Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Metformin-Insulin versus Metformin-Sulfonylurea Combination Therapies in Type 2 Diabetes: A Comparative Study of Glycemic Control and Risk of Cardiovascular Diseases in Addis Ababa, Ethiopia
Authors Gebrie D, Manyazewal T , Ejigu DA, Makonnen E
Received 7 April 2021
Accepted for publication 10 July 2021
Published 24 July 2021 Volume 2021:14 Pages 3345—3359
DOI https://doi.org/10.2147/DMSO.S312997
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Desye Gebrie,1,2 Tsegahun Manyazewal,2 Dawit A Ejigu,3 Eyasu Makonnen2,4
1School of Pharmacy, College of Health Sciences, Mekelle University, Mekelle, Ethiopia; 2Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 3Department of Pharmacology, St Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 4Department of Pharmacology and Clinical Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Desye Gebrie
School of Pharmacy, College of Health Sciences, Mekelle University, P.O. Box 1871, Mekelle, Ethiopia
Tel +251 966-229-236
Fax +251 034-441-66-81
Email [email protected]
Objective: This study aimed to compare glycemic control and risk of cardiovascular outcomes of metformin-insulin versus metformin-sulfonylurea combination therapies in type 2 diabetes mellitus.
Methods: We conducted a comparative cross-sectional study in five tertiary level hospitals in Addis Ababa, Ethiopia. We enrolled 321 patients with type 2 diabetes mellitus who were on continuous treatment follow-up on either metformin-insulin or metformin-sulfonylurea combination therapy. We interviewed the participants and reviewed their medical records to investigate medication efficacy, safety, and adherence. The primary outcome measure was glycemic control and the secondary outcome measures were composite cardiovascular outcomes.
Results: Of the total participants enrolled, 50.5% (n = 162) were those who received metformin-insulin and 49.5% (n = 159) metformin-sulfonylurea combination therapies for a median of 48 months follow-up. The reduction of Hb1Ac levels was comparable between the metformin-insulin (− 1.04 ± 0.96%) and metformin-sulfonylurea (− 1.02 ± 1.03%), p = 0.912. Patients who received metformin-sulfonylurea had 4.3 times more likely to have achieved target HbA1c level compared to those who received metformin-insulin, p < 0.001, adjusted odds ratio (AOR) with 95% CI = 4.31[1.79– 10.32]. Risk of composite cardiovascular outcomes was higher in metformin-insulin group (40.5% versus 34.0%), p = 0.021. Co-morbidities, body mass index, systolic blood pressure, and HbA1c had a significant association with composite cardiovascular outcomes. Reductions of bodyweight, HDL-C, LDL-C, triglycerides levels, and microvascular complications were different between the two groups, p < 0.05.
Conclusion: High proportion of patients who received metformin-sulfonylurea achieved target HbA1c level and had less composite cardiovascular outcomes compared to those who received metformin-insulin. However, these findings have to be confirmed with randomized control trials to determine risks associated with insulin use, while efficacy is maintained as second-line treatment in patients with type 2 diabetes mellitus.
Keywords: glycemic control, cardiovascular diseases, type 2 diabetes mellitus, metformin, insulin, sulfonylurea, glycated hemoglobin A1c (HbA1c)
Introduction
Diabetes mellitus (DM) is one of the top ten causes of mortality and the fastest growing health emergencies of the 21st century, with 463 million people living with it worldwide in 2019, and this number is projected to reach 578 million by 2030 and 700 million by 2045.1 The estimated global direct health expenditure on DM in 2019 was US$ 760 billion and is expected to grow to a projected US$ 825 billion by 2030 and US$ 845 billion by 2045.2 Type 2 diabetes mellitus (T2DM) is the most common and complicated form of the disease and accounts for more than 90% of the estimated cases of DM, affecting the life expectancy, quality of life, and health of an individual.1,3 Yet, there is no cure for T2DM, while its prevalence is largely increasing, with increased risk of complications including diabetic retinopathy, neuropathy, kidney damage, and cardiovascular complications.4–6 Cardiovascular disease (CVD) is a common complication and a major cause of death in patients with T2DM.7,8
Inspite of the introduction of new medicines, treating patients with DM tend to become more challenging due to the progressive nature of the disease.9,10 The American Diabetes Association recommends lifestyle modification as the first step in treating new-onset T2DM patients.11 However, to achieve and maintain patient-specific glycemic targets, the majority of patients require glucose-lowering medications. Nowadays, Metformin is the first-line and commonly used pharmacological drug for patients with T2DM because of its potential advantages, including cardioprotective effect, reduction of weight, and prevention of some comorbid diseases.11–14 If lifestyle modifications and a maximal tolerated dose of metformin therapy fail to achieve the patient’s glycemic target within three months follow-up, the regimen would be changed to combination therapy.11
Metformin and sulfonylureas are the most commonly used combination therapy in treating T2DM.15,16 Sulfonylureas are recommended as second-line treatment regimen in the management of T2DM, while they are still widely used also as a first-line treatment instead of metformin.17 However, treating T2DM patients with a sulfonylureas rather than metformin is associated with a high risk of ischaemic stroke, cardiovascular death, hypoglycemia, and all-cause mortality.17–20 Besides, the use of sulfonylurea as a second-line drug is associated with an increased risk of myocardial infarction, all-cause mortality, and severe hypoglycemia, compared with use of metformin monotherapy; as a result, continuing metformin when introducing sulfonylurea appears to be safer than switching to another drug.21 Such findings led to new requirements from licensing authorities that all new T2DM therapies should show cardiovascular safety.9
Insulin is one of the second-line antidiabetic drug for the treatment of T2DM patients who failed initial metformin monotherapy and lifestyle interventions.22,23 Though insulin has been the preferred drug to be added to metformin when glycated hemoglobin A1c (HbA1c) is markedly elevated, there was no evidence towards improved all-cause mortality or cardiovascular mortality.24 A randomized controlled trial (RCT) in the United Kingdom Prospective Diabetes Study (UKPDS) in patients with T2DM and risk of complications showed that there was no difference in the rates of myocardial infarction and diabetes-related death among participants assigned to sulphonylurea and insulin therapies.25 Nearly one-third of the population initiated a second-line therapy. However, only 15% achieved a HbA1c target <7%, and cardiovascular complication has still been prevalent globally.26 Despite the availability of many new treatment options for T2DM, the proportion of patients achieving their HbA1c target <7.0% remained around 50%, and cardiovascular complications have become high.27
American Diabetes Association’s current standard of care recommends that newly diagnosed T2DM patients whose HbA1c level ≥8.5% should start a combination treatment either metformin with insulin or metformin with sulfonylureas.11 However, there is no clear evidence that shows the relative advantages of either metformin-insulin or metformin-sulfonylurea combination on major treatment outcomes.28 With guidelines moving away from a one-size-fits-all approach and allowing flexibility in choosing a second- or third-line drug, keen individualized medication based on efficacy, risk of hypoglycemia, patient’s comorbid conditions, impact on weight, adverse effects, and cost, management in T2DM has become a challenge.29,30 The benefits of combination therapies for the management of type 2 diabetes are well-documented while the comparative glycemic control and cardiovascular outcomes among the different combination options have not been studied yet. Although most of T2DM patients require combination therapy, the choice of an appropriate second-line drug is a critical issue for the prevention of CVD. Therefore, this study aimed to compare glycemic control and risk of cardiovascular outcomes of metformin-insulin versus metformin-sulfonylureas combination therapies in patients with T2DM.
Methods
Study Design
A comparative cross-sectional study was conducted by reviewing T2DM patient’s medical records retrospectively with the support of prospective patients’ interviews from December 2019 to March 2020.
Participants
Study participants were T2DM patients who were under metformin with insulin or metformin with sulfonylurea combination therapies.
Inclusion and Exclusion Criteria
Volunteers who were ≥18 years old with continuous medical records and continuous follow-up either on metformin with insulin or metformin with sulfonylurea combination therapies were eligible. In terms of exclusion criteria, patients with a history of cardiovascular disease (myocardial infarction, stroke, peripheral vascular disease) before the initiation of the combination therapies, those either on monotherapy or triple therapy of antidiabetic drugs, and those with less than three months of follow-up with the combination therapies were, however, excluded from the study.
Sample Size and Study Area
The required sample size was determined using a 50% estimated proportion of T2DM patients who achieved their HbA1c target less than 7%.27 P = 0.5 and w = 5% and using 95% confidence level: n = Z2 p (1−p)/W2; where, n= sample size, z = statistic for 95% level of confidence; w = precision/margin of error/and p = the estimated proportion of T2DM patients achieving HbA1c level⇒ n = (1.96)2 * (0.5) * (1−0.5)/(0.05)2 = 384. Adding 10% for non-response and considering the emergence of COVID-19 and the state-of-emergency declared as a result, we considered a sample size of 321. We included five tertiary-level governmental hospitals, which were Tikur Anbessa Specialized, St Paul’s Specialized, Yekatit 12, Menelik II, and Zewditu Memorial Hospitals. We used a simple random sampling method to select the study sites. The average monthly T2DM patients’ load was retrieved from the health information management system office of each hospital. Based on the average monthly patients’ load of each hospital, a proportion was made and a total of 321 T2DM patients were included in the study using a convenience sampling method.
Outcomes
The primary outcome measure was glycemic control (reduction in HbA1c level). The secondary outcome measures were composite cardiovascular outcomes (myocardial infarction, stroke, heart failure), hypertension, dyslipidemia, microvascular complications (diabetic neuropathy, retinopathy, and nephropathy), treatment-emergent adverse events (hypoglycemia), and change in bodyweight, blood pressure, fasting blood sugar (FBS) and lipid profiles (low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides).
Statistical Analysis
Statistical analyses were carried out using SPSS version 25. Data were expressed as median [interquartile range (IQR)] for skewed variables, mean ± standard deviation (SD) for normally distributed continuous variables, and the number of cases and percentages for categorical variables. Continuous variables were compared between two groups using an independent sample t-test for normally distributed variables and Mann–Whitney U-test for variables with skewed data. Pearson chi-square was used to categorical variables. Bivariate and multivariate logistics regression analyses were done to assess factors associated with glycemic target level and composite cardiovascular outcomes. The results of the analyses were reported as odds ratios (ORs) with 95% confidence intervals (CIs). Statistical analysis with a p-value <0.05 was considered statistically significant.
Ethical Considerations
Ethical approvals were obtained from the Scientific and Ethics Review Committee of the Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), College of Health Sciences, Addis Ababa University (Ref. No. CDT/18100/19) and Institutional Review Board of Addis Ababa Regional Health Bureau (Ref. No. AAH/7038/227). After securing ethical clearance, an official letter was sent to each hospital to get permission. Full explanation about the purpose of the study was given to authorities of each hospital. Consent was obtained from participants using approved and locally translated informed consent form. Patients were informed about the details of the study, including the general over view, purpose of the study and risk and benefits, and confidentiality was maintained at all stages of the study. This study was conducted in accordance with the Declaration of Helsinki.
Results
Sociodemographic Characteristics of Study Participants
A total of 321 participants with T2DM were enrolled in the study, of whom 50.5% (n = 162) received metformin-insulin combination therapy and 49.5% (n = 159) received metformin-sulfonylurea combination therapy. Among the participants, 43.9% (n = 141) were males. Compared to metformin-insulin combination therapy, metformin-sulfonylurea combination therapy had a greater number of male participants (15.6% vs 28.3%, p < 0.001). The age of the participants was 59.45±10.86 years, and a significant difference was observed between the groups (58.14±9.89 years in the metformin-insulin group and 60.78±11.65 years in the metformin-sulfonylurea group, p = 0.029). Of the study participants, 60.7% (n = 195) attended regular weekly physical exercise (Table 1).
 |
Table 1 Demographic Characteristics of Study Participants (N=321) |
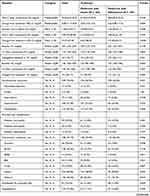
The median follow-up period from baseline to data collection was 48 (24–72) months. Notably, patients in the metformin-insulin group were more likely to have a shorter history of combination therapy than patients in the metformin-sulfonylurea therapy (36 (12–60) months Vs 60 (36–96) months, P < 0.001). Patients who received metformin-insulin combination therapy had more co-morbidities than those who received metformin-sulfonylurea combination therapy (44.9% vs 39.3%; p = 0.022) (Table 2).
 |
 |
 |
Table 2 Clinical Characteristics of Study Participants (N = 321) |
Glycemic Control and Managements
T2DM patient’s HbA1c and FBS level were examined at the beginning of combination therapy and after a median of 48 months follow-up of the combination therapies. Patients who received metformin-insulin combination therapy had a higher HbA1c than those who received metformin-sulfonylurea combination therapy (8.65±1.73% vs 7.46±1.50%, respectively; p < 0.001). However, there was no significant difference in HbA1c changes from baseline value between the groups (−1.04±.96% vs −1.02±1.03%; p = 0.912). After receiving the combination therapies, there was a significant difference in the proportion of patients who achieved the recommended target HbA1c level (<7%) between patients in the metformin-insulin vs metformin-sulfonylurea combination therapies (8.1% vs 24.3%, respectively; p < 0.001) as shown in Table 2. The changes from baseline in median FBS between groups were not significantly different (−24 mg/dl for metformin-insulin vs −29.5 mg/dl for metformin-sulfonylurea; p = 0.370). At baseline and after combination therapy, 13.3% and 32.0% patients, respectively achieved the recommended FBS (<130.00 mg/dl). However, there was no significant difference between patients in the metformin-insulin and metformin-sulfonylurea groups at baseline (6.0% vs 7.3%, respectively; p = 0.330) and after a follow-up of combination therapy (16.3% vs 15.7%, respectively; p = 0.467) as shown in Table 2.
Cardiovascular Risk Factor Control and Managements
Potential cardiovascular risk factors were assessed at baseline and after a follow-up of combination treatment. Patients treated with the metformin-insulin combination had lower baseline bodyweight than those treated with metformin-sulfonylurea combination (70.00±10.68 kg vs 76.48±12.41 kg, respectively; p < 0.001). There was no significant difference in bodyweight after receiving metformin-insulin and metformin-sulfonylurea combination therapies (75.28±11.10 kg vs 73.04±11.69 kg, respectively; p = 0.081). However, the increase in bodyweight with metformin-insulin combination therapy was significantly higher than with metformin-sulfonylurea combination therapy (5.37±5.92 kg vs −3.10±4.68 kg, respectively; p < 0.001) as shown in Table 2.
The reduction from baseline in SBP was not significantly different with both combination therapies (−3.57±16.74mmHg for metformin-insulin vs −5.18±16.88 mmHg for metformin-sulfonylurea; p = 0.415). The reduction from baseline in DBP was not also significantly different in both combination therapies (−5.06±11.79 mmHg for metformin-insulin vs −6.23±11.26 mmHg for metformin-sulfonylurea; p = 0.389). There was no statistically significant difference in the proportion of patients who achieved the recommended target SBP goal (40.0% for metformin-insulin vs 42.0% for metformin-sulfonylurea; p = 0.307) and target DBP goal (48.8% for metformin-insulin vs 48.5% for metformin-sulfonylurea; p = 0.365) as shown in Table 2.
There was a significant reduction from baseline in median HDL-C in patients who received metformin-insulin combination therapy compared to those who received metformin-sulfonylurea combination therapy (−5.00 mg/dl vs 5 mg/dl, respectively; p = 0.004). Metformin-insulin combination treatment significantly increased the mean LDL-C compared with metformin-sulfonylurea treatment from that of the baseline (6.67 mg/dl vs −21.30 mg/dl, respectively; p = <0.001). Similarly, the changes from the baseline in the median triglyceride and cholesterol total between the two treatment groups were significantly different (9 mg/dl for metformin-insulin vs −15 mg/dl for metformin-sulfonylurea; p = 0.005) and (−15 mg/dl for metformin-insulin vs −29 mg/dl for metformin-sulfonylurea; respectively; p = 0.021) as shown in Table 2.
Composite Cardiovascular Outcomes
Of the study participants with known T2DM, 74.5% developed at least one composite cardiovascular outcome over a median of 48 months follow-up. There was a significant difference in the proportion of patients who had CVD between the two treatment groups, with a higher proportion of patients in the metformin-insulin treatment group than metformin-sulfonylurea treatment group (40.5% vs 34.0%, respectively; p = 0.021). Among participants, 4.7% developed myocardial infarction, 1.3% stroke, and 11.5% heart failure. A significantly higher proportion of patients who received metformin-insulin combination therapy had myocardial infarction than those who received metformin-sulfonylurea combination therapy (3.7% vs 0.9%, respectively; p = 0.031). However, there was no significant difference in the proportion of patients who had stroke, and heart failure between the two treatment groups as shown in Table 2.
Microvascular Complications
Among the patients with overall microvascular complications of diabetes, 29.3% had diabetic neuropathy, 9.3% diabetic retinopathy and 4.7% diabetic nephropathy. There was a significant difference in the proportion of patients with diabetic neuropathy between treatment groups (19.0% for metformin-insulin vs 10.3% for metformin-sulfonylurea; p = 0.001) and diabetic retinopathy (7.2% for metformin-insulin vs 2.2% for metformin-sulfonylurea; p = 0.003). However, there was no significant difference in proportion of patients with diabetic nephropathy between the groups (Table 2).
Concomitant Medication Use
Of the study participants, 74.1% used concomitant medications on top of antidiabetic medications. There was no significant difference in the proportion of patients who received concomitant medication between metformin-insulin and metformin-sulfonylurea treatment groups (39.9% vs 34.3%, respectively; p = 0.056). Significant higher proportion of patients in the metformin-insulin treatment group used diuretics (12.8% vs 7.8%, respectively; p = 0.038), angiotensin receptor-blockers (3.7% vs 0.6%, respectively; p = 0.011), Beta-blockers (9.0% vs 4.4%, respectively; p = 0.021), statins (38.9% vs 30.8%, respectively; p = 0.005) and aspirin (26.6% vs 19.1%, respectively; p = 0.010) compared to those in the metformin-sulfonylurea treatment group (Table 2).
Antidiabetic Treatment-Emergent Adverse Events
The proportion of patients self-reported at least one antidiabetic treatment-emergent adverse events during the follow-up period in both metformin-insulin and metformin-sulfonylurea treatment groups were not significantly different (13.4% vs 11.8%, respectively; p = 0.609). A higher proportion of patients reported pain at the injection site and weight gain in the metformin-insulin than metformin-sulfonylurea treatment groups though lower in dyspepsia. Although it was not statistically significant, a slightly large percentage of patients experienced hypoglycemic adverse events in the metformin-insulin than metformin-sulfonylurea treatment groups (17.1% vs 14.6%, respectively; p = 0.405). The median episode of hypoglycemia reported per month was also similar between the two treatment groups (Table 2).
Factors Associated with Glycemic Target Level
The following covariates including sex, place of residence, marital status, level of education, family history of diabetes mellitus, smoking habit, alcohol consumption, khat chewing habit, daily vegetable and fruit consumption, regular exercise, co-morbidities, type of antidiabetic treatment, concomitant medications use, missed doses and source of antidiabetic medications were subjected to bivariate and multivariate logistic regression analysis. Of the covariates, type of antidiabetic treatments had a significant association with glycemic target level (AOR with 95% CI 4.31[1.79–10.32]; p = 0.001) (Table 3).
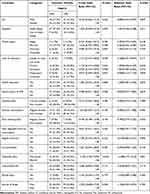 |
Table 3 Bivariate and Multivariate Logistic Regression Analysis of Factors Associated with HbA1c Target Level Among Patients with T2DM |
Factors Associated with Composite Cardiovascular Outcomes
Factors including sex, residence, marital status, level of education, alcohol consumption, regular exercise, type of antidiabetic treatment, co-morbidities, age, HbA1c, FBS, SBP, DBP, HDL-C, LDL-C, cholesterol total and triglycerides were subjected to bivariate logistic regression analysis. Those factors having a p-value <0.20 in the bivariate logistic regression analysis were subjected to multivariate logistic regression analysis. Of the possible factors included in multivariate logistic regression analysis, comorbidities, BMI, HbA1c, and SBP had a significant association with composite cardiovascular outcomes (AOR with 95% CI 0.002[0.000–0.035], p = <0.001; 400.270[2.340–68,469.106], p = 0.022; 0.517[0.304–0.876], p = 0.014 and 0.940[0.892–0.991], p = 0.021), respectively (Table 4).
 |
Table 4 Bivariate and Multivariate Logistic Regression Analysis of Factors Associated with Composite Cardiovascular Outcomes Among Patients with T2DM |
Discussion
Sustainable glycemic control is the major goal of T2DM management to prevent both micro and macrovascular complications of DM.31 There was no significant difference in the mean reduction of HbA1c from the baseline between metformin-insulin and metformin-sulfonylurea combination therapies. In contrast to this finding, study elsewhere32,33 reported that the mean reduction in HbA1c level from the baseline was higher in the metformin-insulin treated groups. This discrepancy might have happened due to differences in study participants and other factors. In the current study, the proportion of patients in the metformin-sulfonylurea treatment group who achieved the HbA1c target level (<7.0%) was higher compared to those in the metformin-insulin treatment group after a median of 48 months follow-up. In contrast to this finding, an open-label RCT34 showed metformin with insulin glargine was as effective as metformin with glimepiride in controlling hyperglycemia perhaps due to the different preparations of insulin used in both studies. Moreover, another study32 showed the proportion of patients who achieved the HbA1c target level (<7.0%) was comparable among different combinations of antidiabetic medications; however, combination with intensified insulin therapy was the most effective treatment in achieving the HbA1c target of 6.5%. The higher proportion of patients in the metformin-sulfonylurea group who achieved the HbA1c target level might be due to the longer duration of follow-up and a greater number of participants attending regular physical exercises. The American diabetes association standard of care guideline recommends that T2DM patient’s FBS target should be less than 130 mg/dl.31 A study elsewhere32 reported a significantly large proportion of patients in the insulin-based combination therapy achieved FBS <130 mg/dl compared with patients in the oral combination therapies. However, the current study showed a non-significant difference in the proportion of patients having achieved FBS (<130.0 mg/dl) between the two treatment groups. This discrepancy might be attributed to the difference in the duration of follow-up.
Obesity is one of the most common risk factors for T2DM and representing a major global health problem.35 It aggravates the pancreatic β-cell failure, insulin resistance, and cardiovascular risk.36 Lowering bodyweight is an indispensable part of T2DM management.35 Both insulin (more pronounced) and sulfonylurea were associated with weight gain by reducing the amount of glucose excreted in the urine, which leads the tissue to reabsorb glucose and store it as fat.37 Other evidences25,34 also revealed that weight gain was significantly higher with insulin than sulfonylurea. In support of these evidences, the current study showed metformin-insulin combination therapy significantly increased from baseline in bodyweight compared with metformin-sulfonylurea combination therapy.
Lowering blood pressure is notably important to reduce the risk of CVD and diabetes-related deaths.38 Compared with sulfonylureas, GLP-1 receptor agonists and SGLT-2 inhibitors added to metformin therapy significantly reduced SBP and DBP from the baseline values.39,40 However, study elsewhere41 reported there was no significant difference in pulse pressure, SBP, and DBP among different treatment groups. In support of the second evidence, the current finding showed no significant difference in the reduction of SBP and DBP from baseline between metformin-insulin and metformin-sulfonylurea combination therapies. Dyslipidemia is a well-known risk factor for CVD in individuals with T2DM.42 Likewise, a cohort study43 conducted elsewhere showed that patients with T2DM who had uncontrolled blood pressure, LDL-C, and HbA1c were at high risk of hospitalization due to CVD, whereas those with blood pressure and LDL-C at target had a lower risk of developing adverse cardiovascular events. Moreover, another evidence44 showed that LDL-C decreased by 5% with the addition of metformin to sulfonylurea. In support of these evidence, the current study showed that metformin-sulfonylurea combination therapy significantly reduced LDL-C compared with metformin-insulin therapy (p < 0.001). Another study elsewhere34 reported that there was no significant difference in lipid profiles between insulin and sulfonylurea added on metformin therapy. In contrast to this finding, the present finding elucidated metformin-insulin combination therapy reduced HDL-C by 5mg/dl, while metformin-sulfonylurea combination therapy increased HDL-C by 5mg/dl from the baseline value (p = 0.004).
CVD is the most common macrovascular complication and a major cause of death in patients with T2DM.7,8 An observational cohort study conducted elsewhere45 showed patients with T2DM who were under metformin-insulin combination therapy were associated with an increased hazard of a composite of nonfatal cardiovascular outcomes and all-cause mortality compared with those who were under metformin-sulfonylurea combination therapy. Another retrospective cohort study46–48 reported that T2DM patients who initiated basal insulin as a second- or third-line therapy had an increased risk of all-cause mortality, cardiovascular disease, and severe hypoglycemia compared with those who received the newer GLP-1 receptor agonists, SGLT-2 inhibitors, and DPP-4 inhibitors. Similar to these findings, the present study showed that a significantly higher proportion of patients had composite cardiovascular outcomes in the metformin-insulin than metformin-sulfonylurea combination therapies. A systematic review of RCTs24 reported that there was no evidence towards improved all-cause mortality or cardiovascular mortality despite insulin being the preferred drug to add to metformin when HbA1c is markedly elevated. Moreover, RCTs25 revealed that there was no difference in the rates of myocardial infarction or diabetes-related death between participants in sulphonylurea and insulin therapies. Inconsistent to these findings, the current study showed a significantly higher proportion of patients under metformin-insulin combination therapy had myocardial infarction than in those under metformin-sulfonylurea combination therapy. More clinical trials should be conducted as there are conflicting results, and the choice of second-line antidiabetic medication should be individualized.
Diabetic retinopathy is the leading cause of visual impairment and blindness in the populations aged 20–74 years as well as in diabetic patients worldwide, and it may affect up to 60% of T2DM patients.49 A study reported elsewhere50 indicates that the prevalence of diabetic retinopathy has sharply increased as the HbA1c level increased. A meta-analysis conducted in Sub-Saharan Africa51 showed that the prevalence of diabetic nephropathy was 41.4% in patients with T2DM. Another study conducted elsewhere52 showed that treatment with sulfonylurea was associated with an increased incidence of microvascular complications, especially neuropathy and retinopathy, compared to treatment with vildagliptin. The low prevalence of microvascular complications observed in the current study might be attributed to poor diagnosis and underreporting of cases. Compared with metformin-sulfonylurea combination therapy, metformin-insulin combination therapy was more likely associated with diabetic neuropathy and diabetic retinopathy.
In the current study, many patients in the metformin-insulin treatment group were more likely to take diuretics, angiotensin receptor blockers, beta-blockers, and lipid-lowering agents compared with those in the metformin-sulfonylurea treatment group. This might be associated to the high prevalence of hypertension, dyslipidemia, and composite cardiovascular outcomes in the metformin-insulin treatment group. In contrast to this study, a retrospective cohort study53 reported that women with T2DM and CVD were more likely to be obese, hypertensive, and have hypercholesterolemia, but were less likely to take statins and angiotensin-converting enzyme inhibitors.
Many studies21,25 have identified patients who were treated with insulin and/or sulfonylurea had experienced hypoglycemia. Another study elsewhere54 reported that prolonged use of both insulin and sulfonylurea may significantly contribute to a greater incidence of hypoglycemia in patients with coronary artery disease. Hypoglycemia affects the cascade of pathophysiology by inducing adrenergic activation and oxidative stress which may lead to worsening of the cardiovascular risk, arrhythmias, and ischemia.55 Despite the different glycemic achievements, a similar prevalence of hypoglycemic adverse events was illustrated between the two treatment groups. A higher episode of hypoglycemia was reported in insulin-treated patients.25 However, this study showed a similar episode of hypoglycemia between groups.
This study reported important information about glycemic control, cardiovascular risk factors, and composite cardiovascular outcomes of metformin-insulin versus metformin-sulfonylurea combination therapies. The study had also many strengths, most importantly it had predefined eligibility criteria for study participants, it was conducted in randomly selected five tertiary level hospitals which could avoid selection bias. Moreover, the data were collected in a mixed approach from the patient through interviewing and reviewing their medical records which could help the study have detailed information about the participants. However, this study did not address the different sulfonylurea drugs and doses. In fact, the most prescribed sulfonylurea as add on to metformin was glibenclamide in the study settings. Another limitation of the study was that some data were incomplete in the patient’s medical record which might have affected the result of the analysis and which might lead to underreporting of adverse events like hypoglycemia. One more limitation was the small sample size of the study which may lack representative of the general population which calls upon careful interpretation.
Conclusion
From the present study, it can be concluded that metformin-sulfonylurea combination therapy could benefit many patients by helping them achieve target HbA1c level (<7%) compared to metformin-insulin. Moreover, metformin-sulfonylurea combination therapy could also reduce many of cardiovascular risk factors and composite cardiovascular outcomes compared to metformin-insulin. More clinical trials, however, have to be conducted to confirm the present finding.
Acknowledgments
The authors would like to acknowledge the Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), College of Health Sciences, Addis Ababa University for funding the study. We also acknowledge staffs and data collectors in the diabetes clinics of all involved hospitals. This manuscript is available online as thesis. http://etd.aau.edu.et/handle/123456789/25627.56
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. International Diabetes Federation. IDF diabetes atlas: 9th ed. Brussels, Belgium: International Diabetes Federation; 2019. Available from: https://diabetesatlas.org/en/resources/.
2. Williams R, Karuranga S, Malanda B, et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108072. doi:10.1016/j.diabres.2020.108072
3. Merino J, Guasch-Ferré M, Ellervik C, et al. Quality of dietary fat and genetic risk of type 2 diabetes: individual participant data meta-analysis. BMJ. 2019;366:l4292. doi:10.1136/bmj.l4292
4. Vujosevic S, Aldington SJ, Silva P, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020;8:337–347. doi:10.1016/S2213-8587(19)30411-5
5. Maddaloni E, Coleman RL, Agbaje O, et al. Time-varying risk of microvascular complications in latent autoimmune diabetes of adulthood compared with type 2 diabetes in adults: a post-hoc analysis of the UK Prospective Diabetes Study 30-year follow-up data (UKPDS 86). Lancet Diabetes Endocrinol. 2020;8(3):206–215. doi:10.1016/S2213-8587(20)30003-6
6. Zweck E, Westenfeld R, Szendroedi J. Oral semaglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;381(21):2075–2076.
7. Cosentino F, Ceriello A, Baeres FMM, et al. Addressing cardiovascular risk in type 2 diabetes mellitus: a report from the European Society of Cardiology Cardiovascular Roundtable. Eur Heart J. 2019;40(34):2907–2919. doi:10.1093/eurheartj/ehy677
8. Steg PG, Bhatt DL, Simon T, et al. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med. 2019;381(14):1309–1320. doi:10.1056/NEJMoa1908077
9. Bellary S, Tahrani AA, Barnett AH. Evidence-based prescribing of diabetes medications: are we getting closer? Lancet Diabetes Endocrinol. 2020;8(3):176–177. doi:10.1016/S2213-8587(20)30020-6
10. Sattar N. Advances in the clinical management of type 2 diabetes: a brief history of the past 15 years and challenges for the future. BMC Med. 2019;17(1):46. doi:10.1186/s12916-019-1281-1
11. American Diabetes Association. Standards of medical care in diabetes-2019: abridged for primary care providers. Clin Diabetes. 2019;37(1):11–34. doi:10.2337/cd18-0105
12. Petrie JR, Rossing PR, Campbell IW. Metformin and cardiorenal outcomes in diabetes: a reappraisal. Diabetes Obes Metab. 2020;22(6):904–915.
13. Zhang K, Yang W, Dai H, et al. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: results from meta-analysis. Diabetes Res Clin Pract. 2020;160:108001. doi:10.1016/j.diabres.2020.108001
14. Powell MK, Cempirkova D, Dundr P, et al. Metformin treatment for diabetes mellitus correlates with progression and survival in colorectal carcinoma. Transl Oncol. 2020;13(2):383–392. doi:10.1016/j.tranon.2019.10.011
15. Sharma M, Beckley N, Nazareth I, et al. Effectiveness of sitagliptin compared to sulfonylureas for type 2 diabetes mellitus inadequately controlled on metformin: a systematic review and meta-analysis. BMJ Open. 2017;7(10):e017260.
16. Foroutan N, Muratov S, Levine M. Safety and efficacy of dipeptidyl peptidase-4 inhibitors vs sulfonylurea in metformin-based combination therapy for type 2 diabetes mellitus: systematic review and meta-analysis. Clin Invest Med. 2016;39(2):E48–E62. doi:10.25011/cim.v39i2.26481
17. Filion KB, Douros A, Azoulay L, et al. Sulfonylureas as initial treatment for type 2 diabetes and the risk of adverse cardiovascular events: a population-based cohort study. Br J Clin Pharmacol. 2019;85(10):2378–2389. doi:10.1111/bcp.14056
18. Laires P, Kurtyka K, Witt EA, et al. Factors associated with physicians’ decision to discontinue or down-titrate sulfonylureas for type 2 diabetes patients. Expert Rev Pharmacoecon Outcomes Res. 2019;19(1):71–79. doi:10.1080/14737167.2018.1510774
19. Douros A, Dell’Aniello S, Yu OHY, et al. Sulfonylureas as second line drugs in type 2 diabetes and the risk of cardiovascular and hypoglycaemic events: population-based cohort study. BMJ. 2018;362:k2693. doi:10.1136/bmj.k2693
20. Whitlock RH, Hougen I, Komenda P, et al. A safety comparison of metformin vs sulfonylurea initiation in patients with type 2 diabetes and chronic kidney disease: a retrospective cohort study. Mayo Clin Proc. 2020;95(1):90–100. doi:10.1016/j.mayocp.2019.07.017
21. Ke C, Morgan S, Smolina K, et al. Mortality and cardiovascular risk of sulfonylureas in South Asian, Chinese and other Canadians with diabetes. Can J Diabetes. 2017;41(2):150–155. doi:10.1016/j.jcjd.2016.08.218
22. American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S90. doi:10.2337/dc19-S009
23. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669. [Epub 2018 Oct 4]. doi:10.2337/dci18-0033
24. Hemmingsen B, Christensen LL, Wetterslev J, et al. Comparison of metformin and insulin versus insulin alone for type 2 diabetes: systematic review of randomised clinical trials with meta-analyses and trial sequential analyses. BMJ. 2012;344:e1771. doi:10.1136/bmj.e1771
25. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. doi:10.1016/S0140-6736(98)07019-6
26. Carracher AM, Marathe PH, Close KL. International diabetes federation 2017. J Diabetes. 2018;10:353–356. doi:10.1111/1753-0407.12644
27. Giugliano D, Maiorino MI, Bellastella G, Esposito K. Glycemic control in type 2 diabetes: from medication nonadherence to residual vascular risk. Endocrine. 2018;61:23–27. doi:10.1007/s12020-017-1517-9
28. Madsen KS, Kähler P, Kähler LK, et al. Metformin and second or third‐generation sulphonylurea combination therapy for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2019;4:CD012368. doi:10.1002/14651858.CD012368.pub2
29. van Baar MJ, van Ruiten CC, Muskiet MH, et al. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. DiabetesCare. 2018;41(8):1543–1556.
30. Wexler DJ. Management of persistent hyperglycemia in type 2 diabetes mellitus. N Engl J Med. 2019;56:355–2477.
31. American Diabetes Association. Glycemic targets: standards of medical care in diabetes-2018. Diabetes Care. 2020;41(Suppl 1):S55–S64. doi:10.2337/dc18-S006.
32. Choi SH, Oh TJ, Jang HC. Comparison of antidiabetic regimens in patients with type 2 diabetes uncontrolled by combination therapy of sulfonylurea and metformin: results of the MOHAS disease registry in Korea. Diabetes Metab J. 2017;41(3):170–178. doi:10.4093/dmj.2017.41.3.170
33. Gitt AK, Bramlage P, Schneider S, Tschöpe D. A real world comparison of sulfonylurea and insulin vs. incretin-based treatments in patients not controlled on prior metformin monotherapy. Cardiovasc Diabetol. 2015;14:13. doi:10.1186/s12933-015-0172-9
34. Moon JS, Ha KS, Yoon JS, et al. The effect of glargine versus glimepiride on pancreatic ß-cell function in patients with type 2 diabetes uncontrolled on metformin monotherapy: open-label, randomized, controlled study. Acta Diabetol. 2014;51:277–285. doi:10.1007/s00592-013-0553-z
35. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36(1):44–58. doi:10.1007/s12325-018-0824-8
36. Cheng V, Kashyap SR. Weight considerations in pharmacotherapy for type 2 diabetes. J Obes. 2011;2011:984245. doi:10.1155/2011/984245
37. Sola D, Rossi L, Schianca GP, et al. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;4(4):840–848. doi:10.5114/aoms.2015.53304
38. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi:10.1016/S0140-6736(15)01225-8
39. Gu J, Meng X, Guo Y, et al. The efficacy and safety of liraglutide added to metformin in patients with diabetes: a meta-analysis of randomized controlled trials. Sci Rep. 2016;6:32714. doi:10.1038/srep32714
40. Chen Z, Li G. Sodium-glucose co-transporter 2 inhibitors compared with sulfonylureas in patients with type 2 diabetes inadequately controlled on metformin: a meta-analysis of randomized controlled trials. Clin Drug Investig. 2019;39(6):521–531. doi:10.1007/s40261-019-00781-w
41. Alemi H, Khaloo P, Mansournia MA, et al. Pulse pressure and diabetes treatments: blood pressure and pulse pressure difference among glucose lowering modality groups in type 2 diabetes. Medicine (Baltimore). 2018;97(6):e9791. doi:10.1097/MD.0000000000009791
42. American Diabetes Association. Cardiovascular disease and risk management: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl1):S103–S123. doi:10.2337/dc19-S010.
43. Nichols GA, Joshua-Gotlib S, Parasuraman S. Independent contribution of A1C, systolic blood pressure, and LDL cholesterol control to risk of cardiovascular disease hospitalizations in type 2 diabetes: an observational cohort study. J Gen Intern Med. 2013;28(5):691–697. doi:10.1007/s11606-012-2320-1
44. Hanefeld M, Brunetti P, Schernthaner GH, Matthews DR, Charbonnel BH. One-year glycemic control with a sulfonylurea plus pioglitazone versus a sulfonylurea plus metformin in patients with type 2 diabetes. Diabetes Care. 2004;27(1):141–147. doi:10.2337/diacare.27.1.141
45. Roumie CL, Greevy RA, Grijalva CG, et al. Association between intensification of metformin treatment with insulin vs sulfonylureas and cardiovascular events and all-cause mortality among patients with diabetes. JAMA. 2014;311(22):2288–2296. doi:10.1001/jama.2014.4312
46. O’Brien MJ, Karam SL, Wallia A, et al. Association of second-line antidiabetic medications with cardiovascular events among insured adults with type 2 diabetes. JAMA Netw Open. 2018;1(8):e186125. doi:10.1001/jamanetworkopen.2018.6125
47. Anyanwagu U, Mamza J, Mehta R, et al. Cardiovascular events and all-cause mortality with insulin versus glucagon-like peptide-1 analogue in type 2 diabetes. Heart. 2016;102:1581–1587. doi:10.1136/heartjnl-2015-309164
48. Nystrom T, Bodegard J, Nathanson D, et al. Second line initiation of insulin compared with DPP-4 inhibitors after metformin monotherapy is associated with increased risk of all-cause mortality, cardiovascular events, and severe hypoglycemia. Diabetes Res Clin Pract. 2017;123:199–200. doi:10.1016/j.diabres.2016.12.004
49. Zhao C, Wang W, Xu D, et al. Insulin and risk of diabetic retinopathy in patients with type 2 diabetes mellitus: data from a meta-analysis of seven cohort studies. Diagn Pathol. 2014;9:130. doi:10.1186/1746-1596-9-130
50. Zhang R, Li Y, Zhang S, et al. The association of retinopathy and plasma glucose and HbA1c: a validation of diabetes diagnostic criteria in a Chinese population. J Diabetes Res. 2016;2016:4034129. doi:10.1155/2016/4034129
51. Wagnew F, Eshetie S, Kibret GD, et al. Diabetic nephropathy and hypertension in diabetes patients of sub-Saharan countries: a systematic review and meta-analysis. BMC Res Notes. 2018;11(1):565. doi:10.1186/s13104-018-3670-5
52. Kolaczynski WM, Hankins M, Ong SH, et al. Microvascular outcomes in patients with type 2 diabetes treated with vildagliptin vs. sulfonylurea: a retrospective study using German electronic medical records. Diabetes Ther. 2016;7(3):483–496. doi:10.1007/s13300-016-0177-8
53. Wright AK, Kontopantelis E, Emsley R, et al. Cardiovascular risk and risk factor management in type 2 diabetes mellitus. Circulation. 2019;139(24):2742–2753. doi:10.1161/CIRCULATIONAHA.118.039100
54. Grenier J, Leiter LA, Langer A, et al. Glycaemic control and cardiovascular risk factor management in patients with diabetes with and without coronary artery disease: insights from the diabetes mellitus status in Canada survey. Eur Heart J Qual Care Clin Outcomes. 2016;2(4):277–284. doi:10.1093/ehjqcco/qcw013
55. Connelly KA, Yan AT, Leiter LA, et al. Cardiovascular implications of hypoglycemia in diabetes mellitus. Circulation. 2015;132(24):2345–2350. doi:10.1161/CIRCULATIONAHA.115.015946
56. Gebire D. Metformin-Insulin versus Metformin-Sulfonylurea Combination Therapies in Type 2 Diabetes: A Comparative Study of Glycemic Control and Risk of Cardiovascular Diseases in Addis Ababa, Ethiopia [MSc thesis]. Addis Ababa: Addis Ababa University; 2020.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
