Back to Journals » Drug Design, Development and Therapy » Volume 10
Methyl salicylate 2-O-β-D-lactoside alleviates the pathological progression of pristane-induced systemic lupus erythematosus-like disease in mice via suppression of inflammatory response and signal transduction
Authors He Y, Yan Y, Zhang H, Lin Y, Chen Y, Yan Y, Wu P, Fang J, Yang S, Du G
Received 7 June 2016
Accepted for publication 22 July 2016
Published 29 September 2016 Volume 2016:10 Pages 3183—3196
DOI https://doi.org/10.2147/DDDT.S114501
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Yang-Yang He,1,2,* Yu Yan,1,3,* Hui-Fang Zhang,1,3 Yi-Huang Lin,3 Yu-Cai Chen,1,3 Yi Yan,4 Ping Wu,1,3 Jian-Song Fang,5 Shu-Hui Yang,2 Guan-Hua Du1,3
1Beijing Key Laboratory of Drug Target Identification and Drug Screening, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 2State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 3State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 4Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, 5Institute of Clinical Pharmacology, Guangzhou University of Traditional Chinese Medicine, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Abstract: Systemic lupus erythematosus (SLE), with a high incidence rate and insufficient therapy worldwide, is a complex disease involving multiple organs characterized primarily by inflammation due to deposition of immunocomplexes formed by production of autoantibodies. The mechanism of SLE remains unclear, and the disease still cannot be cured. We used pristane to induce SLE in female BALB/c mice. Methyl salicylate 2-O-β-D-lactoside (MSL; 200, 400, and 800 mg/kg) was orally administered 45 days after pristane injection for 4.5 months. The results showed that MSL antagonized the increasing levels of multiple types of antibodies and cytokines in lupus mice. MSL was found to suppress joint swelling and have potent inhibitory effect on arthritis-like symptoms. MSL also significantly decreased the spleen index and expression of inflammatory markers in the lupus mice. MSL protected the kidneys of lupus mice from injury through inhibiting the expression of inflammatory cytokines and reducing the IgG and C3 immunocomplex deposits. Further Western blot assays revealed that the downregulation of the intracellular inflammatory signals of NFκB and JAK/STAT3 might be the potential molecular mechanisms of the pharmacological activity of MSL against SLE in vivo. These findings may demonstrate that MSL has the potential to be a useful and highly effective treatment for SLE.
Keywords: methyl salicylate 2-O-β-D-lactoside, systemic lupus erythematosus, inflammatory response, lupus nephritis, signal transduction
Introduction
Systemic lupus erythematosus (SLE) is an intractable, multisystemic, and relapsing autoimmune disease with multiple-organ involvement characterized primarily by the production of autoantibodies against self-antigens.1,2 Symptoms range from rather mild manifestations, such as rash or arthritis, to life-threatening end-organ manifestations. The pathogenesis of SLE includes genetic, environmental, and hormonal factors; however, the cause and mechanism remain unclear.3 It is estimated that 161,000–322,000 people in the US have been diagnosed with SLE, and more than 16,000 new cases of lupus are reported annually across the country.4,5 It is also believed that 5 million people throughout the world have a form of lupus.6 SLE not only harms people’s health and can be fatal but also significantly increases health care utilization and costs.7
Extensive studies have focused on the novel therapeutic options for SLE. Belimumab, a monoclonal antibody that targets BAFF, was the first biological agent approved and first drug approved in 55 years for the treatment of SLE by the US Food and Drug Administration (FDA).8,9 However, including biologics, the current treatment approach can only control SLE, and not cure.
The current treatment approach includes nonsteroidal anti-inflammatory drugs, steroids, antimalarials, immunosuppressive agents, and biologics. Despite novel and modified therapy having ameliorated the prognosis, a subgroup of patients do not respond to routine therapy.10 Therefore, more efficient therapy may be warranted to lower the risk of fatal outcomes and the serious side effects of the current therapies in SLE (gastrointestinal reaction, increased risk of infection, rash, elevated aminotransferase, and marrow suppression).11
Traditional Chinese medicine, as a crucial component of complementary and alternative medicine, has evolved over thousands of years with its own unique system of theories, diagnostics, and therapies.12 Methyl salicylate 2-O-β-D-lactoside (MSL; Figure 1) is a novel salicylic acid analogue extracted from the traditional Chinese herbal medicine Gaultheria yunnanensis that has been widely used for treatment of swelling and various inflammatory responses in the southern regions of the People’s Republic of China.13,14 As one of the main components with excellent anti-inflammatory activities in the herb, MSL has been confirmed to suppress inflammation response in macrophages, microglia, and astrocytes.15,16 Furthermore, in our previous studies, MSL exerted a significant antiarthritis effect, but had hardly any gastrointestinal toxicity in vivo.17 In preclinical pharmacokinetic experiments, MSL has been proved to have good absorption from the gastrointestinal tract in Canidae and primates, and its main metabolite, salicylic acid, presents good pharmacokinetic features after oral administration.18,19 It remains to be elucidated whether the novel salicylic acid analogue is efficacious for the treatment of SLE. The aim of the present study was to investigate the potential therapeutic effect of MSL on SLE, and explore the underlying mechanisms.
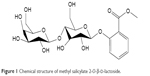  | Figure 1 Chemical structure of methyl salicylate 2-O-β-D-lactoside. |
Materials and methods
Animals
Female BALB/c mice (SPF grade, Certificate No SCXK [Beijing] 2012-0001, List No 11400700048526) aged 7–8 weeks were purchased from Vital River Laboratory Animal Technology Co (Beijing, People’s Republic of China). The mice were maintained in a barrier system with alternating 12 hour light/dark cycles, a relative humidity of 50%±5% and at a constant temperature of 25°C. All procedures involving care and use of the mice conform to the US National Institutes of Health regulations and were reviewed and approved by Chinese Academy of Medical Sciences & Peking Union Medical College Biomedical Research Ethics Committee.
Reagents and instrumentations
MSL with a high purity of 99%, was provided by the Institute of Materia Medica, Chinese Academy of Medical Sciences (Beijing, People’s Republic of China). Pristane, phenylmethanesulfonyl fluoride (PMSF), calf thymus DNA, and histone H1 protein were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Smith (Sm) antigen was purchased from RayBiotech, Inc. (Norcross, GA, USA). Prednisone with a purity of 99% was purchased from National Institutes for Food and Drug Control (Beijing, China). Mouse total immunoglobulin G (IgG), interleukin (IL)-6 tumor necrosis factor (TNF)-α, IL-17A, and monocyte chemoattractant protein-1 (MCP-1) ELISA kits were purchased from Affymetrix Ebioscience (San Diego, CA, USA). Mouse intercellular adhesion molecule-1 (ICAM-1) ELISA kit was purchased from ExCell Bio. (Shanghai, People’s Republic of China). Nucleus dye 4′,6-diamidino-2-phenylindole (DAPI) was purchased from Partec Flow Cytometry technology (Görlitz, Germany). Bead Ruptor Homogenizer was provided by Omni International Company (Kennesaw, GA, USA). Fluorescence microscope Nikon ECLIPSE Ti was purchased from Nikon Corporation (Tokyo, Japan). Laser scanning confocal imaging system was purchased from Leica Microscopy Systems Co. (Shanghai, People’s Republic of China). Radio Immunoprecipitation Assay (RIPA) lysis buffer was purchased from Cell Signaling Technology (CST) Inc. (Boston, MA, USA). Creatinine assay kit was purchased from BioSino Biotechnology & Science Inc. (Beijing, People’s Republic of China). In vivo Imaging System (FX PRO) was purchased from Carestream Health, Inc. (Rochester, NY, USA). Information regarding antibodies used in this study are listed in Table S1.
Treatments of animals
After acclimation for one week, the mice were injected with 0.5 mL of pristane or normal saline (as control group) intraperitoneally. One month after injection, serum was collected from the tail vein of the mice. Type IgG and immunoglobulin M (IgM) auto-antibodies for anti-DNA and anti-Sm, respectively, as well as cytokine IL-6 and total IgG were measured. Positive Sm-antibody or DNA-antibody, or both, demonstrated a successful mouse model after pristane treatment. The successfully induced mice at the time point of 45 days after induction were randomly divided into the following groups: 1) model group; 2) MSL low-dose group (200 mg/kg); 3) MSL medium-dose group (400 mg/kg); 4) MSL high-dose group (800 mg/kg); 5) prednisone group (5 mg/kg). The model group and control group were given appropriate volumes of vehicle with each mouse in different groups having the dose administered orally once-daily.
ELISA for anti-DNA, Sm, and histone antibodies
The auto-antibodies in the serum from each group were detected by an ELISA technique described as Bloom et al.20 96-well ELISA plates were coated with calf thymus DNA (5 μg/mL), Sm antigen (0.5 μg/mL), or histone H1 protein (0.5 μg/mL) overnight at 4°C and preblocked with 2% BSA at room temperature (RT) for 2 hours. After three washes, wells were incubated for 3 hours with murine serum diluted to 1:2,000, 1:200 and 1:400 at RT, respectively. The plates were washed again and then incubated at RT with biotin-conjugated anti-mouse IgG or IgM antibodies for 1 hour.After washing to remove unbound antibodies, the wells were incubated with horseradish peroxidase (HRP)-labeled avidin at RT for 30 minutes. After three washes with phosphate buffered saline with Tween 20 (PBST), a colorimetric assay format was followed, and the absorbance of each well was determined in a single wavelength manner (450 nm) using an ELISA reader (Microplate reader SpectraMax M5). The relative expression levels of the autoantibodies in each group were formulated by the mean enzyme indexes (EI) that were calculated as:21
|
Cytokine and total IgG assays
The (relative) concentrations of TNF-α, IL-6, IL-17A, MCP-1, ICAM-1 and total IgG in the serum or renal tissues were determined using commercial sandwich ELISA kits according to the manufacturer’s instructions.
Behavioral experiments
Spontaneous performance was tested using an autonomic activities tester equipped with eight individual darkrooms and infrared thermo probes. The BALB/c mice were placed in the independent darkroom in quiet conditions for 1 minute to preadapt to the environment. During a following period of 5 minutes, times on spontaneous activity of each mouse was detected by the infrared probes.
Sport coordinate ability of the mice was measured using rotarod test with rotarod treadmills. First, mice were placed on a stationary bar to record whether they could stay on the bar for 2 minutes. Then, the mice were conducted to rotarod test with a constant speed of 25 r/min. The rotarod test was repeated three times on three consecutive days for each mouse, and the mean retention time on the rod per trial was recorded. Each mouse was experimented only once in each period of the test.
Extraction of tissue protein
After euthanizing the mice, the heart, liver, spleen, lung, and kidneys of each mouse were collected and weighed. Total protein was extracted from the tissue using an RIPA lysis method per the manufacturer’s protocol. Using the Omni bead ruptor 24 bead mill homogenizer, the tissue samples were first homogenized in 2 mL Omni bead ruptor tubes prefilled with three metal beads and RIPA buffer plus PMSF (1 mmol/L) at 4°C. The following settings were executed to homogenize the samples: speed of 5 m/s, three cycles of 15 seconds, and a 30 second dwell. After extraction, the tubes were centrifuged at 8,000 r/min to separate soluble protein from homogenate at 4°C, and the protein in the supernatant fluid was quantified and stored for future determination.
Western blotting assays
The activation levels of intracellular signal molecules in tissue from different groups were detected and quantified by western blot assays; 30 μg of a protein extract was resolved on an 8% or 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel, transferred onto a polyvinylidene fluoride membrane, and blocked with 5% BSA in Tris-buffered saline (TBS) for 1 hour at RT. The membrane was washed three times in TBS with 0.1% Tween 20 (TBS-T), and then incubated with anti-phosphorylated STAT3 (p-STAT3), phosphorylated JAK (p-JAK), phosphorylated Erk (p-Erk) or phosphorylated IKKβ (p-IKKβ) antibodies overnight at 4°C. The membrane was washed three times in TBS-T for 10 minutes and incubated with anti-rabbit or anti-mouse IgG (HRP conjugate) in TBS. After another three washes with TBS-T for 10 minutes, membrane was reacted with a chemiluminescence system for 1 minutes, and then was exposed to films. The expression levels of the signal proteins were quantified by scanning densitometry using Quantity One 1-D analysis system.
Renal function and pathological examination
Six months after induction, urine and serum from each mouse were collected, respectively. The creatinine values in the urine and serum were measured using a superoxide dismutase (SOD) enzymatic method, respectively, according to the manufacturer’s instructions. The total protein in the urine was quantified using a bicinchoninic acid method, and the ratio of the total protein and the urine creatinine was calculated.
For immunofluorescence, one kidney was snap-frozen in liquid nitrogen and stored at −80°C. Cryostat sections of 4 μm were fixed using acetone and stained for the deposition of mouse IgG and complement C3. The mouse IgG was directly detected using goat anti-mouse IgG conjugated to DyLight 594, while C3 was detected using rabbit anti-C3 antibody (ab11887) and anti-rabbit IgG conjugated to Fluorescein isothiocyanate. All antibodies were incubated with PBS containing 2% BSA in a humid incubator for 1 hour at RT. Sections were washed five times for 3 min using PBST.Fluorescence intensity was scanned and quantified by ImageJ software using a double-blinded method.
For microscope inspection, renal tissue was fixed in formalin and embedded in paraffin. 4 μm sections from each kidney were stained with hematoxylin-eosin (H&E) and periodic acid-schiff (PAS), respectively.
Arthritis-like changes
Joint swelling was monitored by inspection and scored as follows: 0, no joint swelling; 1, swelling of one finger joint; 2, mild swelling of wrist or ankle; 3, moderate swelling of wrist or ankle; and 4, severe swelling of wrist or ankle. The volume of hind paw of each mouse was measured using a volume meter, and the whole-body imaging technique was conducted to observe the growth of the joint swelling using the Carestream in vivo FX PRO system, according to the directions.
Spleen index and pathological examination
The internal organs index of each mouse was calculated by ratio of organ to body weight (mg/g). The splenic tissue was fixed and stained with H&E and CD68 antibody for immuno-histochemical detection.
Statistical analysis
The statistical significance was assessed by a one-way ANOVA followed by an appropriate post hoc test. P-values <0.05 were considered statistically significant. All calculations were performed and graphs generated using GraphPad Prism Software (GraphPad Software, Inc., La Jolla, California, USA).
Results
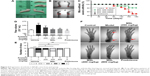
Effects of MSL on autoantibodies
The DNA autoantibody is an antibody that exists widely in SLE patients, and there is a positive relationship between its concentration and the disease activity of SLE.22,23 Using the enzyme-linked immunosorbent assay (ELISA), we measured the two types of DNA antibodies (IgG and IgM) in the serum of BALB/c mice, which were separately collected every 30 days after induction of pristane, and the results showed that both IgG- and IgM-targeted DNA had significantly increased since day 30 after induction (P<0.01, Figure 2A and B). Furthermore, high-dose MSL and prednisone significantly reduced the concentrations of DNA autoantibodies in lupus mice induced with pristane (P<0.05, Figure 2A and B).
The Sm autoantibody, a specific antibody expressed in SLE patients, has important significance in the clinical diagnosis of SLE.24 Using ELISA, we found that both IgG and IgM Sm antibodies increased significantly (P<0.01; Figure 2C and D) in the serum collected from lupus BALB/c mice. After 4.5 months of treatment with high-dose MSL and prednisone, there were significant decreases in Sm autoantibodies (P<0.05, Figure 2C and D).
Similar results were observed in measurements of histone autoantibodies. Compared with the control group, type IgG and IgM antibodies targeting histone had increased significantly from days 60 and 30, respectively. Long-term intervention of MSL and prednisone significantly reduced the relative levels of histone antibodies (P<0.05, P<0.01, P<0.05; Figure 2E and F).
Effects of MSL on total IgG and cytokines in serum
Total IgG was quantified by ELISA. As shown in Figure 3A, total IgG concentrations were maintained at a constant level of 1 mg/mL in control mice, while total IgG in lupus mice increased progressively from month 2 and concentrations were three times more than control values at month 6 (P<0.01, Figure 3A). With the intervention of high-dose MSL, concentrations of total IgG in lupus mice were significantly lower at months 4–6 (P<0.05, P<0.01; Figure 3A). In comparison, prednisone showed a greater effect. Total IgG in the prednisone group showed a significant decrease from day 60, that is, 15 days after exposure to the drugs (P<0.05, Figure 3A).
Cytokine, an endogenous polypeptide produced mainly by immune cells, is known as an important mediator with strong biological effects in multiple immune responses and plays an important role in the process of autoimmune diseases, such as SLE.25,26 On day 60 after induction, the mean concentration of IL-6 in BALB/c lupus mice was 147.2±21.2 pg/mL (Figure 3B), significantly more than the control group (P<0.001). High-dose MSL and prednisone significantly reduced IL-6 concentration in lupus mice (P<0.05, P<0.01, P<0.001; Figure 3B).
On day 180, the concentration of IL-17A in BALB/c lupus mice was 41.7±15 pg/mL, significantly more than the control group (P<0.01, Figure 3C). After 4.5 months of treatment with prednisone, there was a decreased concentration of IL-17A (P<0.05); however, no significant decrease was observed in the MSL groups (Figure 3C).
Similar to the cytokines IL-17A, the adhesion molecule ICAM1 and chemokine MCP1 were also significantly increased (P<0.001, P<0.01), and a significant decrease in ICAM1 expression was observed in the high-dose MSL group (Figure 3D and E). However, no obvious inhibition effect on MCP1 was observed in the prednisone group or the different MSL groups (Figure 3E).
Behavior studies
As shown in Figure 4A, significant decreases in spontaneous activities of BALB/c lupus mice were observed after 2, 4, and 6 months’ exposure to pristane (P<0.01). After the continuous intervention of high-dose MSL and prednisone, the activity of lupus mice improved significantly (P<0.05).
Most of the mice in each group were able to stay on the stationary rod for 2 minutes. The proportion of mice staying on the rod more than 2 minutes in each group showed no obvious difference (Figure 4B). However, on the rotation rod, there were significant differences in performance between the control mice and lupus mice (P<0.01), and the mice in the high-dose MSL group showed significant improvement compared with the lupus BALB/c mice (P<0.05). These results illustrated that MSL and prednisone were able to effectively elevate spontaneous activities and motor-coordination abilities of lupus mice.
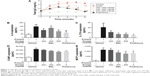
Effects of MSL on spleen index and pathological changes
There were no significant differences among the groups for indices of heart, liver, lungs, or kidneys of BALB/c mice (data not shown), while the spleen index showed a significant difference (Figure 5A and B). The spleen index of the mice that had received 0.5 mL of pristane had nearly doubled, from 4.24±0.19 to 7.92±0.46 (P<0.001), compared with the control mice. High-dose MSL and prednisone significantly reduced the spleen index of lupus mice (P<0.01, P<0.001).
Further pathological examination found that splenic corpuscles abnormally increased, and red pulp widened in spleens collected from lupus mice (Figure 5C). In addition, inflammatory cell infiltration and increased expression of lymphocytes for CD68 antigen could be also observed in spleens collected from lupus mice. High-dose MSL and prednisone obviously improved pathological changes in lupus BALB/c mice (Figure 5C and D).
Effects of MSL on arthritis-like pathological process
Arthritis is a major complication and also considered one of the diagnostic criteria for SLE, which can seriously affect the life quality of patients with SLE.26 In our experiments, arthritis-like symptoms in six lupus mice were observed (Figure 6). After being induced with pristane for 3 months, the first mouse with arthritis (score ≥3) appeared in the model group. In the following days, the numbers of mice with arthritis had increased, which was consistent with previous research.27 Two mice with severe arthritis (score ≥3, 17%) were observed in the low-dose MSL group, and the severe arthritis phenomenon was not observed in other groups (Figure 6C).
Arthritis-like symptoms were mainly represented by symmetrical swelling of the hind legs, and swelling of the forelegs was also observed in some mice with severe arthritis (Figure 6A and B). Under X-ray scanning, natural stretching of the hind limbs of the control mice and other mice without obvious arthritis symptoms was observed. However, dislocation and ankle swelling of mice with severe arthritis symptoms was observed (Figure 6F).
Figure 6D shows that the mice that received pristane alone presented a significant increase in the volumes of hind feet (P<0.01), while the mean paw volumes of mice treated with MSL or prednisone were significantly decreased (P<0.05, P<0.01). Arthritis-score results, as shown in Figure 6E, were in line with the other experiments. These results showed that medium- or high-dose MSL could block the arthritis-like progress of mice induced by pristane.
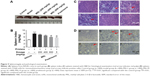
Effects of MSL on lupus nephritis
Clinically, almost all SLE patients manifested some degree of renal injury, and renal failure is the leading cause of death in patients with SLE.28 Under light microscopy, we observed that inflammatory cell infiltration, mesangial proliferation, and thickening of capillary walls were present on sections collected from lupus mice, which showed characteristics typical of glomerulonephritis (hematoxylin and eosin, Figure 7A). Meanwhile, disorganized basement membrane, thickened capillary loops, and renal capsule stenosis were prominent in renal tissues of lupus mice in electron microscopy (PAS, Figure 7B). High-dose MSL and prednisone obviously improved the renal pathological changes of lupus BALB/c mice induced by pristane (Figure 7A and B).
Mean creatinine levels in sera of the mice were calculated by an SOD-enzyme method, and levels in lupus mice were increased by approximately 60%. However, no significant difference was observed among the groups (Figure 7C). Further analysis revealed that the ratio of total protein to urine creatinine in lupus mice was significantly increased compared with the control mice (Figure 7D). Prednisone significantly decreased the ratio in lupus mice. However, the difference in ratios between the model group and each MSL group showed no statistical significance (Figure 7D).
Immunocomplex deposits are a primary cause of lupus nephritis. In our study, the complex deposits were detected by an immunofluorescence assay. Kidneys of lupus mice showed the presence of IgG (Figure 7E) and C3 complexes (Figure 7F) in glomeruli, while kidneys of mice treated with high-dose MSL or prednisone exhibited an obvious decrease in complexes (Figure 7E and F). Further quantitative analysis using fluorescence intensity supported the results. These analyses showed dose-dependent decreases in IgG and C3 complexes in mice receiving various doses of MSL. The fluorescence intensities of IgG and C3 in the high-dose group were significantly different compared with the model group (P<0.05, Figure 7G and H).
Effects of MSL on inflammatory cytokine in kidneys
In our study, we compared renal TNFα, IL-6, IL-17A, and MCP1 in mice from each group following 6 months of induction. TNFα, IL-17A and MCP1 in kidneys of mice that received pristane alone showed a significant increase compared with the control group (Figure 8A, C, and D). Although the mean IL-6 level in renal tissues of BALB/c lupus mice was 24 times higher than that of the control mice, we did not observe a significant difference (Figure 8B). We saw a trend of decrease of cytokines in kidneys from mice that received different doses of MSL compared with the model mice (Figure 8).
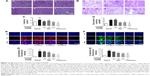
Effects of MSL on signaling pathways in renal tissues
The JAK–STAT signaling pathway is a well-known signaling system that plays an essential role in immune response.29 To determine the effect of MSL on the JAK–STAT3 pathway, the relative levels of p-JAK, p-STAT3, and p-Erk1/2 were examined by Western blot analysis. As shown in Figure 9A–C, the phosphorylation levels of JAK, STAT3, and Erk1/2 were significantly increased, and downregulation of JAK was observed in renal tissues (P<0.01, P<0.001). High-dose MSL and prednisone markedly inhibited the phosphorylation of STAT3 and JAK and downregulation of JAK (P<0.05 and P<0.01). NFκB is known as an important regulator of various genes involved in the production of many proinflammatory enzymes and cytokines related to the inflammatory process.16,30,31 We also examined the inhibiting effect of MSL on the NFκB-signaling pathway. IKK, IκB, and NFκB were observed to be significantly activated in renal tissues of mice that received pristane alone, and treatment with MSL and prednisone significantly reduced the activation levels of signaling molecules (P<0.05, P<0.01, Figure 9D–F).
Discussion and conclusion
SLE is a chronic and complex autoimmune disease and still cannot be completely cured. On one hand, for a long time, many scientific researchers have made much effort to overcome the disease, but the exact mechanism of SLE is still unclear; on the other hand, little progress in research and development for drugs against SLE has been made in this setting. In the past 60 years, only one drug – belimumab – has been approved, by the FDA in 2011.4 It is of great significance to discover and develop new therapeutic drugs.
In this article, we established a classic SLE-like model induced by pristane in BALB/c mice. This is the first time that the therapeutic effects of MSL on SLE have been evaluated systematically and comprehensively with the model. Meanwhile, evaluation indices related to behavior, such as autonomic activity and motor-coordination ability, were applied to the classic mouse model to assess the activity of MSL in vivo.
In order to evaluate the therapeutic effect of MSL, we determined the expression levels of DNA and Sm autoantibodies in the circulation systems of the mice on day 30 after induction. After confirmation of “suffering with” SLE, the mice had been administered MSL. The administration time designed herein was more beneficial to evaluate the therapeutic effect, rather than the preventive effect.
In the latest diagnostic criteria recommended by the American College of Rheumatology, eleven criteria, including autoimmunity and end-organ damage, are presented.31 The mouse model herein successfully simulated some SLE manifestations, such as DNA autoantibodies, Sm autoantibodies, serum-positive antinuclear antibodies, arthritis, and lupus nephritis. These characteristics basically met the diagnostic criteria of the American College of Rheumatology recommendation, consequently providing a basis for the following evaluation.
Using these characteristics, we investigated the role of MSL in the pathogenesis of lupus mice induced by pristane. High-dose MSL significantly reduced the relative expression levels of autoantibodies in lupus mice, postponed the arthritis-like pathological process, and improved the degree of lupus nephritis. In the study, we also found that MSL significantly reduced the expression of inflammatory cytokines in both peripheral circulatory system and renal tissues, and hence inhibited the inflammatory reaction in lupus mice. In a sense, our results explain the protective mechanism of MSL from SLE to some extent through the profiling of inflammation.
For further explanation, we studied the signal transduction associated with inflammatory pathways in kidney tissues, and we found that the signal molecules in these pathways were observably activated in lupus mice. NFκB and JAK–STAT3 signaling have been proved to be crucial pathways in lupus BALB/c mice induced by pristane, which are potential contributors to the development of lupus nephritis. The activation levels of these signaling molecules could be significantly inhibited by drug intervention with MSL, illustrating that the suppression effects of MSL on inflammatory signaling might be a potential action mechanism.
In addition, we examined the therapeutic administration of MSL to prevent and reverse established SLE mice by inhibiting inflammation-signaling pathways. Although more research is needed to assess the activity and the mechanism of MSL, our findings herein suggest that MSL may be an effective agent in the treatment of SLE.
Acknowledgments
This study was supported by the National Scientific and Technological Major Project for Significant New Drug Creation (2013ZX09508104) and China Postdoctoral Science Foundation Project (2015M581026). We are grateful to Dr Peng Du (China Academy of Military Medical Sciences) for suggestions of establishment of the model and measurements of autoantibodies.
Disclosure
The authors report no conflicts of interest in this work.
References
Pisetsky DS. Systemic lupus erythematosus. Med Clin North Am. 1986;70:337–353. | ||
Cogollo E, Silva MA, Isenberg D. Profile of atacicept and its potential in the treatment of systemic lupus erythematosus. Drug Des Devel Ther. 2015;9:1331–1339. | ||
Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol. 2001;2:764–766. | ||
Wiglesworth AK, Ennis KM, Kockler DR. Belimumab: a BLyS-specific inhibitor for systemic lupus erythematosus. Ann Pharmacother. 2010;44:1955–1961. | ||
Riaz M, Afzal N, Mahmud TH, Shahzad F, Rasheed S, Rasheed A. The high percentages of anti-thyroid antibodies positive SLE patients at Sheikh Zayed Hospital, Lahore (Pakistan). Majmaah J Health Sci. 2015;3:28–32. | ||
Kan HJ, Song X, Johnson BH, Bechtel B, O’Sullivan D, Molta CT. Healthcare utilization and costs of systemic lupus erythematosus in Medicaid. Biomed Res Int. 2013;2013:808391. | ||
Aringer M, Smolen JS. Safety of off-label biologicals in systemic lupus erythematosus. Expert Opin Drug Saf. 2015;14:243–251. | ||
Mullard A. 2011 FDA drug approvals. Nat Rev Drug Discov. 2012;11:91–94. | ||
Xiong W, Lahita RG. Pragmatic approaches to therapy for systemic lupus erythematosus. Nat Rev Rheumatol. 2014;10:97–107. | ||
Liao J, Chang C, Wu H, Lu Q. Cell-based therapies for systemic lupus erythematosus. Autoimmun Rev. 2015;14:43–48. | ||
Ling C, Wang L, Wang Y, et al. The roles of traditional Chinese medicine in gene therapy. J Integr Med. 2014;12:67–75. | ||
Alam F, Saqib QN. Pharmacognostic standardization and preliminary phytochemical studies of Gaultheria trichophylla. Pharm Biol. 2015;53:1711–1718. | ||
Zhang D, Liu R, Sun L, et al. Anti-inflammatory activity of methyl salicylate glycosides isolated from Gaultheria yunnanensis (Franch.) Rehder. Molecules. 2011;16:3875–3884. | ||
Zhang T, Sun L, Liu R, et al. A novel naturally occurring salicylic acid analogue acts as an anti-inflammatory agent by inhibiting nuclear factor-κB activity in RAW264.7 macrophages. Mol Pharm. 2012;9:671–677. | ||
Lan X, Liu R, Sun L, Zhang T, Du G. Methyl salicylate 2-O-β-D-lactoside, a novel salicylic acid analogue, acts as an anti-inflammatory agent on microglia and astrocytes. J Neuroinflammation. 2011;8:98. | ||
Xin W, Huang C, Zhang X, et al. Methyl salicylate lactoside inhibits inflammatory response of fibroblast-like synoviocytes and joint destruction in collagen-induced arthritis in mice. Br J Pharmacol. 2014;171:3526–3538. | ||
Zhang D, Ma X, Xin W, et al. Pharmacokinetics of methyl salicylate-2-O-β-D-lactoside, a novel salicylic acid analog isolated from Gaultheria yunnanensis, in dogs. Biomed Chromatogr. 2013;27:1680–1684. | ||
He Y, Yan Y, Zhang T, et al. Lack of dose dependent kinetics of methyl salicylate-2-O-β-d-lactoside in rhesus monkeys after oral administration. J Ethnopharmacol. 2015;164:293–300. | ||
Tan EM, Schur PH, Carr RI, Kunkel HG. Deoxybonucleic [sic] acid (DNA) and antibodies to DNA in the serum of patients with systemic lupus erythematosus. J Clin Invest. 1996;45:1732–1740. | ||
Bloom DD, Davignon JL, Cohen PL, Eisenberg RA, Clarke SH. Overlap of the anti-Sm and anti-DNA responses of MRL/Mp-lpr/lpr mice. J Immunol. 1993;150:1579–1590. | ||
Zhou LL, Wei W, Si JF, Yuan DP. Regulatory effect of melatonin on cytokine disturbances in the pristane-induced lupus mice. Mediators Inflamm. Epub 2010 July 20. | ||
Sciascia S, Bertolaccini ML, Roccatello D, Khamashta MA, Sanna G. Autoantibodies involved in neuropsychiatric manifestations associated with systemic lupus erythematosus: a systematic review. J Neurol. 2014;261:1706–1714. | ||
Yu C, Gershwin ME, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun. 2014;48:10–13. | ||
Spitz C, Winkels H, Bürger C, et al. Regulatory T cells in atherosclerosis: critical immune regulatory function and therapeutic potential. Cell Mol Life Sci. 2016;73:901–922. | ||
Medrano-Campillo P, Sarmiento-Soto H, Álvarez-Sánchez N, et al. Evaluation of the immunomodulatory effect of melatonin on the T-cell response in peripheral blood from systemic lupus erythematosus patients. J Pineal Res. 2015;58:219–226. | ||
Leiss H, Niederreiter B, Bandur T, et al. Pristane-induced lupus as a model of human lupus arthritis: evolvement of autoantibodies, internal organ and joint inflammation. Lupus. 2013;22:778–792. | ||
Austin HA 3rd, Muenz LR, Joyce KM, et al. Prognostic factors in lupus nephritis: contribution of renal histologic data. Am J Med. 1983;75:382–391. | ||
Kawasaki M, Fujishiro M, Yamaguchi A, et al. Possible role of the JAK/STAT pathways in the regulation of T cell-interferon related genes in systemic lupus erythematosus. Lupus. 2011;20:1231–1239. | ||
Burgos P, Metz C, Bull P, et al. Increased expression of c-Rel, from the NF-κB/Rel family, in T cells from patients with systemic lupus erythematosus. J Rheumatol. 2000;27:116–127. | ||
Lech M, Skuginna V, Kulkarni OP, et al. Lack of SIGIRR/TIR8 aggravates hydrocarbon oil-induced lupus nephritis. J Pathol. 2010;220:596–607. | ||
Fu SM, Deshmukh US, Gaskin F. Pathogenesis of systemic lupus erythematosus revisited 2011: end organ resistance to damage, autoantibody initiation and diversification, and HLA-DR. J Autoimmun. 2011;37:104–112. |
Supplementary material
  | Table S1 The information regarding the main antibodies used in this study |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.