Back to Journals » OncoTargets and Therapy » Volume 12
MicroRNA-132 inhibits migration, invasion and epithelial-mesenchymal transition via TGFβ1/Smad2 signaling pathway in human bladder cancer
Received 16 January 2019
Accepted for publication 25 March 2019
Published 23 July 2019 Volume 2019:12 Pages 5937—5945
DOI https://doi.org/10.2147/OTT.S201731
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Xi Chao Wei,1 Zhong Hua Lv2
1Department of Urology, Jining Hospital of Traditional Chinese Medicine, Jining 272000, Shandong, People’s Republic of China; 2Department of Urology, Jining No. 1 People’s Hospital, Jining 272011, Shandong, People’s Republic of China
Background and aim: Increasing evidence shows that microRNAs play an important regulatory role in the development of several types of cancers. However, the role of microRNA-132 (miR-132) in human bladder cancer (BC) metastasis remains unclear. In this research, we aimed to investigate the effect of miR-132 on the cell migration and relate potential mechanism in BC.
Methods: miR-132 expression level was assessed by quantitative real-time PCR (qRT-PCR) in 32 BC tissues and BC cell lines (T24). The function of miR-132 was evaluated by Transwell assay. Gene expression was determined by using qRT-PCR or Western blot.
Results: The results showed that miR-132 had a lower expression in BC tissues than in adjacent normal tissues. At the same time, compared to human normal urethral epithelium cells, the expression level of miR-132 was downregulated in T24 cell lines. miR-132 overexpression significantly inhibited migration and invasion capacities in T24 cells, while downregulation of miR-132 expression strengthened such capacities. Compared with those transfected with miR-132 mimic, EMT-related markers and TGFβ1/Smad2 expression levels were higher in T24 cells transfected with miR-132 inhibitor. Moreover, EMT-related markers and Smad2 expression levels was obviously increased in BC tissues compared to the adjacent normal tissues. The correlation result indicated that the expression of miR-132 and Smad2 was reversed.
Conclusion: In short, our results suggest that miR-132 may play a suppressive role in the metastasis of BC cells via TGFβ1/Smad2 signaling pathway.
Keywords: human bladder cancer, microRNA-132, metastasis, epithelial-mesenchymal transition
Introduction
BC is one of the most common urological malignancy, and its global incidence is rising.1 Although multiple treatments have been gained, the 5-year survival rate of BC patients is still dissatisfied.2 About 33–75% of patients with BC failed to respond to therapy due to the disease relapse or metastasis.3 Biomarkers are surrogate markers of increase or decrease clinicians suspect that future clinically important events, such as cancer episodes, recurrence, progression, or patient death, will or will not occur, and/or specific treatments will reduce the risk of such events.4 Predictive markers identify patients who may benefit or progress from certain additional therapies. Therefore, researchers urgently need to understand the occurrence and progress mechanism of BC disease to make corresponding treatment plans. Owing to about 75% of BC cases are non-muscle invasive.5 Regarding prognosis, 30–80% of non-muscle invasive BC will recur and 1–45% of cases will progress to muscle invasive form within 5 years.6 The risk of recurrence and progression increases with the stage, the grade of malignancy, the size and number of lesions.7 Therefore, we should make corresponding treatment plans for BC patients. Confirmation of specific tumor suppressor gene regulation contributes substantially to the initiation, proliferation and metastasis of BC, and these findings have led researcher to explore new therapies based on targeted gene therapy for cancer.8
MiRNAs are a cluster of small endogenous noncoding RNAs composed of approximately 19~24 nucleotides that regulate target gene expression.9 MiRNAs play a considerable role in tumor cells growth, differentiation, metastasis and apoptosis.10,11 Increasing evidence has showed that miRNAs are participated in the progression of multiple types of cancers, including BC, gastric cancer, hepatocellular carcinoma and glioblastoma.12–15 According to the above research results, miRNAs are considered to be pivotal regulator of gene expression.
In human research, miR-132 has been reported to regulate the progression of cancer cells. In ovarian cancer, miR-132 can suppress the cell proliferation, migration and invasion by targeting E2F5.16 In glioma cells, miR-132 can lead to caspase-dependent apoptotic death.17 In colorectal cancer, downregulation of miR-132 expression is associated with poor prognosis.18 However, there are few studies on the regulation of miR-132 in BC, the potential molecular mechanism of miR-132 in BC progression remains largely unclear.
Previous studies have demonstrated that the TGF-β signaling pathway can promote cancer development and progression by increasing cancer cells motility, invasiveness and metastasis, and inducing epithelial-mesenchymal transition (EMT).19,20 However, previous studies have also shown that this signaling pathway has tumor-promoting activity and tumor-inhibiting activity, so its role is double-sided, depending on the stage of cancer and the cells involved. Of course, previous studies of the TGF-β signaling pathway have been known to play an important role in BC.21 At the same time, TGF-β-induced Smad signaling pathway is also in-depth in controlling tumor metastasis, increasing motility, invasiveness, and complex and diverse responses of EMT.22,23
In this study, we focus on the role of miR-132 in BC cells metastasis. The expression of miR-132 in BC tissues and T24 cells were detected. Based on the result that the mRNA levels of miR-132 in BC and BC cells were downregulated, we analyzed the role of miR-132 in BC cells metastasis and explored the potential molecular mechanism by evaluating the expression of EMT-related markers and EMT-associated signaling pathway.
Materials and methods
Tissue samples
Thirty-two BC samples were obtained from patients. This study was approved by the Ethics Committee of Shandong Jining NO.1 people’s Hospital. Written informed consents were obtained from all patients. All experiments were performed in accordance with the Declaration of Helsinki. The patients’ clinicopathological characteristics were summarized in Table 1.
 |
Table 1 Characteristic of patients with bladder cancer (BC) |
Cell culture
Human BC cell lines (T24) and human normal urothelial cell line (SV-HUC-1) were purchased from Solarbio life sciences (Beijing, China). Cells were cultured in RPMI-1640 (GIBCO BRL,Grand Island, NY, USA) medium supplemented with 10% fetal bovine serum (FBS, GIBCO BRL, Grand Island, NY, USA) and maintained in a 37 °C and 5% CO2 incubator.
Cell transfection and group
The hsa-miR-132 mimics, hsa-miR-132 inhibitor and control mimics (NC) were synthesized from Invitrogen. The sequences were as follows: miR-132 mimics: 5′-UAACAGUCUACAGCCAUGGUCG-3′; miR-132 inhibitor: 5′-CGACCAUGGCUGUAGACUGUUA-3′. Cells in the logar-ithmic growth phase were cultured and the cell confluence reached to 50%, transfection was performed by Lipofectamine 2000 (Life Technologies, Grand Island, NY, USA). Cells were separated into three groups: control mimics (miR-132 NC, cells transfected with nonsense oligonucleotide sequence), miR-132 inhibitor group (miR-132 inhibitor, cells transfected with miR-132 inhibitor), miR-132 mimic group(miR-132 mimic, cells transfected with miR-132 mimic). The transfection efficiency was determined by qRT-PCR.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNAs were obtained from BC tissues and BC cells using TRIzol reagent (Solarbio, Beijing, China). Reverse transcription and qRT-PCR for miR-132 was performed by using miRNAs qRT-PCR kits (Genep-harma, Shanghai, China). Reverse transcription and qRT-PCR for EMT-related markers and Smad2 were performed using PrimeScript™ RT Master Mix and the SYBR® Premix Ex Taq™ II kit (TaKaRa Bio, Otsu, Shiga, Japan). The primers used were as follows: N-Cadherin, sense:5ʹ-TTCCATCCTGCGCGTGAAG-3ʹ, non-sense: 5ʹ-CGGCGTTTCATCCATACCACA-3ʹ; ZEB1, sense: 5ʹ-GCAGATGAAGCAGGATGTAC-3ʹ, non-sense:5ʹ-TCCATTTTCATCATGACCACT-3ʹ; SNAIL, sense: 5ʹ-GAGTGGTTCTTCTGCGCTAC-3ʹ, non-sense:5ʹ-TCCAGAGTTTACCTTCCAGCAG-3ʹ; Vimentin, sense: 5ʹ-ATTGCAGGAGGAGATGCTTC A-3ʹ, non-sense: 5ʹ-GGAT TTCCTCTTCGTGGAGTT-3ʹ; SMAD2, sense: 5ʹ-GACACACCGAGATCCTAACA-3ʹ, non-sense: 5ʹ-GAGAGCCTGTG TCCATACTTT-3ʹ; GAPDH, sense: 5ʹ-AATGAATGGGCAGCCGTTAG GA-3ʹ, non-sense: TCTGATTTGGTCGTATTGGGCG-3ʹ. Bar diagram presented the relative expression normalized to U6 and GAPDH, respectively. The results were figured up using the 2−ΔΔCt method.
Scratch wound-healing assay
Single layer cells were scratched by a sterile pipette tip (200μl). Then, cells were flushed two times with 0.01M PBS, and subjected to a serum‐free RPMI-1640 medium for 24h. Scratched areas were photoed by a microscope (Olympus, Tokyo, Japan). Finally, the closure percentage were evaluated using the ImageJ software.
Cell invasion assay
Transwell assay was utilized to observe the invasive property of T24 cells. The post-transfected cells were plated into the upper chamber, whereas the RPMI 1640 medium consisting of 10% FBS was supplemented to the lower chamber. After incubation for 24 h, the cells remaining on the upper membrane were discarded with cotton swab. The membrane was fixed in 4% polyformaldehyde and stained with 0.1% crystal violet for 15 min at 4 °C. Finally, invaded cells number were counted using photographic images.
Western blot analysis
Cells were lysed with an RIPA lysis buffer (Solar life science, Beijing, China). Protein concentration was quantified by BCA Assay kit (Solar life science, Beijing, China). Proteins (100 μg) for each cell lysate sample were separated by 10% SDS-PAGE, which were then shifted to PVDF membrane (Bio-Rad, Hercules, CA). 5% BSA solution was used to block the membrane for 1h in room temperature. The primary antibodies were as follows: rabbit antibodies against TGFβ1 (1:1000; ab92486), Smad2 (1:800; ab63576), p-Smad2 (1:800; ab53100) and β-actin (1:1000; ab8227; Abcam, Cambridge, MA, UK) were incubated overnight at 4 °C. After washing in TBS containing Tween-20 (TBST) for 15 min, the membrane was incubated with HRP-labeled goat anti-rabbit (IgG-HRP, 1:1000; ab6721; Abcam, Cambridge, MA,UK) at room temperature for 2h. After washing in TBST for 15 min, chemiluminescent detection was performed using a Novex™ ECL Chemiluminescent Substrate Reagent kit (Thermo Fisher Scientific, Inc.). Protein expression level was detected using Bio-Rad Gel Doc XR + system (Bio-Rad, Hercules, CA, USA) and is presented as the density ratio versus β-actin.
Statistical analysis
Statistical analysis was performed using SPSS18.0 software (SPSS Inc., Chicago, IL, USA). The data were presented as mean ± standard deviations (SD). Student’s t-test was used to determine the statistical significance between the groups. Two-tailed Pearson’s correlation was used to assess the relationship between miR-132 and SMAD2 expressions. P<0.05 was considered significant difference.
Results
miR-132 expression was downregulated in BC tissues and cells
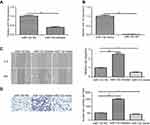
Our finding shown that miR-132 expression level was significantly lower in BC tissues by comparsion with the matched adjacent normal tissues by qRT-PCR (p<0.01; Figure 1A). Furthermore, we studied T24 cell line and SV-HUC-1, the result shown that the mRNA level of miR-132 was also remarkably lower in T24 cells compared with SV-HUC-1 (p<0.01; Figure 1B). These data suggest that downregulation of miR-132 may be related to BC or BC cells metastasis.
miR-132 inhibited the migration and invasion of BC cells
The miR-132 level of human T24 cells transfected with mimic control, miR-132 mimic or inhibitor was determined by qRT-PCR. Figure 2A and B showed that miR-132 expression in T24 cells transfected with miR-132 inhibitor was significantly decreased while transfection of miR-132 mimic remarkably increased the miR-132 level, compared to the NC cells. Next, we investigated the effect of miR-132 on metastasis of T24 cells by wound-healing assay and transwell assay. Scratch assay and transwell assay results showed that overexpression of miR-132 significantly inhibited T24 cells migration and invasion by contrast with the control group (p<0.01), while miR-132 expression inhibition would occur the opposite effect (Figure 2C and D). The results demonstrate that miR-132 can suppress the migration and invasion of T24 cells in vitro.
miR-132 decreased the expression of EMT-related markers
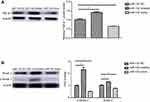
To explore whether overexpression of miR-132 changes EMT, we examined the expression of EMT-related markers in T24 cell lines. The data revealed that miR-132 inhibition dramatically promoted the expression of EMT-related markers (N-Cadherin, Zeb1, Snail and Vimentin). Overexpression of miR-132 can significantly inhibit the expression of EMT-related markers (p<0.05; Figure 3). These results suggest that miR-132 expression involves in EMT in BC cells.
miR-132 downregulation promoted TGFβ1, Smad2 and p-Smad2 expression in BC cell lines
To explore the potential mechanism of the EMT inhibition by miR-132 in BC cells, we have examined TGFβ1/Smad2 expression by Western blot. The results were shown that the expression of TGF-β1, Smad2 and p-Smad2 was significantly increased in T24 cells transfected with miR-132 inhibitor compared with miR-132 NC cells. Consistently, TGFβ1/Smad2 expression was lower in T24 cells transfected with miR-132 mimic than that in NC cells (Figure 4A and B). The results indicate that TGFβ1/Smad2 signaling pathway maybe promote the EMT in BC cells metastasis induced by miR-132.
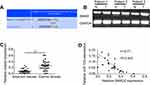
Correlation of the expression of SMAD2 and miR-132 in BC tissues
As a type of conservative miR-132, miR-132-3p is predicted can combine to the 3′UTR of Smad2 on www.targetscan.org (Figure 5A). To further determine the association between Smad2 and miR-132, the expression of SMAD2 in BC tissues was tested by qRT-PCR. The results showed that the expression of SMAD2 was increased in the tumor tissue (Figure 5B). The results revealed that expression of SMAD2 was significantly increased in BC tissues (p<0.01; Figure 5C).
Pearson’s correlation analysis displayed the negative correlation between SMAD2 expression and miR-132 expression (r =−0.71, p<0.001; Figure 5D). The results indicate that SMAD2 maybe one of the target proteins of miR-132 in BC metastasis.
Discussion
Dysregulation of miRNAs was reported involves in many human tumor types, including BC. We detected miR-132 expression and demonstrated the miR-132 role in cells metastasis. Then we detected the EMT-related markers expression in miR-132 overexpression and downregulation of BC cells. Further, TGFβ1/Smad2 changes were explored in BC cells and correlation of SMAD2 and miR-132 was evaluated in BC tissues. Our data showed that the expression level of miR-132 was significantly reduced in BC tissues with lymph node metastasis and BC cells. The migration and invasion capability, EMT-related markers expression and TGFβ1/Smad2 expression were increased in miR-132 inhibited BC cells compared to miR-132 NC cells, and the opposite results were observed in overexpression of miR-132 BC cells. We found a negative correlation between the expression of SMAD2 and miR-132 in BC tissues.
Tumor metastasis is known as a common cause of lethality in cancer including BC. Patients with metastasis in BC cells often have a poor prognosis and high mortality.24 Therefore, the identification of biomarkers for BC with metastasis was very important. In our study, we found miR-132 was significantly reduced in the BC with lymph node metastasis. And cancer cells metastasis capability was increased when inhibiting miR-132 expression. The result was consistent with previous studies,25 suggesting that downregulation of miR-132 may be considered as a specific marker for early progression and prognosis of BC with lymph node metastasis.
EMT is one of the major molecular mechanisms promoting cancer metastasis.26 In the EMT process, the epithelial cells gain the morpho-logy of the mesenchymal cells and gene expression pattern.27 Micro-RNAs can induce EMT in cancer cells in many studies: miR-218 can inhibit EMT and invasion in cervical cancer;28 miR-139-5p was signifi- cantly correlated with the metastasis potential and drug resistance of colon cancer cells by affecting EMT;29 MiR-30a is an important miRNA modulating EMT and cisplatin sensitivity in gastric cancer cells;30 MiR-181a upregulation is associated with EMT in ovarian cancer cells.31
In our study, we first found the expression of mesenchymal cell markers (N-Cadherin, Zeb1, Snail and Vimentin) were suppressed in miR-132 overexpression BC cells, that is, miR-132 can inhibit EMT in BC cells. TGF-β1/Smad signaling is an important regulating pathway of EMT and TGF-β1 induced EMT plays an important role in BC cells invasion. Based on the EMT-related markers results, we further explored the TGF-β1/Smad2 signaling in BC cells to clarify the mechanism of EMT-inducing by miR-132. TGF-β1, Smad2 and p-Smad2 were increased in miR-132 downregulated BC cells. As one target of miR-132, we observed a significant reverse correlation between SMAD2 and miR-132 levels in BC tissues and the results consistent with theoretical speculation. Thus, Smad2 and phosphorylation of Smad2 may be regulated by the expression of miR-132, then the expression of N-Cadherin, Zeb1, Snail and Vimentin were induced, which would promote EMT and BC metastasis. Therefore, our results indicated that miR-132 significantly inhibited the EMT in BC metastasis partly at least via TGFβ1/Smad2 signaling pathway.
In conclusion, miR-132 may play a suppressive role in the metastasis of BC cells by promoting EMT via TGFβ1/Smad2 signaling pathway.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Wu J, Wang J. HMGN5 expression in bladder cancer tissue and its role on prognosis. Eur Rev Med Pharmacol Sci. 2018;22(4):970–975. doi:10.26355/eurrev_201802_14378
2. Hirata H, Hinoda Y, Ueno K, Shahryari V, Tabatabai ZL, Dahiya R. MicroRNA-1826 targets VEGFC, beta-catenin (CTNNB1) and MEK1 (MAP2K1) in human bladder cancer. Carcinogenesis. 2012;33(1):41–48. doi:10.1093/carcin/bgr239
3. Chamie K, Litwin MS, Bassett JC, et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013;119(17):3219–3227. doi:10.1002/cncr.28147
4. Sturgeon CM, Duffy MJ, Stenman UH, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008;54(12):e11–e79. doi:10.1373/clinchem.2008.105601
5. Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66(Suppl 6A):4–34. doi:10.1016/j.urology.2005.07.062
6. van Rhijn BW, Burger M, Lotan Y, et al. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol. 2009;56(3):430–442. doi:10.1016/j.eururo.2009.06.028
7. Losa A, Hurle R, Lembo A. Low dose bacillus Calmette-Guerin for carcinoma in situ of the bladder: long-term results. J Urol. 2000;163(1):68–71.
8. Wu CL, Ho JY, Chou SC, Yu DS. MiR-429 reverses epithelial-mesenchymal transition by restoring E-cadherin expression in bladder cancer. Oncotarget. 2016;7(18):26593–26603. doi:10.18632/oncotarget.8557
9. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi:10.1016/j.cell.2004.12.035
10. Yu X, Li Z, Shen J, et al. MicroRNA-10b promotes nucleus pulposus cell proliferation through RhoC-Akt pathway by targeting HOXD10 in intervetebral disc degeneration. PLoS One. 2013;8(12):e83080. doi:10.1371/journal.pone.0083080
11. Yeung ML, Jeang KT. MicroRNAs and cancer therapeutics. Pharm Res. 2011;28(12):3043–3049. doi:10.1007/s11095-011-0526-2
12. Vinall RL, Ripoll AZ, Wang S, Pan CX, deVere White RW. MiR-34a chemosensitizes bladder cancer cells to cisplatin treatment regardless of p53-Rb pathway status. Int J Cancer. 2012;130(11):2526–2538. doi:10.1002/ijc.26256
13. Kurashige J, Kamohara H, Watanabe M, et al. MicroRNA-200b regulates cell proliferation, invasion, and migration by directly targeting ZEB2 in gastric carcinoma. Ann Surg Oncol. 2012;19(Suppl 3):S656–S664. doi:10.1245/s10434-012-2217-6
14. Zhang X, Hu S, Zhang X, et al. MicroRNA-7 arrests cell cycle in G1 phase by directly targeting CCNE1 in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2014;443(3):1078–1084. doi:10.1016/j.bbrc.2013.12.095
15. Huber RM, Rajski M, Sivasankaran B, Moncayo G, Hemmings BA, Merlo A. Deltex-1 activates mitotic signaling and proliferation and increases the clonogenic and invasive potential of U373 and LN18 glioblastoma cells and correlates with patient survival. PLoS One. 2013;8(2):e57793. doi:10.1371/journal.pone.0057793
16. Tian T, Hou L, Xiong YM, et al. miR-132 targeting E2F5 suppresses cell proliferation, invasion, migration in ovarian cancer cells. Am J Transl Res. 2016;8(3):1492–1501.
17. Li Y, Zhang J, He J, Zhou W, Xiang G, Xu R. MicroRNA-132 cause apoptosis of glioma cells through blockade of the SREBP-1c metabolic pathway related to SIRT1. Biomed Pharmacother. 2016;78:177–184. doi:10.1016/j.biopha.2016.01.022
18. Mokutani Y, Uemura M, Munakata K, et al. Down-regulation of microRNA-132 is associated with poor prognosis of colorectal cancer. Ann Surg Oncol. 2016;12:23.
19. Akhurst RJ, Derynck R. TGF-beta signaling in cancer-a double- edged sword. Trends Cell Biol. 2001;11(11):S44–S51.
20. Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29(2):117–129. doi:10.1038/ng1001-117
21. Wei H, Kamat AM, Aldousari S, et al. Genetic variations in the transforming growth factor beta pathway as predictors of bladder cancer risk. PLoS One. 2012;7(12):e51758. doi:10.1371/journal.pone.0051758
22. Pardali K, Moustakas A. Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007;1775(1):21–62. doi:10.1016/j.bbcan.2006.06.004
23. Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12(1):22–29.
24. Zhang Q, Miao S, Han X, et al. MicroRNA-3619-5p suppresses bladder carcinoma progression by directly targeting β-catenin and CDK2 and activating p21. Cell Death Dis. 2018;9(10):960. doi:10.1038/s41419-018-1111-y
25. Abba M, Patil N, Leupold JH, Allgayer H. MicroRNAs from metastasis prediction to metastasis prevention? Mol Cell Oncol. 2015;3(2):e1074336. doi:10.1080/23723556.2015.1074336
26. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
27. Chen W, Zhao K, Miao C, et al. Silencing Trim59 inhibits invasion/migration and epithelial-to-mesenchymal transition via TGF-β/smad 2/3 signaling pathway in bladder cancer cells. OncoTargets Ther. 2017;10:1503–1512. doi:10.2147/OTT.S130139
28. Jiang Z, Song Q, Zeng R, et al. MicroRNA-218 inhibits EMT, migration and invasion by targeting SFMBT1 and DCUN1D1 in cervical cancer. Oncotarget. 2016;7:29. doi:10.18632/oncotarget.9850.
29. Li Q, Liang X, Wang Y, et al. miR-139-5p inhibits the epithelial-mesenchymal transition and enhances the chemotherapeutic sensitivity of colorectal cancer cells by down- regulating BCL2. Sci Rep. 2016;6:27157. doi:10.1038/srep27157
30. Wang LL, Zhang XH, Zhang X, Chu JK. MiR-30a increases cisplatin sensitivity of gastric cancer cells through suppressing epithelial-to-mesenchymal transition(EMT). Eur Rev Med Pharmacol Sci. 2016;20(9):1733–1739.
31. Li L, Xu QH, Dong YH, et al. MiR-181a upregulation is associated with epithelial-to-mesenchymal transition (EMT) and multidrug resistance (MDR) of ovarian cancer cells. Eur Rev Med Pharmacol Sci. 2016;20(10):2004–2010.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.