MiR-26a and miR-138 block the G1/S transition by targeting the cell cycle regulating network in prostate cancer cells. J Cancer Res Clin Oncol. 2016;142(11):2249–2261. | ||
Zhang Y, Zhang P, Wan X, et al. Downregulation of long non-coding RNA HCG11 predicts a poor prognosis in prostate cancer. Biomed Pharmacother. 2016;83:936–941. | ||
Corcoran C, Friel AM, Duffy MJ, Crown J, O’Driscoll L. Intracellular and extracellular microRNAs in breast cancer. Clin Chem. 2011;57(1):18–32. | ||
Dijkstra JR, Mekenkamp LJ, Teerenstra S, De Krijger I, Nagtegaal ID. MicroRNA expression in formalin-fixed paraffin embedded tissue using real time quantitative PCR: the strengths and pitfalls. J Cell Mol Med. 2012;16(4):683–690. | ||
Faltejskova P, Svoboda M, Srutova K, et al. Identification and functional screening of microRNAs highly deregulated in colorectal cancer. J Cell Mol Med. 2012;16(11):2655–2666. | ||
Slaby O, Bienertova-Vasku J, Svoboda M, Vyzula R. Genetic polymorphisms and microRNAs: new direction in molecular epidemiology of solid cancer. J Cell Mol Med. 2012;16(1):8–21. | ||
Knyazev EN, Samatov TR, Fomicheva KA, Nyushko KM, Alekseev BY, Shkurnikov MY. MicroRNA hsa-miR-4674 in hemolysis-free blood plasma is associated with distant metastases of prostatic cancer. Bull Exp Biol Med. 2016;161(1):112–115. | ||
Shkurnikov MY, Makarova YA, Knyazev EN, et al. Plasma level of hsa-miR-619-5p microRNA is associated with prostatic cancer dissemination beyond the capsule. Bull Exp Biol Med. 2017;163(4):475–477. | ||
Zhang C, Shu L, Kim H, et al. Phenethyl isothiocyanate (PEITC) suppresses prostate cancer cell invasion epigenetically through regulating microRNA-194. Mol Nutr Food Res. 2016;60(6):1427–1436. | ||
Kong Q, Chen XS, Tian T, Xia XY, Xu P. MicroRNA-194 suppresses prostate cancer migration and invasion by downregulating human nuclear distribution protein. Oncol Rep. 2017;37(2):803–812. | ||
Das R, Gregory PA, Fernandes RC, et al. MicroRNA-194 promotes prostate cancer metastasis by inhibiting SOCS2. Cancer Res. 2017;77(4):1021–1034. | ||
Muhammad N, Bhattacharya S, Steele R, Ray RB. Anti-miR-203 suppresses ER-positive breast cancer growth and stemness by targeting SOCS3. Oncotarget. 2016;7(36):58595–58605. | ||
An J, Lv W, Zhang Y. LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. OncoTargets Ther. 2017;10:5377–5390. | ||
Miao J, Wang W, Wu S, et al. miR-194 suppresses proliferation and migration and promotes apoptosis of osteosarcoma cells by targeting CDH2. Cell Physiol Biochem. 2018;45(5):1966–1974. | ||
Yang H, Wang L, Zhao J, et al. TGF-beta-activated SMAD3/4 complex transcriptionally upregulates N-cadherin expression in non-small cell lung cancer. Lung Cancer. 2015;87(3):249–257. | ||
Han K, Zhao T, Chen X, et al. microRNA-194 suppresses osteosarcoma cell proliferation and metastasis in vitro and in vivo by targeting CDH2 and IGF1R. Int J Oncol. 2014;45(4):1437–1449. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.