Back to Journals » Infection and Drug Resistance » Volume 15
Microbiological Quality of Selected Local and Imported Non-Sterile Pharmaceutical Products in Dar es Salaam, Tanzania
Authors Myemba DT , Bwire GM , Sangeda RZ
Received 24 December 2021
Accepted for publication 7 April 2022
Published 21 April 2022 Volume 2022:15 Pages 2021—2034
DOI https://doi.org/10.2147/IDR.S355331
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
David T Myemba,1 George M Bwire,2 Raphael Z Sangeda2
1Department of Pharmaceutics and Pharmacy Practice, School of Pharmacy, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania; 2Department of Pharmaceutical Microbiology, School of Pharmacy, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
Correspondence: David T Myemba, Department of Pharmaceutics and Pharmacy Practice, School of Pharmacy, Muhimbili University of Health and Allied Sciences, 9 United Nations Road, Upanga West, P.O. Box 65013, Dar es Salaam, 11103, Tanzania, Tel +255767445565, Email [email protected]
Background: Pathogenic and non-pathogenic microbial contaminants can cause physical–chemical alterations of pharmaceuticals and medicine-related infections. This study aimed to examine the microbiological quality of selected local and imported non-sterile pharmaceutical products in the Dar es Salaam market and the antibiogram of the isolated microorganisms.
Methods: Samples were collected between April and June 2021 and analysed for microbial content as per the harmonised methods of the European Pharmacopoeia (EP). Antibiotic susceptibility of the microbial isolates was studied using Kirby-Bauer disc diffusion method.
Results: Fifty percent (50%) of the samples failed both bacterial and fungal enumeration tests. In this study, local products recorded lower microbial counts than imported products. Major bacterial contaminants isolated were P. aeruginosa (45.5%), S. epidermidis, (45.5%) and K. pneumoniae, while major fungal contaminants were A. flavus (58.3%), followed by A. fumigatus (25%) and Penicillium spp (16.7%). The isolated bacterial contaminants recorded high resistance levels to commonly used antibiotics.
Conclusion: The tested products were contaminated with microorganisms at different levels, most of them exceeding the maximum acceptable colony counts. Syrups or suspensions were more contaminated than tablets and capsules. The isolated bacterial contaminants were highly resistant to commonly used antibiotics.
Recommendations: We recommend that pharmaceutical manufacturers abide by good manufacturing, distribution and storage practices to limit contamination and cross-contamination of products. Responsible drug regulatory authorities should heighten the frequency of inspection of manufacturing facilities and regularly conduct post-marketing surveillance (PMS) of registered products to assess continued conformity to GMP guidelines. Future studies should involve samples collected directly from manufacturing sites.
Keywords: microbiological analysis, pharmaceutical quality, pharmaceutical analysis, pharmaceutical contamination, microbial contamination, microbial contaminants
Introduction
Contamination and cross-contamination of pharmaceutical products with microbes may pose a public health threat since microorganisms can spoil the quality of the products, in addition to the possibility of pathogenic organisms causing infections in consumers.1
Contaminants can enter a production process stream from several sources such as personnel, buildings and facilities, incoming ventilation air, machinery and other equipment for production, raw material and semi-finished material, packaging material, utilities such as water, different media used in the production process as well as for cleaning and cleanroom clothing.1,2 However, such entry can be limited by following Good Manufacturing Practices (GMP), which involves strict sanitation programs as well as prevention of contamination and cross-contamination. Furthermore, in addition to the control of manufacturing processes, strict control must be exercised during storage and distribution.1,3
Unlike parenteral products, which should be completely sterile, certain microbial levels may be tolerated for non-sterile products such as tablets, capsules, and syrups. The acceptance criteria of pharmaceuticals should be strictly maintained according to the recommended specifications given by the USP or EP.4–7 For instance, the total aerobic microbial count (TAMC) should be under 103 CFU/g and the total yeast and mould count (TYMC) should not exceed 102 CFU/g within the finished products of oral non-aqueous preparations.4–7 Likewise, the finished products of oral aqueous preparations should not go over the limit of 102 CFU/mL for TAMC and 10 CFU/mL for TYMC. In addition, Escherichia coli (E. coli) must be absent from both categories of oral preparations.5,7 Although this study focuses on oral non-sterile products, microbial limits are also set for topical semisolid products and are among the most important critical quality attributes (CQAs) emphasised by the quality-by-design (QbD) manufacturing approach for these products.8
Provided that the microenvironment within the final product is favourable, microbial contaminants can proliferate and colonize the product for a considerable amount of time until the product finally reaches the final consumer.9,10 Microbial contaminants in pharmaceutical products beyond acceptable limits have detrimental effects on both the product manufacturer and consumers. It is widely known that microbial spoilage of pharmaceutical products may result in physicochemical deterioration of both the active and inactive ingredients of the preparation. Ultimately, less effective, or toxic constituents may be formed. The presence of microbes may also have a direct hazardous effect on the consumer’s health by causing infections. Microbial contaminants, particularly on antimicrobial products, may give rise to resistant strains, contributing to antimicrobial resistance. In addition, microbial toxins also pose risks to the individual’s health.1,11 Massive outbreaks of medicine-related infections have resulted in product recalls on several occasions.12,13 The common hazardous microorganisms found in pharmaceutical products and premises include, but are not limited to, Klebsiella spp,14 Escherichia coli (E. coli), Salmonella spp., Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus (S. aureus),15 Burkholderia spp., Alcaligenes spp., Flavobacterium spp., Chromobacter spp., Serratia spp., Bacillus subtilis, Bacillus megaterium, Enterobacter aerogenes and Enterobacter cloacae, Proteus spp., Streptococcus faecalis, Clostridium spp,16 and the other opportunistic bacterial pathogens.1,17
Studies have reported contamination of pharmaceutical products before. For example, eleven years ago, a study in Tanzania by Mgoyela and Mwambete found that pharmaceutical products dispensed to patients in a public tertiary hospital were “heavily” contaminated. In that study, the predominant contaminants were Klebsiella, Bacillus, and Candida species.14 Product contamination beyond acceptable limits has also been reported in studies conducted elsewhere in Africa,15,16,18–20 Asia,17,21 and Europe.22 However, there is generally a scarcity of studies from the East African region.
It has been more than a decade since the last study in Tanzania to document contamination in pharmaceutical products. To our knowledge, no other studies were conducted before or after that study. Additionally, no comparison has been made between locally manufactured and imported products to compare conformity to good manufacturing, distribution, and storage practices. Therefore, the objective of this study was to analyse samples of selected tablets, capsules, and syrups of local and imported non-sterile pharmaceutical products for microbial quality and quantity to provide a clue about conformity to GMP guidelines during manufacturing, storage, and distribution. Furthermore, the antibiotic susceptibility profile of the isolated microorganisms was determined.
Materials and Methods
Collection of Pharmaceutical Samples
The study involved tablets, capsules and syrups commonly dispensed to patients. Commonly used analgesics, cough/cold preparations, and medicines for the treatment of erectile dysfunction were covered. In each category, both local and imported products (mainly from India) were collected. Indian products contribute most (54%) of the pharmaceutical importations into Tanzania, while the selected groups are among the fastest moving and most imported products into Tanzania.23 Samples of the selected products were collected from registered local representatives of pharmaceutical companies. Generally, one supplier is registered for every product in the market, and these are called Marketing Authorization Holders (MAH).22 To minimise chances of procuring counterfeits and the influence of storage conditions, samples were collected from reputable registered suppliers with well-established distribution chains and storage facilities. Nowadays, most products are packed mainly in blister packs and entry-resistant containers to limit the entry of microbial contaminants during transportation and handling of the products. Additionally, physical inspection of the products (including package integrity), as well as of the facilities, was conducted before collection, whereas only those samples passing physical inspection were collected in bulky. Test samples were prepared in the lab by randomly picking the bulky containers and then pooling together the contents to make up to the amount required for each test. Refer to Table 1 for sampling details.
 |
Table 1 Sampling Details |
Laboratory Procedures
All laboratory tests were conducted at the Pharmaceutical Microbiology Laboratory of MUHAS by trained personnel. Collected samples were picked randomly to make test samples, and test samples were given unique code numbers. Sample preparation and microbiological examinations were carried out as per the harmonised methods as described in the European Pharmacopeia (microbial enumeration tests,4 and tests for specified microorganisms.5) These methods were developed in co-operation with the Japanese Pharmacopoeia (JP) and the United States Pharmacopeia (USP) to achieve harmonised requirements. Similar procedures have been prescribed by the International Pharmacopeia (IP),24 and the WHO.25 Furthermore, antibiotic susceptibility testing (AST) was performed following the protocol by the National Committee for Clinical Laboratory Standards (NCCLS).26 Each microbiological assay was performed in triplicate for consistency of results and statistical purposes.
Sample Preparation
Samples were selected at random from the bulk material or the available containers of the preparation. First, tablets and capsules were carefully ground to make powders. Then, 10g (for tablets and capsules) or 10mL (for syrups and suspensions) of each sample to be examined were taken with precautions to avoid extrinsic contamination. These were dissolved in sterile sodium chloride-solution pH 7.0 to make 1:10 and 1:100 dilutions.
Examination of the Samples
Microbial Enumeration Tests
Samples were examined using a surface-spread plate-count method. Using Petri dishes, 15–20mL of liquefied nutrient agar (NA) medium (Accumix®, Microexpress®- India) for the cultivation of bacteria and a liquefied Sabouraud Dextrose Agar (SDA) medium (HIMEDIA®, HiMedia Laboratories- India) for the cultivation of fungi were added at about 45 °C to each Petri dish and allowed to solidify. The plates were dried in a hot air oven. A measured volume of 0.2 mL of the samples prepared were spread over the surface of the media. Three Petri dishes (triplicates) were used for each medium and each level of dilution. The plates were incubated at 30–35 °C and five days for bacteria and 20–25 °C, seven days for fungi unless a reliable count was obtained in a shorter time. Plates corresponding to a single dilution showing the highest number of colonies less than 300 (100 for fungi) were selected for counting. The arithmetic average of the counts was taken and the number of colony-forming units (CFU) per gram or millilitre was calculated.
Tests for Specified Microorganisms
There were separate tests to identify the presence of specific pathogenic microorganisms. Test preparations (0.2 mL) were inoculated on MacConkey Agar (MCA, Crystal Violet- and NaCl- free) (Candalab®, Laboratorios Canda- Spain). This suitable selective medium supports the growth of indicator pathogenic microorganisms such as Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Salmonella spp. These indicator organisms are associated with the most common sources of contamination in pharmaceutical production, such as contaminated water, surfaces, air and skin surfaces. Additionally, they represent the most common causes of bacterial infections with public health importance. The presence of such bacteria was confirmed by using colony morphology and specific biochemical tests. Fungal identification was done using macroscopic and microscopic features of the isolates from the Sabouraud Dextrose Agar (SDA) medium.
Antibiotic Susceptibility Testing (AST)
Antibiotic susceptibility testing was performed on Muller-Hinton Agar (MHA) using the Kirby-Bauer disc diffusion method. Individual growth colonies were transferred and sub-cultured in suitable conditions overnight.
Inoculums were prepared from the obtained pure cultures by picking 3–5 similar colonies of the isolated bacteria using a sterile loop and transferring this growth to a tube of saline. The saline tube was compared to a 0.5 McFarland turbidity standard (approx. cell density 1.5 x10^8 CFU/mL). The density of the test suspension was adjusted to that of the standard by adding more bacteria or more sterile saline. The plates were inoculated by dipping a sterile swab into the inoculum. The excess inoculum was removed by pressing and rotating the swab firmly against the side of the tube above the level of the liquid. The swab was streaked all over the surface of the medium three times, rotating the plate through an angle of 60° after each application. Finally, the swab was passed through the edge of the agar surface. The inoculum was left to dry for a few minutes (at least 3 to 5 minutes, but no more than 15 minutes) at room temperature with the lid closed.
Two classes of commonly used antibiotics (cell wall targeting antibiotics and protein synthesis inhibitors were tested for susceptibility against the isolates. Appropriate antimicrobial-impregnated disks were placed on the surface of the agar. Each disc was gently placed down to ensure complete contact with the agar surface. The plates were placed in an incubator at the appropriate temperature, time and conditions depending on the species tested. After overnight incubation, the diameter of each zone (including the diameter of the disc) was measured using a ruler on the under-surface of the plate without opening the lid and recorded in mm. Results were recorded as sensitive (S), intermediate (I), or resistant (R).
Quality Control
The American Type Culture Collection (ATCC) standard bacteria such as Escherichia coli; ATCC 25922, Pseudomonas aeruginosa; ATCC 27853, and Staphylococcus aureus: ATCC 25923, corresponding to each clinical isolate were used as control microorganisms. All reagents, equipment and apparatus used were sterilized before use, while the working surfaces were thoroughly disinfected before and after the start of each procedure.
Negative Controls
To verify testing conditions, such as to check for external contamination, negative controls were performed using the chosen diluent (sterile sodium chloride) in place of the test preparation in each set of tests. No growth of microorganisms was observed in all the negative controls.
Positive Controls
These were done to check for the growth promotion properties of the media. Each batch of the ready-prepared medium was tested for the capacity to support microbial growth. Media plates were inoculated with a small number (not more than 100 CFU) of standard microorganisms. The plates were incubated under specified conditions and observed for visible microbial growth. All positive controls showed significant microbial growth.
Interpretation of Results
Microbial Counts
The total aerobic microbial count (TAMC) was equivalent to the number of CFU found using a general medium (NA) for bacterial growth. The total combined yeasts/mould count (TYMC) was equivalent to the number of CFU found using the SDA medium. When an acceptance criterion for microbiological quality was prescribed, it was interpreted as follows:
— 101 CFU: maximum acceptable count = 20;
— 102 CFU: maximum acceptable count = 200;
— 103 CFU: maximum acceptable count = 2000, and so forth.
Further details on microbial limit specifications are shown in Table 2
 |
Table 2 Recommended Acceptance Criteria for Microbiological Quality of Non-Sterile Dosage Forms (European Pharmacopeia) |
Antibiotic Susceptibility Testing
Using the published Clinical and Laboratory Standards Institute (CLSI) guidelines (performance standards for antibiotic susceptibility testing),26 the susceptibility or resistance of the organism to each drug tested was determined. For each drug, it was indicated on the recording sheet whether the zone size was susceptible (S), intermediate (I), or resistant (R) based on the interpretation chart. In addition, the numerical value for each zone of inhibition for each isolate was also recorded.
Data Processing and Presentation
The data were processed using Microsoft Excel. Isolated MOs were reported to genus or species level, while microbial counts were reported as Colony Forming Units per gram or per mL (CFU/g or CFU/mL).
Results
Total Microbial Counts
In general, all samples analysed were found to be contaminated with microorganisms, albeit at different levels, while only one product (local cough/cold capsules) (10%) passed both tests for total aerobic microorganisms and total yeast and mould counts. Fifty percent (50%) of the samples failed both the microbial enumeration tests. In this study, local products were found to have lower microbial counts than imported products (Table 3).
 |
Table 3 Average Total Counts of the Microbial Contaminants Found in Selected Locally Produced and Imported Non-Sterile Pharmaceutical Products |
Total Aerobic Microbial Count (TAMC)
Only three out of ten products (30%) passed the microbiological tests for aerobic microorganisms. These included two locally made products (sildenafil citrate with a mean 6.0×102 CFU/g and cough/cold capsules with a mean of 3.0×102 CFU/g). The rest of the products were found to be contaminated with aerobic microbes beyond the maximum acceptable levels. Two out of the five analysed local products (40%) and just one out of five (20%) of the imported products passed the TAMC test. While some products were found to contain just marginal excess from the maximum acceptable level (MAL), for instance, the imported sildenafil citrate tablets (2.5 x 103 CFU/g, 1.25-fold from the MAL), others were found to be heavily contaminated, such as the local paracetamol and imported cough syrups (8.0 x 103 CFU/mL, 40 folds and 8.5×103 CFU/mL, 42.5 folds, respectively). Local products had lower bacterial counts than imported products. The total aerobic microbial counts for local products ranged from 3.0×102 to 8.0×103 CFU/g or mL (average 3.5×103 CFU/g or mL) while those for imported products ranged from 1.3×103 to 1.4×104 CFU/g or mL (average 6.3×103 CFU/g or mL) (Table 3).
Total Yeast and Mould Count (TYMC)
Three products (30%) passed the test for total combined yeast and mould count. Two local products (paracetamol tablets and cough/cold capsules, both with 1.5×102 CFU/g of fungi) passed the test, while only one of the imported products (sildenafil tablets with less than 1.5×102 CFU/g) passed the test. These counts are less than the 200 MAL. Tested samples exceeded the MAL by 2.25 folds (local sildenafil tablets, 4.5×102 CFU/g) to a staggering 625 folds (imported paracetamol syrups, 1.3×104 CFU/mL). Local products had lower fungal counts than imported products. The total combined yeasts and moulds counts for local products ranged from 1.5×102 to 2.4×103 CFU/g or mL (average 9.7×102 CFU/g or mL), while those for imported products ranged from 1.5×102 to 1.3×104 CFU/g or mL (average 4.8×103 CFU/g or mL) (Table 3).
Specified Microbial Contaminants
Bacterial Contaminants
Bacterial identification was done by using colony morphology, Gram staining and specified biochemical tests. In general, eleven isolates were obtained from the tested samples and majority of these were either Pseudomonas aeruginosa 5 (45.5%) or Staphylococcus epidermidis 5 (45.5%). The remaining one isolate was Klebsiella pneumoniae. Only one set of products (imported paracetamol tablets, 10%) showed no growth of colonies on a selective medium. Generally, no sample contained E. coli (Table 4).
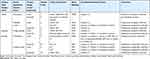 |
Table 4 Identification of Bacterial Contaminants Isolated from the Products |
Fungal Contaminants
Fungal identification was done by using macroscopic and microscopic features of the isolates. Twelve isolates were obtained, and majority were Aspergillus flavus (7, 58.3%), followed by Aspergillus fumigatus (3, 25%) and Penicillium spp (2, 16.7%). No candida spp or any other yeast cells were identified from the isolates (Table 5).
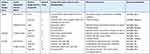 |
Table 5 Identification of Fungal Contaminants Isolated from the Products |
Microbial Quality by Dosage Forms
Compared to the non-aqueous/solid dosage forms (tablets and capsules), all the aqueous/liquid dosage forms (syrups/suspensions) failed both quality tests for bacterial and fungal counts. On average, syrups/suspensions recorded higher TAMC (6.6 x 103 versus 1.9×103) and TYMC (4.4 x 103 versus 1.9×103) than tablets and capsules. Between the solid dosage forms, tablets recorded higher TAMC (5.8 x 103 versus 7.8×102) but lower TYMC (1.6 x 103 versus 2.6×103) than capsules. All the capsule products passed the TAMC test. The solid and liquid dosage forms did not contain E. coli, but most P. aeruginosa isolates (4/5) were from tablets and capsules. Out of the seven Aspergillus flavus isolates, five (71.4%) were from the non-aqueous products, while all Aspergillus fumigatus isolates were from these products.
Antibiogram of the Isolated Pathogens
The reader is advised to read this section concurrently with Table 6. The study identified Pseudomonas aeruginosa, Staphylococcus epidermidis and Klebsiella pneumoniae as the predominant contaminants. Generally, the isolated microorganisms were highly resistant to common antibiotics.
 |
Table 6 Susceptibility Patterns (%) of the Isolated Pathogens Against Common Cell-Wall-Targeting Antibiotics and Protein Synthesis Inhibitors |
Susceptibility Profile of Cell Wall Targeting Antibiotics
About 70% and 60% of the Pseudomonas aeruginosa and Staphylococcus epidermidis isolates, respectively, were resistant against common penicillins. Although amoxicillin-clavulanic acid is commonly used today to treat Gram-positive infections, especially those caused by Staphylococcal and Streptococcal spp, the isolated Staphylococcus epidermidis worryingly displayed 80% resistance against the drug. Piperacillin showed somewhat encouraging results among the penicillins, with 60% sensitivity and 60% intermediate susceptibility towards Staphylococcus epidermidis and Pseudomonas aeruginosa, respectively.
Cephalosporins (cephems) showed the best susceptibility profile in this class. The Pseudomonas aeruginosa and Staphylococcus epidermidis isolates both showed 100% sensitivity against ceftriaxone and cefoxitin, respectively.
Vancomycin is most often used as a reserved antibiotic in our settings, and it proved to be 100% sensitive towards the isolated Staphylococcus epidermidis.
Susceptibility Profile of Protein Synthesis Inhibitors
Pseudomonas aeruginosa was 40 to 100% resistant against protein synthesis inhibitors, with 100% resistance seen against trimethoprim-sulfamethoxazole, while its greatest sensitivity was against gentamicin (60%). Similarly, Staphylococcus epidermidis displayed 40 to 100% resistance against protein synthesis inhibitors. Erythromycin is one of the most important drugs in treating skin and soft tissue staphylococcal infections, but there was 60% resistance against the drug. The highest sensitivity of the Staphylococcus epidermidis isolate was noted against nitrofurantoin (100%).
Lastly, the most resistant isolate was Klebsiella pneumoniae, with 100% resistance against almost every antibiotic tested and a slightly intermediate sensitivity seen against only one antibiotic, nalidixic acid (Table 6).
Discussion
This study found the tested pharmaceutical products to be contaminated with microorganisms, albeit at different levels, with only one set of products (local cough/cold capsules) (10%) passing both tests for total aerobic microorganisms and total yeast and mould counts. The total aerobic microbial counts of up to 1.4×104 CFU/g were observed, while the total combined yeasts and moulds counts of up to 1.3×104 CFU/mL were recorded. Fifty percent (50%) of the tested products failed both of the microbial enumeration tests. Other studies have reported similar findings. A similar study in Tanzania showed that 50% of all tested products were “heavily” contaminated, with total viable counts (TVC) of up to 6×103 CFU/mL observed.14 In another study, some paediatric anti-malarial and cough preparations sold in retail outlets were found to be heavily contaminated with microbial agents, with bacterial counts as high as 2.7×107 CFU/mL reported.15 Herbal preparations are known to be prone to microbial attacks, with one study in Nigeria showing that solid and liquid herbal preparations were “heavily” contaminated with bacteria and fungi at levels far above the officially stipulated limits for oral pharmaceutical preparations.20 A string of other studies have reported remarkable deviations from the acceptable microbial limits.16,19,21,27 In contrast, a study in Poland demonstrated that the percentage of non-compliant samples was just 1.87%, with most samples passing the quality tests.22 This high level of compliance to microbiological standards might be contributed by the stringent drug regulations that are in force in Europe and the developed world. High levels of contamination are undesirable for pharmaceutical products. Microbial agents may cause physicochemical degradation of the product, causing the formation of ineffective and/or toxic by-products. Meanwhile, consumers may be affected by suffering medicine-related infections, especially when they have compromised immune functions.1,11,16 On a more serious note, contaminated medicines have resulted in mass outbreaks of infections and thus necessitated product recalls. United States Food and Drugs Authority (FDA) enforcement reports from 2012 to 2019 showed that Gram-negative bacteria were the most common microbial contaminants of non-sterile drugs in the United States. Burkholderia cepacia was the number one culprit for non-sterile drug recalls with 102 recalls, followed by Ralstonia pickettii (45 recalls) and the USP indicator, Salmonella species (28 recalls). Unidentified microbial contamination accounted for 77% of non-sterile and 87% of sterile drug recalls indicating extremely poor microbiology practices. The presence of yeast and mould was the reason for 52 recalls of sterile and non-sterile drugs, with only 12% providing any information at the genus or species level.12,13 Contaminated products, especially those with antimicrobial action, may contribute to the rise of antimicrobial resistance.
This study identified Pseudomonas aeruginosa and Staphylococcus epidermidis as the predominating contaminants of the non-sterile products tested. Pseudomonas aeruginosa is a pathogenic bacterium and can cause infections and toxin-related health problems to final consumers, especially those with unfit immune systems. Staphylococcus epidermidis is a human skin normal flora and its presence in the products might suggest a possible shedding from the personnel to the products. Although not highly pathogenic, individuals with compromised immune systems may be affected when these bacteria are consumed in large quantities. One product (a local paracetamol syrup) was found to contain Klebsiella pneumoniae. Such an organism’s presence is worrying as it might indicate that raw materials or finished products were contaminated with human digestive waste. Improper hand hygiene and sanitation could be the source. Meanwhile, Aspergillus flavus followed by Aspergillus fumigatus and Penicillium spp were suspected among the fungal isolates. No Candida spp or any other yeast cells were identified from the isolates. Reports of pathogenic bacteria being found in pharmaceutical products have been there before. A study from Tanzania found Klebsiella, Bacillus, and Candida species as predominant contaminants.14 Both Gram-positive and negative organisms were identified in an Egyptian study, with major contaminants belonging to Micrococcaceae, while other isolates contained Enterobacteriaceae and Bacillaceae.16 Similarly, human normal flora and airborne organisms (such as moulds including Aspergillus spp., Penicillium spp., Fusarium spp. and Acremonium spp) have been reported.18,21,27 This indicates irregularities during manufacturing, packaging and repackaging. Although not exhaustive, the most common hazardous microorganisms found in pharmaceutical products and premises include Escherichia coli (E. coli), Salmonella spp., Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus (S. aureus), Burkholderia spp., Alcaligenes spp., Flavobacterium spp., Chromobacter spp., Serratia spp., Bacillus subtilis, Bacillus megaterium, Enterobacter aerogenes and Enterobacter cloacae, Proteus spp., Streptococcus faecalis, Clostridium spp. and the opportunistic bacterial pathogens.15–22,27
The aqueous/liquid dosage forms (syrups/suspensions) failed both quality tests for the bacterial and fungal count in this analysis. On average, syrups/suspensions recorded higher bacterial and fungal counts than tablets and capsules. Between the solid dosage forms, tablets recorded higher bacterial but lower fungal counts than capsules. Capsule products recovered the lowest bacterial levels among the dosage forms tested. These findings are expected as aqueous products have high water activity and thus can favour the growth of microbes. The aforementioned Tanzanian study showed that glycodin® in cough syrup was the most heavily contaminated, showing a bacterial load of 6.0×103 CFU/mL.14 Similarly, an analysis of paediatric anti-malarial and cough syrups/suspensions found the total bacterial counts ranging from 6.00×102 to 2.70×106 CFU/mL.15 This was even higher than in this study, where the highest total aerobic microbial count among syrups was 8.5×103 CFU/mL. In another study in Pakistan, the highest microbial load was observed in syrups, with counts up to 8.4×106 CFU/mL recorded, while the lowest count was observed in tablets (1.5×103 CFU/g).21 Further studies have reported either higher microbial loads among syrups/suspensions followed by tablets and capsules,20,27 or more contaminated samples of liquid medications than solid medications.18,19
Generally, the isolated microbial contaminants were resistant to common cell-wall targeting antibiotics and protein synthesis inhibitors. Except for piperacillin, susceptibility for all isolates was generally poor against penicillins, including amoxicillin-clavulanic acid. Cephalosporins (cephems) showed the best susceptibility with Pseudomonas aeruginosa and Staphylococcus epidermidis, showing 100% sensitivity against ceftriaxone and cefoxitin, respectively. Vancomycin is most often used as a reserved antibiotic in our settings and it proved to be 100% sensitive towards the isolated Staphylococcus epidermidis. Regarding protein synthesis inhibitors, Pseudomonas aeruginosa showed 100% resistance against trimethoprim-sulfamethoxazole, while its lowest resistance was against gentamicin (60%). The staphylococcal isolate showed the greatest sensitivity against nitrofurantoin (100%) but lower sensitivity against erythromycin, one of the most important drugs in treating staphylococcal infections. Klebsiella pneumoniae was the most resistant isolate with 100% resistance against almost every antibiotic tested and a slightly intermediate sensitivity seen against only nalidixic acid. One report indicated that Bacillus spp isolated from pharmaceuticals were resistant to amoxicillin-clavulanic acid and cloxacillin.14 If these products end up causing medicine-related infections to consumers, such infections would indeed be challenging to treat using common antibiotics. A literature search indicated a shortage of antibiotic susceptibility patterns for pharmaceutical microbial contaminants, but similar patterns have been reported for clinical isolates. A recent report demonstrated that Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa were resistant to amoxicillin-clavulanic acid (≥90%) and third generation cephalosporins, particularly ceftazidime and ceftriaxone. In comparison, nitrofurantoin was 100% resistant against Pseudomonas aeruginosa isolates.28
In this study, local products were less contaminated than imported products on average counts. Two local products passed each of the total bacterial and fungal tests, while only one imported product passed each test. Imported products (generally from India) might have been manufactured or distributed in less controlled environments than local products. Contamination in the products tested in this study might have risen from various sources, including raw materials (particularly water and natural origin), processing, cleaning and maintenance equipment, air and the environment, personnel, and packaging materials. Although microorganisms might gain entry during distribution and storage, the fact that these products are packed in blisters and entry-resistant containers means that chances of contamination occurring at these later stages are low. However, uncontrolled storage conditions can favour the proliferation of microorganisms and thus, the influence of storage conditions at any particular stage in the distribution chain cannot be understated.
As a limitation, these results cannot ascertain whether 100% contamination occurred at the production stage because product samples were not directly collected from manufacturing sites. There are chances of microbes getting in if the products are not handled well along the distribution channel, particularly if the supply chain is long. In a measure to mitigate this limitation, samples were obtained from reputable suppliers with well-established distribution channels. For imported products, samples were procured from marketing authorisation holders (MAH), while local products were obtained from primary distribution points. In addition, samples were subjected to physical inspections before they were procured.
Conclusions
All products studied were contaminated with microorganisms, with most of the products exceeding the maximum acceptable counts. Syrups/suspensions were more contaminated than tablets and capsules. Major contaminants were identified to be Pseudomonas aeruginosa, Staphylococcus epidermidis and Klebsiella pneumoniae. The isolated contaminants were found to be highly resistant to common cell-wall targeting antibiotics and protein synthesis inhibitors. Good susceptibility was seen against piperacillin, vancomycin ceftriaxone, cefoxitin and nitrofurantoin.
Recommendations
Pharmaceutical manufacturers should follow good manufacturing, distribution, and storage practices to avoid contamination and cross-contamination of their products. Relevant medicine regulatory authorities should regularly inspect the manufacturing facilities and conduct post-marketing surveillance (PMS) of the registered products to assess conformity to GMP guidelines. Future studies should involve samples collected directly from manufacturing sites and further extend to assessing the impact of microbial contamination on pharmaceutical products, including medicine-related infections.
Data Sharing Statement
Data may be available from the corresponding author upon reasonable request.
Ethical Declaration
This study went through an ethical review process at Muhimbili University of Health and Allied Sciences (MUHAS), Research Ethics Committee (REC), and obtained an ethical clearance certification numbered MUHAS-REC-05-2021-630. All the tests and interpretations were performed by trained and experienced research staff. In addition, this research was conducted following MUHAS ethical regulations and requirements.
Acknowledgments
The authors extend special thanks to the MUHAS Pharmaceutical Microbiology Lab staff for their technical and intellectual support when conducting this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Muhimbili University of Health and Allied Sciences (MUHAS) in terms of research materials. MUHAS was not involved in the design, conductance or preparation and submission of the manuscript of the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Denyer SP, Hodges NA, Gorman SP, et al. Hugo and Russell’s Pharmaceutical Microbiology.
2. Sandle T. Cleanrooms and associated controlled environments - biocontamination control: dissecting the standard. Available from: https://www.ivtnetwork.com/article/cleanrooms-and-associated-controlled-environments-biocontamination-control-dissecting-standa.
3. WHO Expert Committee on Specifications for Pharmaceutical Preparations. Annex 2 WHO good manufacturing practices for pharmaceutical products: main principles; 2011:77–135.
4. European Pharmacopeia 2.6.12. Examination of non-sterile products (Total Viable Aerobic Count). Farmacopea europea. 2005;1:11–12.
5. European Pharmacopeia 8.0. Examination of non-sterile products: test for specified microorganisms. Test. 2010;2:167–171.
6. USP 61. Cleaning-In-Place: Dairy, Food and Beverage Operations.
7. USP 31. Microbiological examination of nonsterile products: tests for specified microorganisms. Microbiol Tests. 2009:1–5. Available from: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/generalChapter62.pdf.
8. Namjoshi S, Dabbaghi M, Roberts MS, Grice JE, Mohammed Y. Quality by design: development of the quality target product profile (QTPP) for semisolid topical products. Pharmaceutics. 2020;12(3):287. doi:10.3390/pharmaceutics12030287
9. Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:1–8. doi:10.1186/1471-2334-6-130
10. Khan JA, Khan IU, Iqbal Z, et al. Microbial spoilage, instability risk of antacid suspension in the presence of commonly used preservative system. Pak J Pharm Sci. 2015;28(5):1637–1646.
11. Kamil OH, Lupuliasa D. Modern aspects regarding the microbial spoilage of pharmaceutical products. Farmacia. 2011;59(2):133–146.
12. Jimenez L. Analysis of FDA enforcement reports (2012–2019) to determine the microbial diversity in contaminated non-sterile and sterile drugs. Am Pharm Rev. 2019;4:1–21.
13. Tavares M, Kozak M, Balola A, Sá-Correia I. Burkholderia cepacia complex bacteria: a feared contamination risk in water-based pharmaceutical products. Clin Microbiol Rev. 2020;33:e00139–19.
14. Mugoyela V, Mugoyela V, Mwambete KD. Microbial contamination of nonsterile pharmaceuticals in public hospital settings. Ther Clin Risk Manag. 2010;443. doi:10.2147/tcrm.s12253
15. Adeshina GO, Ajayi S, Onaolapo JA. Microbiological quality of some commercially available paediatric anti-malarial and cough preparations in ilorin. Nigeria. 2009;8(1):109–117.
16. Eissa ME. Distribution of bacterial contamination in non-sterile pharmaceutical materials and assessment of its risk to the health of the final consumers quantitatively. Beni-Suef Univ j Basic Appl Sci. 2016;5(3):217–230. doi:10.1016/j.bjbas.2016.08.005
17. Noor R, Zerin N, Das KK. Microbiological quality of pharmaceutical products in Bangladesh: current research perspective. Asian Pac J Trop Dis. 2015;5(4):264–270. doi:10.1016/S2222-1808(14)60781-7
18. Kilani AM, Olaifa KW. Microbiological quality of selected non-sterile pharmaceutical products sold in retail outlets in dutsinma metropolis, Katsina State, Nigeria. J Public Health Developing Countries. 2017;3(1):339–346.
19. El-Houssieny RS, Aboulwafa MM, Elkhatib WF, Hassouna NAH. Recovery and detection of microbial contaminants in some non-sterile pharmaceutical products. Arch Clin Microbiol. 2013;4(6):1–14. doi:10.3823/278
20. Esimone CO, Chah KF, Ikejide SC. Microbiological quality of herbal preparations marketed in South East Nigeria. J Nat Remedies. 2002;42:42–48.
21. Rauf A, Erum A, Noreen S, Shujaat J, Ashraf MU, Afreen S. Microbiological quality control of some non-sterile preparations commonly used in Pakistan. Pak J Pharm Sci. 2018;31:1237–1242.
22. Ratajczak M, Kubicka M, Kamińska D, Sawicka P, Długaszewska J. Microbiological quality of non-sterile pharmaceutical products. Saudi Pharm J. 2015;23(3):303–307. doi:10.1016/j.jsps.2014.11.015
23. Wande DP, Sangeda RZ, Tibalinda P, et al. Pharmaceuticals imports in Tanzania: overview of private sector market size, share, growth and projected trends to 2021. PLoS One. 2019;14(8):1–17. doi:10.1371/journal.pone.0220701
24. The International Pharmacopoeia 9th Ed. Microbiological quality of non-sterile products: recommended acceptance criteria for pharmaceutical preparations; 2019:1–2.
25. World Health Organization Expert, Preparations P, Pharmacopoeia. Supplementary information, S. 3. 7 microbiological quality of non-sterile products: recommended acceptance criteria for pharmaceutical preparations: final text for revision of the international pharmacopoeia; 2012.
26. CLSI. Performance Standards for Antimicrobial Susceptibility Testing.
27. Gad GFM, Aly RAI, Din Ashour MSE. Microbial evaluation of some non-sterile pharmaceutical preparations commonly used in the Egyptian market. Trop J Pharm Res. 2011;10(4):437–445. doi:10.4314/tjpr.v10i4.9
28. Minzi OM, Kilonzi M, Mikomangwa WP, et al. Update on bacterial and antibiotic susceptibility profiles among patients attending a tertiary referral hospital in Tanzania. J Glob Antimicrob Resist. 2021;8:87–88. doi:10.1016/j.jgar.2021.02.030
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
