Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 14
Micronutrient Deficiencies in Systemic Sclerosis: A Scoping Review
Authors Nguyen AD, McMahan ZH, Volkmann ER
Received 24 December 2021
Accepted for publication 15 April 2022
Published 17 December 2022 Volume 2022:14 Pages 309—327
DOI https://doi.org/10.2147/OARRR.S354736
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Chuan-Ju Liu
Audrey D Nguyen,1 Zsuzsanna H McMahan,2 Elizabeth R Volkmann1
1Division of Rheumatology, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; 2Division of Rheumatology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Correspondence: Elizabeth R Volkmann, Division of Rheumatology, Department of Medicine, David Geffen School of Medicine at UCLA, 1000 Veteran Ave, Ste 32-59, Los Angeles, CA, 90095, USA, Tel +1 310-825-2448, Email [email protected]
Purpose: The primary aim is to identify the micronutrient deficiencies commonly reported in SSc. The exploratory aim is to evaluate associations between micronutrient deficiencies and SSc clinical manifestations.
Patient and Methods: We conducted a scoping review of all published reports on SSc and nutrition in PubMed from its inception to August 2020. Clinical trials, observational studies, meta-analyses, and case series (with ≥ 20 cases) containing data on nutritional deficiency and SSc were included. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for reporting our findings. Two reviewers (ADN and ERV) studied the titles and abstracts of all search results with pre-specified inclusion and exclusion criteria.
Results: Among 790 retrieved publications, 35 full-length articles and 3 abstracts met the inclusion/exclusion criteria. Included studies took place across multiple geographic locations and included patients with both diffuse and limited cutaneous SSc. Vitamin D deficiency was the most commonly reported deficiency described in SSc, followed by vitamin B12, vitamin B9, selenium, zinc, and iron. In addition, some small studies found deficiencies in vitamins B1, B6, C, E, and A. While some studies reported associations between specific micronutrient deficiencies and SSc disease features (eg, interstitial lung disease was commonly associated with vitamin D deficiency and elevated homocysteine [Hcy]), the evidence to support these associations was not robust.
Conclusion: Micronutrient deficiencies are common in SSc and are associated with specific SSc features. Routine screening for micronutrient deficiencies may lead to early detection of malnutrition. Future studies are needed to understand how interventions to replete micronutrient deficiencies affect patient outcomes in SSc.
Keywords: systemic sclerosis, micronutrient deficiency, vitamin deficiency, malnutrition, nutritional deficiency
Plain Language Summary
Deficiencies in micronutrients, which generally include vitamins and minerals, are a hallmark of malnutrition in systemic sclerosis (SSc). Previous studies have shown that nutritional decline occurs in SSc and is associated with disease morbidity, including decreased quality of life. To our knowledge, no studies have systematically explored the literature to determine the most common micronutrient deficiencies in SSc. The primary aim of this scoping review is to therefore identify the micronutrient deficiencies present in patients with SSc. The exploratory aim is to evaluate associations between micronutrient deficiencies and SSc clinical features. Thirty-eight articles met eligibility criteria for full-text review. This review found that micronutrient deficiencies occur commonly in patients with SSc. Vitamin D deficiency was the most reported deficiency described, followed by vitamin B12, B9, selenium, zinc, and iron. In addition, micronutrient deficiencies can exist across different SSc subgroups (eg, limited versus diffuse cutaneous disease) and in patients with both early- and late-stage disease. Routine screening for micronutrient deficiencies may lead to early detection of malnutrition. Future studies are needed to explore the relationship between nutritional status and SSc features, as well as how interventions to replete micronutrient deficiencies can affect patient outcomes in SSc.
Introduction
Systemic sclerosis (SSc) is a rare and incurable autoimmune disease with the highest cause-specific mortality of all connective tissue diseases.1,2 After the skin, the gastrointestinal tract (GIT) is the most commonly affected internal organ in SSc3 and a leading cause of morbidity and mortality.4,5 The spectrum of GIT involvement varies in SSc, with some patients reporting no significant symptoms and others presenting with disabling and life-altering symptoms.5,6 Over 90% of the patients will report some GIT symptoms during the SSc disease course, and while the precise etiology of SSc-GIT involvement is not fully understood, dysmotility is the predominant clinical feature.7 Deemed to result from a complex interplay between perturbations in the GIT vasculature, autonomic nervous system and immune system, GIT dysfunction in SSc may also be associated with changes in the gut microbiota.8
Among the various SSc-GIT manifestations, malnutrition is a serious complication that can affect all aspects of a patient’s health, from impaired wound healing to inability to maintain life-sustaining weight. Studies suggest that the prevalence of malnutrition in SSc ranges from 10% to 21% and is more likely to be associated with disease activity than nutritional consumption.9 Up to 18% of the SSc patients may be at risk for malnutrition, and contributing factors to malnutrition in SSc are poorly understood.10,11 Moreover, among SSc patients with diffuse cutaneous disease and severe GIT involvement, including malabsorption syndromes and problems requiring hyperalimentation, only 15% survive beyond 9 years after disease onset.12 In addition, numerous studies have demonstrated that GIT symptoms contribute to decreased quality of life in patients with SSc.3,13–15
Micronutrient deficiencies are a hallmark of malnutrition and can adversely affect immune function and homeostasis in SSc.16 Micronutrients generally include vitamins and minerals,17 in contrast to macronutrients, which are defined as carbohydrates, fats, and proteins.18 Henceforth, we will refer to micronutrients in reference to vitamins and minerals only. In SSc, the severity and extent of micronutrient deficiencies remain unclear. It is unknown whether these deficiencies arise from decreased oral intake, depression, underlying SSc-related gastrointestinal disease, medications, and/or other factors. To our knowledge, no studies have systematically probed the literature to identify the types of SSc patients at greater risk of malnutrition and the most common deficiencies in SSc along with their associated clinical manifestations. For example, it is unclear whether SSc patients with interstitial lung disease (ILD), have specific micronutrient deficiencies compared to patients without ILD.
To that end, the primary aim of this scoping literature review is to identify micronutrient deficiencies in SSc. The exploratory aim is to evaluate associations between micronutrient deficiencies and SSc clinical features, including SSc subtype, severity, and clinical manifestations (eg, ILD, pulmonary hypertension [PH], etc). Understanding micronutrient deficiencies in patients with SSc may help guide clinicians about appropriate nutritional screening and management of SSc patients.
Materials and Methods
We conducted a scoping review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for scoping reviews (Supplementary Table S1).19 We chose to conduct a scoping review with the intention of identifying all available literature to better inform patients and providers of relevant nutritional considerations in patients with SSc.
Study Eligibility
Study inclusion and exclusion criteria were formulated using the PICOS framework (population, intervention, comparisons, outcomes, and study settings and designs) (Table 1).20 The following inclusion criteria was used: published original articles (including meta-analyses) that discussed SSc and nutrition, vitamins and/or diet. Our exclusion criteria consisted of review articles (except for meta-analyses), letters, editorials, case reports or studies with <20 patients, opinion articles, and abstracts lacking a published full article.
 |
Table 1 PICOS Structure for Study Inclusion and Exclusion |
Data Sources
Studies were selected for initial review via searching of the electronic databases PubMed/MEDLINE for articles published prior to August 2020. Studies were also selected for initial review by reviewing the references of retrieved articles. A comprehensive search strategy involving search terms derived from PICOS concepts was used. Keyword search terms included “scleroderma” OR “systemic sclerosis” AND “diet”; “vitamins” OR “vitamin deficiency”; “nutrients” OR “nutrition” OR “nutrient deficiency”. No PubMed filters or limits were applied to maintain a broad search strategy. To broaden the scope, we also conducted a manual search through references listed in relevant studies or review articles on nutrition and SSc to identify additional relevant articles for further consideration based on title.
Data Abstraction and Synthesis
The following information was collected from included studies: study characteristics (authors, year of publication, study design and location, sample size); type of nutrients investigated; SSc subtype (limited and/or diffuse); mean or median nutrient levels and proportion of study population that was nutrient deficient among study groups (eg, patients, healthy controls); and major clinical manifestations grouped by general organ system (eg, skin, cardiovascular, pulmonary, gastrointestinal, musculoskeletal, miscellaneous). Data were synthesized according to the type of nutrient deficiencies reported, the number of included studies that found a nutrient deficiency in their SSc cohort, and the clinical correlates and SSc-related manifestations frequently associated with particular nutrient deficiencies. Deficiencies in the following nutrients and their associated manifestations were evaluated from the selected studies: vitamins D, B12, C, E, B6, B1, and B9; homocysteine (Hcy); and minerals including iron, zinc (Zn), copper (Cu), and selenium (Se).
Results
Search Results
Search of electronic databases yielded 782 articles published between 1946 and 2020 for review of titles and abstracts (Figure 1). Eight additional articles were identified from other sources, including references of included studies or suggested articles by PubMed. Sixty articles met the inclusion criteria based on title and abstract review. These eligible articles were reviewed by two reviewers (ERV and ADN) for relevance based on title and abstract. The search culminated in 38 total articles meeting eligibility for full-text review, among which 35 had a full-text that could be accessed by the study authors. Three had published a full-text; however, the full-text could not be accessed by the authors, and thus, only the abstracts were reviewed for these three studies.
 |
Figure 1 Article selection for scoping review. |
The 38 reviewed studies took place across multiple geographic locations and consisted of case–control, retrospective cohort, and prospective cohort study designs. The total number of included patients in the reviewed studies combined was 3732, with both diffuse and limited SSc subtypes represented.
Vitamin D
Vitamin D is a fat-soluble vitamin absorbed in the small intestine from dietary consumption and produced in the skin upon absorption of ultraviolet B radiation. In homeostasis, vitamin D functions to promote gastrointestinal absorption of calcium and phosphorus and renal absorption of calcium. Studies have demonstrated deficiencies in vitamin D in patients with various autoimmune diseases.21,22 There is evidence that vitamin D plays a key role in bone mineralization and immune function.23
For SSc, the majority of reviewed studies investigated associations between vitamin D and SSc (23 studies included for full-text analysis) (Table 2). Most studies defined normal vitamin D levels as >30 ng/mL, insufficiency as 10–30 ng/mL, and deficiency as <10 ng/mL. Vitamin D levels were consistently reported below the normal range in SSc patients, with a higher proportion of vitamin D insufficiency or deficiency within the SSc cohorts.24–33 A number of studies also found significantly lower vitamin D levels compared with healthy controls.34–46 One of these studies reported hypovitaminosis in both SSc patients (n=64) and controls (n=35), but found that the healthy subjects still had significantly higher vitamin D levels compared with SSc patients.36
 |  |  |  |
Table 2 Characteristics of Included Studies Investigating Nutrient Levels Between SSc Patients and Healthy Controls, or Between SSc Patient Sub-Groups |
Among studies that reported serum vitamin D levels, the mean level among patients ranged from 9.8 ± 4.1 ng/mL to 27.4 ± 16.2 ng/mL24–27,31,33,37 (Table 2). The median vitamin D level ranged from 13.1 ng/mL to 43.7 ng/mL.32,33,38,42,43 Among studies reporting the prevalence of vitamin D deficiency, the actual prevalence varied widely with a median prevalence of 24.55%.24–28,30,31,33,35–39,42,43,45,47 However, it is important to note that some of these studies used different thresholds and definitions of vitamin D deficiency. In general, the threshold for vitamin D deficiency was <30 ng/mL, whereas the threshold for insufficiency was between 20 and 30 ng/mL.
Select studies evaluated the potential impact of geographic location and season on vitamin D levels. For example, two studies found no regional/latitude difference in vitamin D levels between cohorts from different countries: Italy and Belgium,24 and France and Italy.30 In fact, Vacca et al30 investigated vitamin D levels in two independent cohorts (French and Italian) and found comparably high proportions of vitamin insufficiency (>80%) in SSc patients from both regions. In contrast, one study comparing vitamin D levels in SSc patients between two regions in Spain found that SSc patients in the North of Spain had lower levels of vitamin D compared to those in the South of Spain (20.7±11.0 ng/dL vs 27.4±16.2 ng/dL).27 Another study demonstrated that vitamin D levels remain significantly lower in SSc patients compared with healthy controls across all seasons.37
B Vitamins (B12, B9, B6) and Homocysteine (Hcy)
Vitamin B12 (cobalamin), vitamin B9 (folate), and Hcy were the next most frequently explored micronutrients. These micronutrients, along with vitamin B6 (pyridoxine), are interrelated due to their roles in methionine metabolism. Vitamins B9 and B12 are both enzymatic cofactors in the synthesis of methionine from Hcy.50,51 Meanwhile, vitamin B6 is a cofactor for enzymes that convert Hcy to cysteine.51 Deficiencies in these B vitamins may therefore result in elevated Hcy levels.
In vivo, vitamin B12 forms a complex with intrinsic factor that is absorbed in the distal ileum and helps to maintain neurological function.50 Serum vitamin B12 >300 pg/mL is typically considered normal, 200–300 pg/mL borderline, and <200 pg/mL defines deficiency.52 Among the reviewed SSc studies, however, thresholds for vitamin B12 deficiency varied. The average vitamin B12 levels in SSc patients ranged from 197.90 ± 60.68 to 323.6 ± 291.5 pg/mL,53,54 while the proportion of patients with vitamin B12 deficiency ranged from 13.2% to 71% (Table 2).54,55 One study further demonstrated that vitamin B12 levels were lower in SSc patients compared with healthy controls.56
Few studies have evaluated the deficiency of vitamin B9 in SSc. Vitamin B9 is absorbed in the duodenum and jejunum and plays an essential role in the synthesis of DNA purines and pyrimidines. As a result, it is essential for rapidly growing cells and tissues. It is also important in maintaining proper brain function, playing a key role in mental and emotional health.57 Because vitamin B9 levels were reported in different units across studies, reference ranges were more difficult to establish (Table 2). One case–control study in Italy reported that patients with SSc have decreased vitamin B9 levels compared to healthy controls,56 while another case–control study in Iran demonstrated that serum vitamin B9 was comparable between SSc patients and healthy individuals.53 These disparities could be due to regional or dietary differences in the study population. In one study evaluating micronutrient deficiencies in SSc patients with established versus very early disease, vitamin B9 was the most frequently detected deficit, affecting 17.9% of the established group and 4.3% of the very early SSc group.55
In addition to vitamins B12 and B9, vitamin B6 was investigated in one study. Vitamin B6 is absorbed in the small intestine and catalyzes transamination and decarboxylation reactions for the synthesis of important molecules, including neurotransmitters and histamine.58 Among 82 SSc patients who underwent a micronutrient workup, vitamin B6 deficiency (≤30 nmol/L) was found in 62% of the patients.59 Further studies are needed to evaluate the relationship between vitamin B6 deficiency and SSc.
Finally, elevated Hcy was present in some study populations. Hcy is an amino acid derived primarily from methionine, and elevated Hcy levels may impair endothelial cell function and contribute to the development of vascular disease, increasing the risk of stroke and atherosclerosis, which are reported to be elevated in patients with SSc.60–62 Although definitions varied, elevated Hcy in the context of vitamin deficiency was generally defined as >15 μmol/L. The mean plasma Hcy level in SSc patients with vitamin B12 deficiency ranged from 9.2 μmol/L to 19.43 ± 7.205 μmol/L.48,53,54,63,64 Among vitamin B12-deficient patients, the proportion of elevated Hcy ranged from 9.3% to 72.7%.48,54,65 While some studies demonstrated that serum Hcy was significantly higher in SSc compared with healthy controls,48,49,54,56,66 other studies found no significant difference.63–65 In fact, Nazarinia et al53 reported Hcy to be significantly higher in controls than in SSc patients, though reasons for this finding are unclear. Therefore, the evidence for elevated Hcy in SSc is conflicting.
Other Vitamins (B1, C, E, A)
Other reported vitamin deficiencies included vitamin B1 (thiamine), vitamin C (ascorbic acid), vitamin E (alpha-tocopherol), and vitamin A (retinol). Vitamins C and E are antioxidants that have various biological functions. Vitamin C is known for its role in collagen synthesis and the maintenance of neuronal structure and neurotransmission,67 while vitamin E protects nerves from oxidative stress and preserves immune function.68 Vitamin A is essential for cell growth and reproduction, vision under poor lighting, and the maintenance of epithelial tissue to ensure barriers to infection.69 Lastly, vitamin B1 is important for maintaining the structure and function of nerves.70 These vitamins are primarily absorbed throughout the small intestine, a portion of the GIT commonly impacted by SSc.
Given the relatively few studies investigating these nutrients and varied units of measurement, the actual proportions of these vitamin deficiencies were difficult to assess (Table 2). Lower concentrations of vitamin C, vitamin E, and carotene (source of vitamin A) were reported in SSc patients compared to healthy controls in one study.71 In a study comparing SSc patients with malnutrition versus those without malnutrition (defined by the French National Health Authority statement), 73% of the SSc patients were deficient in at least one of the evaluated micronutrients (vitamin C, Se, Zn, B1, B6, B9, B12), and 68% of the non-malnourished SSc patients were still deficient in at least one nutrient.59 Thus, SSc patients may have deficiencies in numerous vitamins that affect nerve function and physiology.
Minerals
The reviewed studies investigated the following minerals: Zn, Cu, Se, and iron. Zn, Cu, and Se are absorbed primarily throughout the small intestine and are essential in maintaining cellular membranes and regulating oxygen utilization by promoting the elimination of free radicals.72 Though results are mixed, it has been shown that Se levels are altered among patients with cancer,73 thyroid disease,74,75 and cardiovascular disease,73,76 which are often reported among SSc patients.
In the reviewed studies, the prevalence of Zn deficiency ranged from 10.9% to 48%,55,59,77 with one study reporting reduced Zn levels in SSc patients with PH compared to healthy controls.77 Meanwhile, no significant difference was found in serum Cu between SSc patients and controls.77,78 Therefore, SSc patients may have Zn deficiencies, but further studies are needed to elucidate whether SSc patients have lower Zn levels compared to healthy individuals.
Interestingly, decreased Se has been reported in SSc patients compared to healthy controls.71,77,78 The prevalence of Se deficiency in SSc patients ranged from 9.1% to 41.5% across several cohorts55,59,77 (Table 2). Notably, Se levels may be related to presence of PH. Sun et al77 reported a progressive reduction in Se levels in healthy controls, SSc patients, and SSc patients with PH (who had the lowest Se levels averaged across three measures of Se). Se deficiency in SSc may thus be tied to the presence of SSc-related PH.
Iron is absorbed primarily in the duodenum and upper jejunum. In the body, iron is essential for the synthesis of oxygen transport proteins, including hemoglobin and myoglobin.79 Iron deficiency anemia is seen among patients with SSc, often in the context of abnormalities in the gut mucosa and vasculature (eg, esophageal ulcers, vascular malformations in the intestine), malignancies, and/or malnutrition due to abnormal GIT function. Although anemia is common in SSc, few studies investigated the prevalence and severity of iron deficiency among SSc patients. One study found no significant differences in serum iron between patients and healthy controls.78 Another study found that the prevalence of iron deficiency was significantly greater in SSc patients with PH compared to those without PH.80 The latter study also demonstrated increased hepcidin, a protein that regulates iron homeostasis, in the SSc cohort.80
Relationship Between Micronutrient Deficiencies and SSc Features
A summary of findings regarding the association of micronutrient deficiencies and SSc clinical manifestations is shown in Supplementary Table S2. SSc features including disease subtype, duration, and severity were examined in several of the included studies. Overall, most studies found no significant association between micronutrient deficiencies and SSc subtype or disease duration (Table 3). Specifically, vitamin D deficiency was not associated with SSc subtype (limited versus diffuse cutaneous)24,26,30,31,33,38–42,45,47 or increased disease duration.24,28,31,36,40,42 Similarly, deficiencies in vitamin B1254,55 and vitamin B948,55,71 were not found to be associated with SSc subtype or disease duration. Elevated Hcy was not found to be associated with SSc subtype,48,64,65 while the evidence for disease duration was conflicting.48,64 Deficiencies in Se, Cu, or Zn were not found to be associated with SSc subtype.55,59,77 Due to relatively few studies, there was not enough evidence to draw robust conclusions on the relationship between vitamins C, E, A and disease subtype, duration, or severity.59,71
 |
Table 3 Nutrient Deficiencies and Their Clinical Correlates in SSc |
The relationship between micronutrient deficiencies and SSc disease severity is contradictory and difficult to synthesize due to differing metrics used to assess disease severity. When disease severity was based on the presence of organ system involvement (eg, presence of cardiopulmonary manifestations), some studies reported an association between vitamin D deficiency and increased SSc-related organ involvement.26,39,45 However, when validated measures were used to assess disease severity (eg, Medsger Disease Severity Scale [DSS]), the relationship between vitamin D deficiency and disease severity was unclear.24,30,39,44
The relationship between micronutrient deficiencies and pulmonary involvement is contradictory (Table 3). Although some studies found vitamin D deficiency to be associated with ILD,24,26,30 others found no significant relationship.33,43 There is also conflicting evidence on the relationship between vitamin D deficiency and PH, with some studies reporting significance26,27,30,39,44 and others finding no significance.24,27,34,35,40,47 Similarly, there was contradictory evidence regarding Hcy and lung involvement. Some studies detected a significant association between increased Hcy and presence of ILD65 and PH,48,64 while others found no correlation between Hcy levels and lung involvement.53,63
Furthermore, there does not appear to be a significant relationship between specific micronutrient deficiencies (eg, vitamin D, Hcy) and skin involvement, including modified Rodnan skin score (mRSS),26,33,34,36,38–40,42,44,47 Raynaud phenomenon,35 digital ulcers,24,26,33,38–40,42,43,47 or calcinosis.30,44 Lastly, few studies endeavored to evaluate the relationship between micronutrient deficiency and GIT involvement. Studies did not find an association between GIT involvement and deficiencies in vitamin B12 and B953,54 or Hcy.53 For vitamin D deficiency, lower vitamin D levels correlated with higher GIT and kidney parameters of the Medsger DSS.24 However, some studies found no correlations between vitamin D levels and GIT involvement (undefined),38,40 GIT ulcers,34 or gastroesophageal reflux disease.33 Vitamin D levels were also not found to be associated with other markers of impaired gut absorption.30
Discussion
Malnutrition is a serious complication among SSc patients that impacts their disease course and quality of life, requiring some individuals to rely on total parenteral nutrition (TPN).10,15 The investigation of micronutrient deficiencies in SSc is therefore crucial in clinical management and patient outcomes. In line with previous studies on nutrient deficiencies in SSc,16,59,78,81 this scoping review found that micronutrient deficiencies commonly occur in SSc patients compared to healthy individuals. Vitamin D deficiency was the most frequently studied and reported deficiency, followed by vitamins B12, B9, Se, Zn, and iron. Micronutrient deficiencies have also been shown to exist across different SSc subgroups (eg, SSc patients with and without PH, limited versus diffuse) and in patients with both early and advanced disease.
There was limited robust evidence regarding the exploratory aim to investigate micronutrient deficiencies and associated clinical features. Deficiencies in vitamin D and Se appear to indicate greater risk of SSc-related organ involvement, such as advanced cardiopulmonary manifestations. Though evidence was contradictory, some studies reported an association between ILD and vitamin D deficiency, as well as elevated Hcy. Vitamin D could potentially affect ILD progression by exerting various anticoagulant and anti-inflammatory effects,82 while Hcy modulates reactive oxygen species that lead to endothelial dysfunction,83,84 potentially amplifying vascular abnormalities already present in the setting of SSc. In addition, iron and Se deficiency were frequently investigated in the setting of PH, suggesting the important role of nutritional antioxidants in modulating pulmonary vascular function.85,86 However, further studies are needed to investigate these associations to yield more robust conclusions.
Interestingly, evidence linking micronutrient deficiencies and GIT involvement was also limited. The lack of a strong relationship could be attributed to the chosen disease outcome measures or the sample sizes of the reviewed studies, which may not have been powered to effectively identify such associations. Still, the small bowel is commonly involved in SSc, and 20% of the patients with small bowel involvement may actually be asymptomatic.4 Moreover, a study examining abnormal GIT transit and Medsger DSS in SSc patients found that all patients requiring TPN have delayed small bowel transit.87 Since many micronutrients are absorbed in the small bowel, additional studies with increased statistical power are needed to determine whether small bowel dysfunction is associated with specific micronutrient deficiencies.
The reasons for micronutrient deficiencies in SSc are likely multifactorial. Low levels of micronutrients in SSc may occur due to reduced oral intake, malabsorption, or psychological factors. Intake of antioxidants such as vitamin C has been comparable in both SSc patients and healthy controls.88,89 However, SSc patients generally report lower intake of dietary fiber (eg, foods with coarse structure) and lower intake of vegetables and fruit.71 In addition, intestinal malabsorption due to SSc-related GIT manifestations, including small intestinal bacterial overgrowth and dysmotility secondary to fibrous infiltration of the gut wall, may contribute to poor nutrient absorption.4 Lastly, mental health may play a role in micronutrient deficiencies. One study of 98 SSc patients found greater risk of malnutrition in patients with mild-to-severe depressive symptoms and hypothesized that depression could be either causative or a consequence of malnourishment.90
Some limitations of the included studies were that most were relatively small, single-center studies that lacked validation cohorts. In addition, with the exception of vitamin D, the majority of micronutrients were investigated in only a few studies, making it difficult to establish a robust relationship between a specific micronutrient and SSc features. Moreover, thresholds used for defining micronutrient deficiencies varied in some cases, and the clinical criteria used for defining specific manifestations also varied (eg, some studies evaluated pulmonary pathology using pulmonary function tests, while others defined pulmonary involvement based on diagnostic imaging).
This scoping review has several strengths. First, the included studies occurred across different geographic regions, representing the breadth of patients with SSc and accounting for possible differences in environmental factors that may contribute to nutrient deficiencies in this disease, such as diet or sunlight exposure. Second, the included studies investigated SSc patients with different features, enabling us to evaluate the relationship between specific vitamin deficiencies and clinical phenotypes. Third, the present study employed robust methodology to conduct a comprehensive literature search using the standardized PRISMA framework and established inclusion and exclusion criteria following the predetermined PICOS structure.
There are several important clinical implications of the present findings. Because micronutrient deficiencies were found across different SSc groups, routine screening for these deficiencies may be important for the clinical care of SSc patients.59 Since testing for micronutrient deficiencies is non-invasive and highly feasible in most clinical settings, all SSc patients, regardless of their disease subtype and duration, should be routinely screened for the presence of these deficiencies. The scoping review also found that even SSc patients with early disease and without GIT symptoms are likely to have nutritional impairments. Approximately 33% of the patients with very early SSc had at least one deficiency in either Zn, Se, vitamin B9, while about half of patients with established SSc had at least one nutrient deficiency.55 Effective nutrient screening should thus be initiated early upon clinical suspicion or diagnosis, and perhaps a standardized method of identifying high-risk patients and screening for deficiencies must be further studied.
Nutritional education may also be important for patients to manage their health status. Table 4 shows common nutrient deficiencies in SSc and healthy food sources that may help guide patients and providers in supplementing these nutrients. Figure 2 shows locations along the GIT where these nutrients are mainly absorbed. Micronutrient screening and greater understanding of nutrient absorption may help with the detection and management of micronutrient deficiencies and potentially lead to earlier identification of malnutrition.
 |
Table 4 Sources for Vitamins and Nutrients Commonly Depleted in Patients with SSc |
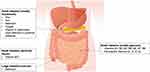 |
Figure 2 Main locations of micronutrient absorption in the gastrointestinal tract. |
Regarding future research directions, larger studies are needed to examine the nutritional etiologies and associated clinical presentations of micronutrient deficiencies in SSc. The relationship between nutritional status, diet, demographic factors, and psychological symptoms in SSc should also be more thoroughly investigated. Lastly, it is necessary to further explore how interventions to replete nutrient deficiencies may affect patient outcomes. Understanding micronutrient deficiencies not only enables more informed management of disease-related complications but also has potential to greatly improve the health and quality of life in SSc patients.
Conclusions
Micronutrient deficiencies are common in SSc and have been associated with specific SSc clinical features. Screening for micronutrient deficiencies among symptomatic patients may lead to the identification and effective management of malnutrition in SSc. Larger studies are needed to evaluate the relationship between nutritional status and SSc symptoms and to determine whether repletion of micronutrient deficiencies can affect patient outcomes in SSc.
Abbreviations
SSc, systemic sclerosis; GIT, gastrointestinal tract; Hcy, homocysteine; Zn, zinc; Cu, copper; Se, selenium; PH, pulmonary hypertension; ILD, interstitial lung disease.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from NIH/NHLBI: K23 HL150237 to ERV; NIH/NIAMS K23 AR071473, Scleroderma Research Foundation, Rheumatology Research Foundation K23 Supplement, and Jerome L Greene Foundation to ZM. The study sponsors had no role in the study design, data collection, data synthesis, and interpretation of data, nor in the writing of the report and in the decision to submit the paper for publications. None of the authors have been paid to write this article by a pharmaceutical company or other agency.
Disclosure
Elizabeth R Volkmann reports grants and personal fees from Boehringer Ingelheim, and grants from Kadmon, Horizon, Corbus, and Forbius, outside the submitted work. The authors report no other potential conflicts of interest or competing interests in this work. The abstract of this paper with interim findings was published in the “Abstract Book” of the Annual European Congress of Rheumatology EULAR 2021, an official supplement to Annals of the Rheumatic Diseases (http://dx.doi.org/10.1136/annrheumdis-2021-eular.1420)
References
1. Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–1699. doi:10.1016/S0140-6736(17)30933-9
2. Poudel DR, Derk CT. Mortality and survival in systemic sclerosis: a review of recent literature. Curr Opin Rheumatol. 2018;30(6):588–593. doi:10.1097/BOR.0000000000000551
3. Franck-Larsson K, Graf W, Rönnblom A. Lower gastrointestinal symptoms and quality of life in patients with systemic sclerosis: a population-based study. Eur J Gastroenterol Hepatol. 2009;21(2):176–182. doi:10.1097/MEG.0b013e32831dac75
4. McFarlane IM, Bhamra MS, Kreps A, et al. Gastrointestinal manifestations of systemic sclerosis. Rheumatology. 2018;8. doi:10.4172/2161-1149.1000235
5. Sallam H, McNearney TA, Chen JDZ. Systematic review: pathophysiology and management of gastrointestinal dysmotility in systemic sclerosis (scleroderma). Aliment Pharmacol Ther. 2006;23(6):691–712. doi:10.1111/j.1365-2036.2006.02804.x
6. Nagaraja V, McMahan ZH, Getzug T, Khanna D. Management of gastrointestinal involvement in scleroderma. Curr Treat Options Rheumatol. 2015;1(1):82–105. doi:10.1007/s40674-014-0005-0
7. McMahan ZH, Paik JJ, Wigley FM, Hummers LK. Determining the risk factors and clinical features associated with severe gastrointestinal dysmotility in systemic sclerosis. Arthritis Care Res. 2018;70(9):1385–1392. doi:10.1002/acr.23479
8. Volkmann ER, Chang YL, Barroso N, et al. Association of systemic sclerosis with a unique colonic microbial consortium. Arthritis Rheumatol. 2016;68(6):1483–1492. doi:10.1002/art.39572
9. Caporali R, Caccialanza R, Bonino C, et al. Disease-related malnutrition in outpatients with systemic sclerosis. Clin Nutr. 2012;31(5):666–671. doi:10.1016/j.clnu.2012.02.010
10. Harrison E, Herrick AL, McLaughlin JT, Lal S. Malnutrition in systemic sclerosis. Rheumatology. 2012;51(10):1747–1756. doi:10.1093/rheumatology/kes160
11. Baron M, Hudson M, Steele R, et al. Malnutrition is common in systemic sclerosis: results from the Canadian Scleroderma Research Group database. J Rheumatol. 2009;36(12):2737–2743. doi:10.3899/jrheum.090694
12. Steen VD, Medsger TA
13. Bodukam V, Hays RD, Maranian P, et al. Association of gastrointestinal involvement and depressive symptoms in patients with systemic sclerosis. Rheumatology. 2011;50(2):330–334. doi:10.1093/rheumatology/keq296
14. Omair MA, Lee P. Effect of gastrointestinal manifestations on quality of life in 87 consecutive patients with systemic sclerosis. J Rheumatol. 2012;39(5):992–996. doi:10.3899/jrheum.110826
15. Preis E, Franz K, Siegert E, et al. The impact of malnutrition on quality of life in patients with systemic sclerosis. Eur J Clin Nutr. 2018;72(4):504–510. doi:10.1038/s41430-018-0116-z
16. Wan YN, Yan JW, Peng WJ, et al. Micronutrients, their potential effect on patients with systemic sclerosis. Mod Rheumatol. 2014;24(5):709–714. doi:10.3109/14397595.2013.844383
17. Shenkin A. Micronutrients in health and disease. Postgrad Med J. 2006;82(971):559–567. doi:10.1136/pgmj.2006.047670
18. Carreiro AL, Dhillon J, Gordon S, et al. The macronutrients, appetite, and energy intake. Annu Rev Nutr. 2016;36(1):73–103. doi:10.1146/annurev-nutr-121415-112624
19. Moher D, Liberati A, Tetzlaff J, Altman DG; Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. PLOS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
20. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi:10.1136/bmj.b2700
21. Murdaca G, Tonacci A, Negrini S, et al. Emerging role of vitamin D in autoimmune diseases: an update on evidence and therapeutic implications. Autoimmun Rev. 2019;18(9):102350. doi:10.1016/j.autrev.2019.102350
22. Gatenby P, Lucas R, Swaminathan A, Vitamin D deficiency and risk for rheumatic diseases: an update. Curr Opin Rheumatol. 2013;25(2):184–191. doi:10.1097/BOR.0b013e32835cfc16
23. Chauhan K, Shahrokhi M, Huecker MR. Vitamin D. In: In: StatPearls [Internet]. StatPearls Publishing; 2021.
24. Trombetta AC, Smith V, Gotelli E, et al. Vitamin D deficiency and clinical correlations in systemic sclerosis patients: a retrospective analysis for possible future developments. PLoS One. 2017;12(6):1–13. doi:10.1371/journal.pone.0179062
25. Sampaio-Barros MM, Takayama L, Sampaio-Barros PD, Bonfá E, Pereira RMR. Low vitamin D serum levels in diffuse systemic sclerosis: a correlation with worst quality of life and severe capillaroscopic findings. Rev Bras Reumatol. 2016;56(4):337–344. doi:10.1016/j.rbre.2016.05.006
26. Caramaschi P, Dalla Gassa A, Ruzzenente O, et al. Very low levels of vitamin D in systemic sclerosis patients. Clin Rheumatol. 2010;29(12):1419–1425. doi:10.1007/s10067-010-1478-3
27. Rios-Fernández R, Callejas-Rubio JL, Fernández-Roldán C, et al. Bone mass and vitamin D in patients with systemic sclerosis from two Spanish regions. Clin Exp Rheumatol. 2012;30(6):905–911.
28. Braun-Moscovici Y, Furst DE, Markovits D, et al. Vitamin D, parathyroid hormone, and acroosteolysis in systemic sclerosis. J Rheumatol. 2008;35(11):2201–2205. doi:10.3899/jrheum.071171
29. Grabowski G, Grant JP. Nutritional support in patients with systemic scleroderma. J Parenter Enter Nutr. 1989;13(2):147–151. doi:10.1177/0148607189013002147
30. Vacca A, Cormier C, Piras M, Mathieu A, Kahan A, Allanore Y. Vitamin D deficiency and insufficiency in 2 independent cohorts of patients with systemic sclerosis. J Rheumatol. 2009;36(9):1924–1929. doi:10.3899/jrheum.081287
31. Giuggioli D, Colaci M, Cassone G, et al. Serum 25-OH vitamin D levels in systemic sclerosis: analysis of 140 patients and review of the literature. Clin Rheumatol. 2017;36(3):583–590. doi:10.1007/s10067-016-3535-z
32. Paolino S, Pacini G, Schenone C, et al. Nutritional status and bone microarchitecture in a cohort of systemic sclerosis patients. Nutrients. 2020;12(6):1–10. doi:10.3390/nu12061632
33. Gambichler T, Chrobok I, Höxtermann S, Kreuter A. Significantly decreased serum 25-hydroxyvitamin D levels in a large German systemic sclerosis cohort. J Rheumatol. 2011;38(11):2492LP–2493 . doi:10.3899/jrheum.110695
34. An L, Sun MH, Chen F, Li JR. Vitamin D levels in systemic sclerosis patients: a meta-analysis. Drug Des Devel Ther. 2017;11:3119–3125. doi:10.2147/DDDT.S144860
35. Zhang L, Duan Y, Zhang TP, et al. Association between the serum level of vitamin D and systemic sclerosis in a Chinese population: a case control study. Int J Rheum Dis. 2017;20(8):1002–1008. doi:10.1111/1756-185X.12794
36. Corrado A, Colia R, Mele A, et al. Relationship between body mass composition, bone mineral density, skin fibrosis and 25(OH) vitamin D serum levels in systemic sclerosis. PLoS One. 2015;10(9):e0137912–e0137912. doi:10.1371/journal.pone.0137912
37. Seriolo B, Molfetta L, Cutolo M. Seasonal variations in serum levels of 25-hydroxyvitamin D in patients with systemic sclerosis. Clin Rheumatol. 2011;30(3):445–446. doi:10.1007/s10067-011-1684-7
38. Calzolari G, Data V, Carignola R, Angeli A. Hypovitaminosis D in systemic sclerosis. J Rheumatol. 2009;36(12):2844LP–2844 . doi:10.3899/jrheum.090439
39. Groseanu L, Bojinca V, Gudu T, et al. Low vitamin D status in systemic sclerosis and the impact on disease phenotype. Eur J Rheumatol. 2016;3(2):50–55. doi:10.5152/eurjrheum.2015.0065
40. Arnson Y, Amital H, Agmon-Levin N, et al. Serum 25-OH vitamin D concentrations are linked with various clinical aspects in patients with systemic sclerosis: a retrospective cohort study and review of the literature. Autoimmun Rev. 2011;10(8):490–494. doi:10.1016/j.autrev.2011.02.002
41. Ahmadi R, Hajialilo M, Ghorbanihaghjo A, et al. FGF-23, klotho and vitamin D levels in scleroderma. Iran J Public Health. 2017;46(4):530–536.
42. Gupta S, Mahajan VK, Yadav RS, et al. Evaluation of serum vitamin D levels in patients with systemic sclerosis and healthy controls: results of a pilot study. Indian Dermatol Online J. 2018;9(4):250–255. doi:10.4103/idoj.IDOJ_328_17
43. Park EK, Park JH, Kweon SM, Kim GT, Lee SG. Vitamin D deficiency is associated with digital ulcer but not with atherosclerosis or arterial stiffness in patients with systemic sclerosis: a pilot study. Clin Rheumatol. 2017;36(6):1325–1333. doi:10.1007/s10067-017-3622-9
44. Atteritano M, Santoro D, Corallo G, et al. Skin involvement and pulmonary hypertension are associated with vitamin D insufficiency in scleroderma. Int J Mol Sci. 2016;17(12):1–8. doi:10.3390/ijms17122103
45. Ibn Yacoub Y, Amine B, Laatiris A, Wafki F, Znat F, Hajjaj-Hassouni N. Bone density in Moroccan women with systemic scleroderma and its relationships with disease-related parameters and vitamin D status. Rheumatol Int. 2012;32(10):3143–3148. doi:10.1007/s00296-011-2150-1
46. Hax V, Gasparin AA, Schneider L, et al. Vitamin D and cytokine profiles in patients with systemic sclerosis. JCR J Clin Rheumatol. 2019;1:548.
47. Caimmi C, Bertoldo E, Pozza A, et al. Vitamin D serum levels and the risk of digital ulcers in systemic sclerosis: a longitudinal study. Int J Rheum Dis. 2019;22(6):1041–1045. doi:10.1111/1756-185X.13554
48. Caramaschi P, Martinelli N, Biasi D, et al. Homocysteine plasma concentration is related to severity of lung impairment in scleroderma. J Rheumatol. 2003;30(2):298–304.
49. Zhang YJ, Zhang L, Huang XL, Duan Y, Yang LJ, Wang J. The association between homocysteine and systemic sclerosis: a review of the literature and meta-analysis. Mod Rheumatol. 2018;28(4):681–689. doi:10.1080/14397595.2017.1386844
50. O’Leary F, Samman S. Vitamin B12 in health and disease. Nutrients. 2010;2(3):299–316. doi:10.3390/nu2030299
51. Fratoni V, Brandi ML. B vitamins, homocysteine and bone health. Nutrients. 2015;7(4):2176–2192. doi:10.3390/nu7042176
52. Ankar A, Kumar A. Vitamin B12 Deficiency. In: StatPearls [Internet]. StatPearls Publishing; 2021.
53. Nazarinia M, Shams M, Sarvestani EK, Shenavande S, Khademalhosseini M, Khademalhosseini Z. Serum homocysteine level in patients with scleroderma. Iran Red Crescent Med J. 2013;15(1):29–31. doi:10.5812/ircmj.3672
54. Tas Kilic D, Akdogan A, Kilic L, et al. Evaluation of vitamin B12 deficiency and associated factors in patients with systemic sclerosis. J Clin Rheumatol. 2018;24(5):250–254. doi:10.1097/RHU.0000000000000686
55. Läubli J, Dobrota R, Maurer B, et al. Impaired micronutrients and prealbumin in patients with established and very early systemic sclerosis. Clin Exp Rheumatol. 2020;29(3):120–126.
56. Marasini B, Casari S, Bestetti A, et al. Homocysteine concentration in primary and systemic sclerosis associated Raynaud’s phenomenon. J Rheumatol. 2000;27(11):2621–2623.
57. Froese DS, Fowler B, Baumgartner MR. Vitamin B12, folate, and the methionine remethylation cycle—biochemistry, pathways, and regulation. J Inherit Metab Dis. 2019;42(4):673–685. doi:10.1002/jimd.12009
58. Abosamak N, Gupta V. Vitamin B6 (Pyridoxine). StatPearls Publishing; 2021.
59. Dupont R, Longué M, Galinier A, et al. Impact of micronutrient deficiency & malnutrition in systemic sclerosis: cohort study and literature review. Autoimmun Rev. 2018;17(11):1081–1089. doi:10.1016/j.autrev.2018.05.010
60. Kumar A, Palfrey HA, Pathak R, Kadowitz PJ, Gettys TW, Murthy SN. The metabolism and significance of homocysteine in nutrition and health. Nutr Metab (Lond). 2017;14(1):78. doi:10.1186/s12986-017-0233-z
61. Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum. 2011;63(7):2078–2090. doi:10.1002/art.30380
62. Ungprasert P, Sanguankeo A, Upala S. Risk of ischemic stroke in patients with systemic sclerosis: a systematic review and meta-analysis. Mod Rheumatol. 2016;26(1):128–131. doi:10.3109/14397595.2015.1056931
63. Nazarinia M, Zare A, Fallahi MJ, Shams M. Association of serum homocysteine level and interstitial lung disease in systemic sclerosis: a case-control study. Curr Rheumatol Rev. 2019;15(1):74–78. doi:10.2174/1573397114666180628162907
64. Szamosi S, Csiki Z, Szomják E, et al. Plasma homocysteine levels, the prevalence of methylenetetrahydrofolate reductase gene C677T polymorphism and macrovascular disorders in systemic sclerosis: risk factors for accelerated macrovascular damage? Clin Rev Allergy Immunol. 2009;36(2):145–149. doi:10.1007/s12016-008-8105-y
65. Motegi S, Toki S, Yamada K, Uchiyama A, Ishikawa O. Elevated plasma homocysteine level is possibly associated with skin sclerosis in a series of Japanese patients with systemic sclerosis. J Dermatol. 2014;41(11):986–991. doi:10.1111/1346-8138.12642
66. Caramaschi P, Canestrini S, Martinelli N, et al. Scleroderma patients nailfold videocapillaroscopic patterns are associated with disease subset and disease severity. Rheumatology. 2007;46(10):1566–1569. doi:10.1093/rheumatology/kem190
67. Figueroa-Méndez R, Rivas-Arancibia S. Vitamin C in health and disease: its role in the metabolism of cells and redox state in the brain. Front Physiol. 2015;6:397. doi:10.3389/fphys.2015.00397
68. Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51(5):1000–1013. doi:10.1016/j.freeradbiomed.2011.05.017
69. Gilbert C. What is vitamin A and why do we need it? Community Eye Health. 2013;26(84):65.
70. Frank LL. Thiamin in clinical practice. J Parenter Enter Nutr. 2015;39(5):503–520. doi:10.1177/0148607114565245
71. Lundberg AC, Akesson A, Akesson B. Dietary intake and nutritional status in patients with systemic sclerosis. Ann Rheum Dis. 1992;51(10):1143LP–1148 . doi:10.1136/ard.51.10.1143
72. Chan S, Gerson B, Subramaniam S. The role of copper, molybdenum, selenium, and zinc in nutrition and health. Clin Lab Med. 1998;18(4):673–685. doi:10.1016/S0272-2712(18)30143-4
73. Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168(4):404–410. doi:10.1001/archinternmed.2007.74
74. Rasmussen LB, Schomburg L, Köhrle J, et al. Selenium status, thyroid volume, and multiple nodule formation in an area with mild iodine deficiency. Eur J Endocrinol. 2011;164(4):585–590. doi:10.1530/EJE-10-1026
75. Drutel A, Archambeaud F, Caron P. Selenium and the thyroid gland: more good news for clinicians. Clin Endocrinol (Oxf). 2013;78(2):155–164. doi:10.1111/cen.12066
76. Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr. 2006;84(4):762–773. doi:10.1093/ajcn/84.4.762
77. Sun Q, Hackler J, Hilger J, et al. Selenium and copper as biomarkers for pulmonary arterial hypertension in systemic sclerosis. Nutrients. 2020;12(6):1894. doi:10.3390/nu12061894
78. Tikly M, Channa K, Theodorou P, Gulumian M. Lipid peroxidation and trace elements in systemic sclerosis. Clin Rheumatol. 2006;25(3):320–324. doi:10.1007/s10067-005-0013-4
79. Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19(2):164–174.
80. Ruiter G, Lanser IJ, Deman FS, et al. Iron deficiency in systemic sclerosis patients with and without pulmonary hypertension. Rheumatol. 2014;53(2):285–292. doi:10.1093/rheumatology/ket331
81. Westerman MP, Martinez RC, Medsger TA, Totten RS, Rodnan GP. Anemia and scleroderma: frequency, causes, and marrow findings. Arch Intern Med. 1968;122(1):39–42. doi:10.1001/archinte.1968.00300060041007
82. Ma D, Peng L. Vitamin D and pulmonary fibrosis: a review of molecular mechanisms. Int J Clin Exp Pathol. 2019;12(9):3171–3178.
83. Majors A, Ehrhart LA, Pezacka EH. Homocysteine as a risk factor for vascular disease. Arterioscler Thromb Vasc Biol. 1997;17(10):2074–2081. doi:10.1161/01.ATV.17.10.2074
84. Perła-Kaján J, Twardowski T, Jakubowski H. Mechanisms of homocysteine toxicity in humans. Amino Acids. 2007;32(4):561–572. doi:10.1007/s00726-006-0432-9
85. Babitt JL. Ironing out pulmonary arterial hypertension. Proc Natl Acad Sci. 2019;116(26):12604LP–12606 . doi:10.1073/pnas.1908298116
86. Ostojic P, Damjanov N. Effects of micronutrient antioxidants (alpha-tocopherol and ascorbic acid) on skin thickening and lung function in patients with early diffuse systemic sclerosis. Rheumatol Int. 2011;31(8):1051–1054. doi:10.1007/s00296-010-1398-1
87. McMahan ZH, Tucker AE, Perin J, et al. The relationship between gastrointestinal transit, Medsger GI severity, and UCLA GIT 2.0 symptoms in patients with systemic sclerosis. Arthritis Care Res. 2020. doi:10.1002/acr.24488
88. Herrick AL, Worthington H, Rieley F, et al. Dietary intake of micronutrient antioxidants in relation to blood levels in patients with systemic sclerosis. J Rheumatol. 1996;23(4):650–653.
89. Teh LS, Johns CW, Shaffer JL, et al. Ascorbic acid absorption in patients with systemic sclerosis. J Rheumatol. 1997;24(12):2353–2357.
90. Türk İ, Cüzdan N, Çiftçi V, Arslan D, Doğan MC, Unal İ. Malnutrition, associated clinical factors, and depression in systemic sclerosis: a cross-sectional study. Clin Rheumatol. 2020;39(1):57–67. doi:10.1007/s10067-019-04598-y
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
