Back to Journals » International Journal of Nanomedicine » Volume 18
Metal–Phenolic Networks for Chronic Wounds Therapy
Authors Wang D, Xing J, Zhang Y, Guo Z, Deng S, Guan Z, He B, Ma R, Leng X, Dong K, Dong Y
Received 8 August 2023
Accepted for publication 27 October 2023
Published 8 November 2023 Volume 2023:18 Pages 6425—6448
DOI https://doi.org/10.2147/IJN.S434535
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor R.D.K. Misra
Danyang Wang,1,2 Jianfeng Xing,2 Ying Zhang,2 Ziyang Guo,2 Shujing Deng,2 Zelin Guan,2 Binyang He,2 Ruirui Ma,2 Xue Leng,2 Kai Dong,2 Yalin Dong1
1Department of Pharmacy, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China; 2School of Pharmacy, Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China
Correspondence: Kai Dong, School of Pharmacy, Xi’an Jiaotong University, 76 Yanta West Road, Xi’an, Shaanxi, 710061, People’s Republic of China, Tel/Fax +86-29-82655139, Email [email protected] Yalin Dong, Department of Pharmacy, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China, Te1 +86-29-85323241, Fax +86-29-85323240, Email [email protected]; [email protected]
Abstract: Chronic wounds are recalcitrant complications of a variety of diseases, with pathologic features including bacterial infection, persistent inflammation, and proliferation of reactive oxygen species (ROS) levels in the wound microenvironment. Currently, the use of antimicrobial drugs, debridement, hyperbaric oxygen therapy, and other methods in clinical for chronic wound treatment is prone to problems such as bacterial resistance, wound expansion, and even exacerbation. In recent years, researchers have proposed many novel materials for the treatment of chronic wounds targeting the disease characteristics, among which metal–phenolic networks (MPNs) are supramolecular network structures that utilize multivalent metal ions and natural polyphenols complexed through ligand bonds. They have a flexible and versatile combination of structural forms and a variety of formations (nanoparticles, coatings, hydrogels, etc.) that can be constructed. Functionally, MPNs combine the chemocatalytic and bactericidal properties of metal ions as well as the anti-inflammatory and antioxidant properties of polyphenol compounds. Together with the excellent properties of rapid synthesis and negligible cytotoxicity, MPNs have attracted researchers’ great attention in biomedical fields such as anti-tumor, anti-bacterial, and anti-inflammatory. This paper will focus on the composition of MPNs, the mechanisms of MPNs for the treatment of chronic wounds, and the application of MPNs in novel chronic wound therapies.
Keywords: metal–phenolic networks, chronic wounds, antimicrobial, antioxidant, reactive oxygen species scavenging, revascularization
Introduction
Chronic wounds are pathological disorders of skin healing characterized by wound ulceration, bacterial infection, persistent inflammation, and proliferation of reactive oxygen species (ROS) levels.1–3 With a lingering course, most chronic wounds do not regain their tissue morphology and functional integrity even after 3 months.4,5 Due to the colonization of bacteria in chronic wounds, they possess a more severe inflammatory response and a difficult wound remodeling process than normal wounds.6,7 Currently, more than 2% of the global population suffers from chronic wounds with a five-year mortality rate approaching 50%.4,8,9 As the aging of the population, chronic wounds caused by chronic diseases such as diabetes will increasingly affect people’s lives and health.10
Clinical treatments used for chronic wounds are mainly mechanical debridement, which can quickly and effectively remove inactivated tissue from wounds but may damage normal tissue.11,12 Autolytic debridement with endogenous hydrolases is also possible, but its onset of action is slow (within 72 hours) and it should not be used in septic patients.13 For bacterial infection, short-term use of antimicrobial drugs is effective in sterilization, but long-term use is prone to drug resistance.14 The short-term use of topical anti-inflammatory drugs can quickly regulate the level of inflammatory factors and accelerate wound healing, but long-term use may inhibit platelet aggregation, prolong bleeding time and cause skin lesions.15 In addition, hyperbaric oxygen therapy is also an effective method to improve wound hypoxia and oxidative stress and inhibit anaerobic bacteria, which can increase blood oxygen concentration, effectively improve microcirculation, and promote capillary regeneration and wound healing, but may also cause oxygen toxicity, pneumatic pressure injury, and decompression sickness.16,17
In recent years, researchers have developed several novel materials for wound hemostasis or wound remodeling applications.18–22 Tissue adhesives (eg, cyanoacrylate-based glues, purified bovine serum albumin and glutaraldehyde, fibrin, and synthetic polymer sealants) are highly adhesive and can be used for wound hemostasis,23 they suffer from problems of mechanically inappropriate, cytotoxic, and biocompatible, and their removal can easily cause secondary damage.24,25 Some bioengineered materials, such as epidermal and dermal substitutes can mimic real biological structures and activate the healing cascade in patient wounds, but they are expensive to produce and have a relatively slow speed of clinical translation.26,27 Nanotechnology has opened up a new field of application in the treatment of chronic wounds, and some researchers have proposed nanomaterials with their therapeutic properties as “drug-loaded” formulations to further improve the efficacy of nanomedicines in the treatment of chronic wounds.5,28–31 However, it also suffers from cumbersome synthesis steps and difficulties in clinical translation.32,33 Therefore, there is an urgent need to explore the development of novel therapeutic options.34,35
Metal–phenolic networks (MPNs) are supramolecular amorphous molecular networks formed by the coordination of multivalent metal ions with polyphenols.36 Structurally flexible MPNs-based nanoparticles or coatings can be constructed by various methods, such as the “one-step” assembly method, “multi-step” assembly method, and sol-gel method.37–41 The excellent physicochemical properties can be used in various biomedical fields such as photothermal therapy and Fenton reaction. In the last decade, the research on MPNs has been increasing day by day.42 In the decade following this, the research on MPNs has been increasing gradually.41,43–45 One of its main components, natural polyphenols, have been shown to have various effects such as anti-tumor,46–48 anti-inflammatory,49–51 anti-bacterial52–54 and anti-thrombotic,55 and many of the polyphenols have been approved by the US Food and Drug Administration (FDA) as ingredients in food or drugs.41 In chronic wound treatment, polyphenols can promote wound healing by alleviating wound oxidative stress and modulating excessive inflammatory responses. Metal ions have long been widely used in antimicrobial applications, and recent studies have shown that they can not only kill bacteria through direct contact and the production of reactive oxygen species but also promote neovascularization at the wound site. The abundance of polyphenols and metal ions endows MPNs with a variety of properties that broaden their application in biomedical therapies. MPNs can also play a therapeutic role through some novel ROS-based therapies, such as chemodynamic therapy (CDT), photodynamic therapy (PDT), and sonodynamic therapy (SDT).56–59 In addition, some studies in recent years have shown that MPNs formed by some polyphenols have photothermal effects.38,60,61 Therefore, MPNs can also realize multiple therapeutic effects by photothermal therapy (PTT).62 There have been several papers summarizing and organizing the applications of MPNs in antibacterial and antitumor applications.36,63–65 However, their role in the treatment of chronic wounds has not yet been carefully discussed; therefore, in this paper, we will systematically summarize the composition of MPNs, their mechanisms for the treatment of chronic wounds, and the application of MPNs in novel chronic wound therapies.
Composition of MPNs
MPNs are composed of a wide variety of metal ions and polyphenols, and their use in chronic wound therapy in recent years is summarized in Table 1. This section will next summarize the typical metal ions and polyphenols that makeup MPNs and the role they play in chronic wound therapy, as reported in recent studies.
 |
Table 1 Different Combinations of MPNs for Chronic Wound Treatment |
Metal ions released from metal-containing compounds have significant therapeutic properties for chronic wounds. The metal ions in MPNs are mainly multivalent metal ions, including some noble metal ions, alkaline earth metal ions, transition metal ions, rare earth metal ions, and some common metal ions, and their roles in MPNs are significantly correlated with the properties of the metal ions themselves (Table 1). For example, precious metals, especially silver (Ag), gold (Au), and platinum (Pt) have excellent physicochemical properties and biochemical functions which allow them to be used as antimicrobials in chronic wound therapy.83–88 Calcium (Ca), an alkaline earth metal, is useful in all phases of chronic wound treatment.89 Calcium ions (also known as coagulation factor IV) can trigger an endogenous coagulation cascade reaction with other coagulation factors during the hemostatic phase, accelerating the synthesis of thrombin to promote coagulation.79 During the inflammatory phase, calcium regulates neutrophil function,90 and it is also a major regulator of multiple signaling pathways that promote keratinocyte differentiation and regulate angiogenesis during the proliferative and remodeling phases.91 Some transition metal ions also have positive implications for chronic wound treatment. For example, iron ions can sterilize wounds by local production of ROS via the Fenton reaction,56 and copper plays a key role in skin regeneration and angiogenesis and accelerates the healing process through induction of vascular endothelial growth factor (VEGF) and angiogenesis by hypoxia-induced factor 1-alpha (HIF-1α) action.92
Polyphenols are secondary metabolites that are widely distributed in fruits and vegetables.93 Natural polyphenols are plant-derived organic molecules whose structure is characterized by the presence of two or more phenol units.36,43 Depending on the chemical structure, natural polyphenols can be divided into different classes: flavonoids, phenolic acids, stilbene, lignans, etc.64 Common natural polyphenols used in chronic wound treatment include epigallocatechin gallate (EGCG), epigallocatechin gallate (EGC), epigallocatechin gallate (ECG), CAT, TA, quercetin, proanthocyanidins, gallic acid (GA), etc.94 These natural polyphenols have excellent biomedical functions, and they can be used not only as therapeutic agents for antitumor, antioxidant, anti-inflammatory, and antibacterial diseases but also as major component modules that play an important role in novel drug delivery systems.95–98 For example, Polyphenols can protect key cellular components from reactive free radical damage, primarily due to their properties of activating antioxidant enzymes and mitigating oxidative stress and inflammation.97 It also inhibits or isolates reactive oxygen species and transfers electrons to free radicals, thereby avoiding cellular damage.99 It has a regulatory role in glucose metabolism and holds great promise in diabetes prevention and intervention. It also prevents cardiovascular disease by regulating blood pressure and lipids.100 The multiple hydroxyl groups of natural polyphenols allow them to exhibit strong adhesion not only with inorganic molecules (eg, silicon-based materials and metals) through covalent or non-covalent interactions,41 but also easily with biomolecules through hydrogen bonding and non-covalent interactions for better attachment to the wound surface for a longer-lasting therapeutic effect of the material.101
The assembly of MPNs is mainly based on the pH dependence of the ligand bonding between the catechol moiety and the metal ion as the crosslinking unit.37 The presence of catechol or catechol groups in phenolic compounds provides binding sites for the chelation of metal ions. Depending on the pH, monocomplexes, bicomplexes or tricomplexes can be formed.102 Two adjacent phenol groups in polyphenols can be complex with metal ions in the form of oxygen-negative ions to form stable five-membered cyclic chelates. The third phenol light group in the structure of o-benzenetriol, although not involved in the complexation, can promote the dissociation of the other two phenol light groups, thus contributing to the formation and stabilization of the final complex.103
Treatment Mechanisms of MPNs for Chronic Wounds
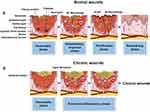
Organismal wound healing is a dynamic process consisting of four consecutive, overlapping, and precisely regulated phases: the hemostatic phase, the inflammatory response phase, the proliferative phase, and the remodeling phase104–106 (Figure 1). Hemostasis is the first stage of wound healing. When the skin is injured, rapid vasoconstriction and collagen exposure in the subendothelial matrix cause platelet aggregation and platelet plug formation.107 In addition, the coagulation cascade is activated and soluble fibrinogen is converted to insoluble chains and forms a fibrin network, which binds to the platelet plugs to form a thrombus, thereby stopping bleeding.108 Next, the normal wound enters the inflammatory response phase, where its microenvironment contains a variety of reactive cells and their regulatory factors that adequately and accurately promote neovascularization.109 For example, leukocytes recruited to the wound may secrete appropriate concentrations of ROS and proteases to help fight bacteria and foreign pathogens, while wound endothelial cells also secrete growth factors that promote extracellular matrix (ECM) remodeling.9 During the proliferative phase of wound healing, healing processes such as re-epithelialization and neovascularization occur simultaneously. Fibroblasts synthesize new ECM and aid in wound contraction and also act as scaffolds for newly synthesized ECM, neovascularization, and inflammatory cells to promote wound tissue reconstruction.110,111 The remodeling phase includes the degradation of neovascularization, periodic deposition of ECM, and subsequent remodeling of granulation and scar tissue.112
However, in chronic wounds, due to the presence of bacteria and endotoxins, the levels of inflammatory factors are abnormally elevated, causing the wound to enter a vicious cycle of inflammatory response. Bacterial biofilms interact with the host immune system through the activation of pro-inflammatory macrophages and neutrophils, leading to the accumulation of inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), as well as metallo-matrix protease (MMP) and promoting sustained inflammation.3 Failure of normal secretion of inflammatory factors and macrophage differentiation at the wound site leads to programmed anti-inflammatory modulation and antimicrobial failure.113,114 In addition, poor circulation and impaired angiogenesis in the wound site camp lead to inadequate nutrient and oxygen supply, aging of fibroblasts, reduced cell proliferation, impaired collagen production and deposition, and failure of ECM remodeling.109,115 In brief, chronic wounds suffer from three main problems: first, bacterial infection; second, a chronic state of inflammatory imbalance; and third, the lack of a suitable environment to promote cell migration, proliferation, and angiogenesis.4 Ingeniously, recent studies have shown that MPNs can simultaneously achieve multiple goals required for wound healing, including antimicrobial, immunomodulatory, and regenerative effects, enhancing a beneficial healing environment while disrupting pathogenic mechanisms.
Hemostasis
Hemostasis is a complex process. In this process, vasoconstriction accelerates the formation of coagulation factors, which in turn activates platelets to form platelet hemostatic plugs for wound blockage, realizing initial hemostasis. Then, the soluble fibrinogen in plasma will be converted into insoluble fibrin and intertwined into a network to reinforce the hemostatic thrombus and achieve effective hemostasis. Chronic wounds, however, have poor coagulation function, low activity of coagulation factors, and fragile blood vessels at the wound site, leading to repeated wound rupture and continuous blood seepage, causing a delay in wound healing. Therefore, hemostasis is one of the first key problems that should be overcome in the treatment of chronic wounds.69
TA is a hemostatic adhesive that can adhere to tissues around wounds and directly interact with proteins in the blood to promote hemostasis. Based on this, Chen et al prepared mesoporous silica nanoparticles (Ca-TA-MSN@Ag) modified by tannic acid (TA), silver nanoparticles, and calcium ions. During the coagulation process, TA plays the role of vasoconstriction, and Ca-TA-MSN@Ag with a high specific surface area and large pore volume can induce erythrocyte aggregation and form a three-dimensional network structure with fibrin to accelerate coagulation. In addition, calcium ions as coagulation factor IV and the negative charge of Ca-TA-MSN@Ag accelerated the coagulation cascade reaction (Figure 2A and B). These three synergistic effects on an animal model showed that Ca-TA-MSN@Ag reduced the hemostatic time by nearly 50% compared to mesoporous silica. The good biocompatibility and bacteriostatic activity (~99%) of Ca-TA-MSN@Ag suggest that it is a promising biomaterial for clinical applications.79
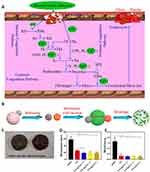 |
Figure 2 MPNs treat chronic wounds by hemostasis. Potential hemostatic (A) and antimicrobial (B) mechanisms of Ca-TA-MSN@Ag. Reprinted from Chen JW, Qiu LP, Li QL, et al. Rapid hemostasis accompanied by antibacterial action of calcium crosslinking tannic acid-coated mesoporous silica/silver Janus nanoparticles. Mat Sci Eng C-Mater. 2021;123:111958, Copyright 2021, with permission from Elsevier.79 (C) CS/Ag/TA cryogel before and after blood absorption. Hemostasis time (D) and blood loss (E) in a rat liver injury model treated with gauze, CS and CS/Ag/TA cryogel. **p-value < 0.01. Reprinted from Xu G, Xu N, Ren TJ, et al. Multifunctional chitosan/silver/tannic acid cryogels for hemostasis and wound healing. Int J Biol Macromol. 2022;208:760–771, Copyright 2022, with permission from Elsevier.80 |
The ability to enrich blood cells is also a key factor in assessing the hemostatic function of hemostatic materials. Therefore, Xu et al prepared a multifunctional cryogel CS/Ag/TA, which has great potential in hemostasis and wound healing. The water absorption and hemocyte adhesion functions of CS/Ag/TA enable it to capture a large number of hemocytes in a short period, which provides strong hemostatic ability (Figure 2C). The porosity of CS/Ag/TA ensures that the material has good mechanical strength, which gives it the shape memory capability while creating a comfortable microenvironment for cell proliferation. In addition, Ag NPs introduced as antimicrobial materials can effectively inhibit Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli with good antimicrobial effect and low cytotoxicity. The natural polyphenol TA in CS/Ag/TA can scavenge free radicals and control oxidative stress in wounds, in addition to acting as a cross-linking agent to improve the mechanical properties of the gel. The above advantages suggest that it is expected to be used as a hemostatic and pro-wound healing material for clinical applications.80
Antibacterial
Antimicrobial therapy is a priority in the treatment of chronic wounds because they are often accompanied by bacterial infections.4 Currently, widely acceptable antimicrobial mechanisms of MPNs include: 1) release of metal ions to disrupt bacterial cell membranes; 2) generation of ROS to damage bacteria and their biofilms; and 3) interaction with proteins, polypeptides, deoxyribonucleic acid (DNA), and other substances to damage and affect their functions.
Antimicrobial metal ions have been used in the treatment of infections and can damage bacteria by direct contact with them and their biofilms, but their application has been limited by greater cytotoxicity. Recent studies have reduced the undesirable cytotoxic and genotoxic effects caused by Ag NPs alone as well as bimetallic antimicrobial systems (eg, Fe-Ag, Pt-Pd, Co-Au, etc.) through the introduction of MPNs doped with polyphenolic compounds or metal ions (Fe3+, Al3+, Sm3+, etc.), which have dramatically improved their biocompatibility and made it possible to design safer and more effective materials for biomedicine.116–120 For example, in the study of Hu et al, silver nanoparticles modified with gallic acid (GA@AgNPs) were enriched with carboxyl groups, which could chelate with divalent ions in the gel network and form ionic bonds with sodium alginate (SA), strongly immobilizing the silver nanoparticles in the hydrogel network. Ag+ was sustainably released from the GA@AgNPs-SA hydrogels, with a cumulative leaching rate of 8.74% over seven days. Cellular experiments showed that it possessed good cytocompatibility, and the bacterial morphology treated with the gel exhibited significant cell membrane rupture and cell shrinkage or rupture, suggesting that the GA@AgNPs-SA hydrogel exhibited excellent bactericidal ability.81
Due to the presence of multiple phenolic hydroxyl groups in polyphenols, they exert antioxidant effects mainly by scavenging reactive oxygen species when used alone.121 However, when polyphenols are used as constituent structures to prepare with metal ions to obtain MPNs, polyphenols can enhance the Fenton reaction by reducing metal ions in high oxidation states to metal ions in low oxidation states, which improves the reutilization of metal ions,122 thus produce more ROS to cause damage to bacteria. For example, Arakawa et al showed that the tea polyphenol EGCG can react with dissolved oxygen in an alkaline aqueous solution and act as a pro-oxidant for auto-oxidation by generating hydrogen peroxide (H2O2).123,124 Based on this, Chen et al synthesized eight metal-EGCG networks and experimentally verified that metal ions can accelerate the production of H2O2 from EGCG, while Fe3+ can further catalyze the decomposition of hydrogen peroxide to generate ·OH, which is effective against bacteria and their pathogenic biofilms125 (Figure 3). Xie et al synthesized Ag@Cu alloy nanostructures (Ag@Cu-MPNNC) employing iron (III)-tannic acid (FeIII-TA) MPN support, which could damage bacteria by producing high levels of ROS, while the released Ag+ ions disrupted the bacterial cell membranes further contributing to the effective penetration of ROS into the cytoplasm and the oxidized intracellular contents. The coating exhibited effective antibacterial activity against both Gram-positive and Gram-negative bacteria without any off-target effects on mammalian cells. Importantly, Ag@Cu-MPNNC also showed significant therapeutic effects on wound healing in infected animals with infected skin wounds by reducing inflammation and promoting hemotransfusion remodeling of injured tissues.66
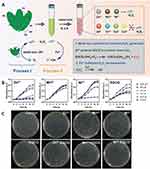 |
Figure 3 MPNs treat chronic wounds with antimicrobial therapy. (A) Schematic diagram of the MPN formed by 8 metal ions and EGCG to promote H2O2 production by EGCG. (B) Antimicrobial screening experiments. Growth inhibition curves of EGCG, Zn2+, Mn2+, Ni2+ against E. coli at specified time and concentration. (C) Bacterial culture experiment of E. coli with MEN. Reprinted from Chen S, Yan Y, Yu Y, et al. Ferric Ions as a Catalytic Mediator in Metal-EGCG Network for Bactericidal Effect and Pathogenic Biofilm Eradication at Physiological pH. Adv Mater Interfaces. 2021;8(23):2101605, Copyright 2021, with permission from John Wiley and Sons.125 |
In recent years, studies on antibacterial mechanisms have gradually increased, and researchers have gradually devoted their attention to killing bacteria by delivering some agents that interact with other components such as bacterial membrane proteins and secreted proteins. For example, siderophore is a low molecular weight substance secreted by microorganisms, such as bacteria and fungi, that efficiently binds iron in the surrounding environment to form stable chelates with it. Since Ga3+ is similar to Fe3+ in terms of radius, electronegativity, charge, and coordination number, it can replace iron as a “Trojan horse” against bacteria. Ga3+ in the environment can be taken up by bacteria through their iron carrier system, and since Ga3+ is not reducible, the continuous oxidation and reduction cannot continue, preventing the critical oxidation of iron (from Fe2+ to Fe3+), which in turn affects the normal physiological metabolism of bacteria.126,127 Based on this, Ghalayani Esfahani et al designed chitosan (CS)/gallium (Ga) composite coatings, which were applied to implant surfaces by electrophoretic deposition. It was shown that the CS/Ga coatings on orthopedic implants showed good bactericidal activity and biocompatibility, and could effectively reduce infections in orthopedic implant applications.82 It has also been shown that rare earth ions ytterbium ions, whose ionic radius is close to that of calcium ions, can displace calcium ions in the binding sites on cell membranes and thus inhibit microbial growth. Natural polyphenolic compounds possess a large number of phenolic hydroxyl groups, which can be used to capture metal ions and immobilize them on the substrate, giving new properties to the materials. Thus, Liu et al synthesized an antimicrobial membrane material by covalently bonding ytterbium ions (Yb3+) to the plant polyphenols catechin (CAT), EGCG, and black catechin (BWT) and immobilizing them on a polyamide membrane, which can prevent biofilm-induced associated infections in a safe, inexpensive, and sustainable manner.78
Anti-Inflammatory
Inflammation is a natural defense response to irritants such as infection, injury, and toxins.128–130 However, excessive and uncontrolled inflammation has been shown to contribute to many diseases such as cardiovascular disease, hepatitis, nephritis, and delayed wound healing.9,131 For chronic wounds, infection by bacteria and their biofilms, failure of the body’s immune regulation, and hypoxia in the wound microenvironment all contribute to the persistence of wound inflammation. Macrophages are the most abundant immune cells during the inflammatory phase and are crucial in the healing process.132,133 However, failure of macrophages to polarize from pro-inflammatory (M1 phenotype) to reparative (M2 phenotype) phenotypes may also lead to chronic wounds.3,134,135 At this time, macrophages of the M1 phenotype are impaired in scavenging apoptotic neutrophils, and overexpressed inflammatory mediators [eg, IL-17, TNF-α, inducible nitric oxide synthase (iNOS), and ROS] accumulate in the wound, which will negatively affect the wound microenvironment. In contrast, macrophages of the M2 phenotype, known as healing-associated macrophages, can secrete chemokines, cytokines, and growth factors, such as TGF-α, TGF-β, and VEGF, which are beneficial for stimulating the proliferation of wound cells and initiating angiogenesis.3 Polyphenols are one of the important anti-inflammatory components in MPNs, some of which can act directly on inflammatory response pathways to affect macrophage polarization, for example, by inactivating the nuclear factor-kappa light-chain enhancer (NF-κB), which activates B-cells, and by regulating the mitogen-activated protein kinase (MAPK) and arachidonic acid pathways.136 For example, Corrêa et al investigated the effects of a Brazilian red propolis extract that contains several polyphenolic fractions (catechins, flavonols, chalcones, isoflavones, isoflavans, pterocarpans, and bioflavonoids) on inflammation and wound healing in mice, and found that the extract could down-regulate the expression of the inflammatory transcription factor pNF-κB protein and reduce the secretion of inflammatory cytokines (eg, TGF-β, TNF-α) and IL-6, which had a positive effect in the wound healing process.137 In addition, due to their antioxidant activity, polyphenols can exert anti-inflammatory effects by scavenging ROS or inhibiting certain enzymes involved in the production of ROS, such as xanthine oxidase and NADPH oxidase, thereby slowing down the induction of ROS on macrophages, promoting the shift of macrophages from a pro-inflammatory M1 phenotype to a pro-repairing M2 phenotype, and facilitating wound remodeling.136,138
As the most abundant polyphenol in green tea, EGCG has been reported to be a potent anti-inflammatory agent through the repolarization of macrophages from M1 to M2 phenotype.70,139,140 Unfortunately, its poor bioavailability and rapid metabolism severely limit its application. To address this problem, Xu et al designed Fe3+/EGCG-based photothermite nanoparticles (BSA@MPN) by self-assembly of Fe3+ and EGCG using BSA as a nanoreactor and colloidal stabilizer. Based on the high photothermal conversion efficiency of Fe3+/EGCG-based MPNs in the NIR region and the fact that BSA@MPN can be decomposed in infected tissues with the continuous release of acidic pH after photothermal sterilization treatment. The delivery of EGCG through this nanosystem enhanced the colloidal stability and biosafety of EGCG. Due to the release of EGCG, BSA@MPN repolarized M1 macrophages to M2 phenotype, which successfully achieved a satisfactory therapeutic effect of combined photothermal antimicrobial and anti-inflammatory treatment70 (Figure 4A).
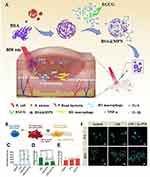 |
Figure 4 MPNs treat chronic wounds through anti-inflammation. (A) Schematic representation of the synthesis of BSA@MPN and its application in antibacterial and anti-inflammatory therapy. Reprinted from Xu YY, Cai YJ, Xia Y, et al. Photothermal nanoagent for anti-inflammation through macrophage repolarization following antibacterial therapy. Eur Polym J. 2023;186:111840, Copyright 2023, with permission from Elsevier.70 (B) Scheme illustrates that stimulated by LPS, M0 macrophages transformed to M1 phenotype in the pro-inflammatory phase. Treated with Cu-PTA NPs, the macrophages experience polarization and differentiate into M2 phenotype. (C) Quantitative analysis of TNF-α levels using ELISA kit in LPS-treated RAW264.7. (D and E) Fluorescent intensity quantitated by Image J culturing in LPS with Cu-PTA NPs for 24 h with immuno-fluorescent stained iNOS and CD206. (F) Representative morphology of macrophage under LPS stimulating and skeletal polarization over 4/24 h in the absence or presence of 25 μg mL−1 Cu-PTA NPs. Scale bar = 20 μm; ns, p-value > 0.05, **p-value < 0.01, ***p-value < 0.001. Reprinted from Li DY, Li JR, Wang SW, Wang QM, Teng W. Dually Crosslinked Copper-Poly (tannic acid) Nanoparticles with Microenvironment-Responsiveness for Infected Wound Treatment. Adv Healthc Mater. 2023;12(17):2203063, Copyright 2023, with permission from John Wiley and Sons.139 |
In addition, due to the antioxidant activity of polyphenols, they can indirectly exert anti-inflammatory effects by scavenging ROS. For example, Li et al developed a TA-engineered non-porous copper (II) nanoplatforms (Cu-PTA NPs) for antibacterial and anti-inflammatory synergistic therapeutic use. Cu-PTA NPs have a ROS-stimulated responsive release mode, which is a structure that allows for ROS-responsive degradation of the bacterial infected region and on-demand release of Cu2+, significantly prolonging the antimicrobial period and minimizing side effects. In addition, the degradation of Cu-PTA NPs scavenges ROS, which can subsequently promote macrophage M2 polarization and facilitate angiogenesis by upregulating M2 phenotype-related gene expression, thereby systematically promoting infected wound healing139 (Figure 4B–F).
Removal of Excessive ROS
ROS, which are produced early in wound formation and are essential for wound healing, are the primary reactive substances for the immune system to exert antimicrobial activity during inflammatory processes. Trace amounts of ROS can recruit neutrophils and other leukocytes to the wound site and act as signaling molecules to activate epithelial signaling pathways involved in epidermal regeneration.77,141 Subsequently, these ROS are usually rapidly neutralized by non-enzymatic and enzymatic antioxidants, thereby maintaining oxidant/antioxidant balance and hence tissue homeostasis.142 However, oxidative stress from uncontrolled ROS production or depletion of the antioxidant system may lead to inappropriate or defective wound responses and promote chronic injury and inflammation. In chronic wounds, neutrophils and monocytes produce excess ROS, activate NF-κB, and upregulate MMP-9. Excess unregulated MMP-9 disrupts the ECM and prevents wound healing. In addition, advanced glycosylation end products (AGEs) in diabetic wounds can induce excessive ROS production, leading to severe oxidative damage and aging of the ECM and cell membranes, which ultimately results in poor angiogenesis and re-epithelialization, insufficient growth factor production, and prolonged inflammatory response.131,141
To achieve sustained release of the polyphenol EGCG, which can play a role in scavenging ROS,143 Zeng et al designed and prepared a multifunctional hydrogel (SC-E@Cu) dressing containing N-methacryloyl true silk phenanthrene, k-type carrageenan (kCA), EGCG and Cu2+. The polyphenol-metal functional complexes and ion-covalent entanglement networks were formed by introducing Cu2+, and the mechanical properties of the gels were enhanced. In addition, Cu2+ chelated with EGCG to form an EGCG-Cu2+ complex, which could continuously release EGCG. The phenolic hydroxyl structure of EGCG makes it easy to release protons and can effectively capture ROS. It was shown that the structure has sufficient mechanical strength, excellent antimicrobial properties, controlled antioxidant action, and good biocompatibility, providing a new strategy for chronic wound care74 (Figure 5).
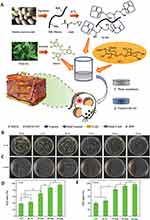 |
Figure 5 MPNs treat chronic wounds by removing ROS. (A) Preparation of SC-E@Cu hydrogels with multifunctional properties for potential application in chronic wound treatment. Plate images of (B) E. coli and (C) S. aureus after co-culture with each dosing group. Relative bactericidal rates of each dosing group against (D) E. coli and (E) S. aureus. *p ≤ 0.5. Reprinted from Zeng ZW, Guo CP, Lu DH, Geng ZJ, Pei DT, Yu S. Polyphenol-Metal Functionalized Hydrogel Dressing with Sustained Release, Antibacterial, and Antioxidant Properties for the Potential Treatment of Chronic Wounds. Macromol Mater Eng. 2022;307(10):2200262, Copyright 2023, with permission from John Wiley and Sons.74 |
Oxygen free radicals play a key role in inflammatory processes; therefore, nanocarriers with free radical scavenging properties may have anti-inflammatory and cytoprotective effects. Inspired by MPNs, Qin et al introduced a metal-phenolic assembly strategy into the field of starch science to prepare a novel multifunctional modified starch. Two series of novel multifunctional modified starch nanoparticles (MPN@DBS-NPs and MPN@SNCs) were obtained based on the introduction of TA and iron ions. The functionalized cellular assay confirmed the non-toxicity and high biocompatibility of MPN@DBS-NP, which has good prospects for application.68
TA is a common antioxidant that has been reported to eliminate various types of free radicals and is beneficial for the healing of damaged skin.41 MPNs represented by iron tannate (TA-Fe) complexes have shown many fascinating properties in skin wound care, including bactericidal activity, photothermal properties, free radical scavenging ability, anti-inflammatory effects, etc. Li et al reported an alginate-based aerogel dressing (TA-Fe MPN) with application to photo-thermally-responsive antimicrobial wound dressings. It combines photothermal bactericidal activity, hemostatic properties, and free radical scavenging for disinfecting skin wounds and accelerating wound healing. After 10 days of treatment of MRSA-infected whole-skin wound model mice with this gel, the state of wound healing and recovery of new hairs on the skin in the treated group showed comparable results to clinical antibiotic drugs, providing a viable route for photoactivated wound dressings.69
Promotion of Revascularization
The formation of neovascularization or new blood vessels is essential for wound healing. It provides oxygen, growth factors, and immune support to the healing tissues, thus allowing cell proliferation and tissue regeneration to occur.144 However, in chronic wounds, excessive inflammatory response prevents or impairs the initial formation of microvasculature, further disrupting ECM remodeling and causing delayed wound healing. For example, in diabetic patients, a number of them develop a chronic wound complication, diabetic foot ulcer (DFU), caused by microvascular or macrovascular injury.8,145 Therefore, promoting wound angiogenesis and establishing normal tissue cell microcirculation at the wound site is also a critical component in the treatment of chronic wound injury.146
VEGF is a key dynamic molecule in angiogenesis, and several studies have shown that metal ions released from metal–phenolic networks have a significant promotional effect on VEGF production.147–149 Zinc-based metal-epigallocatechin gallate capsules (EGCG/ZnPs) prepared by Chen et al could reduce the in vitro cytotoxicity of VEGF by slow and sustained release of zinc ions (Zn2+) and cytotoxicity in vitro and in vivo, enhance VEGF secretion, and promote angiogenesis. EGCG can scavenge ROS and realize the improvement of microenvironmental oxidative stress. In a mouse limb ischemia model, EGCG/ZnPs significantly promoted angiogenesis and cell proliferation in ischemic tissues.75
Liu et al developed a controlled-release antimicrobial composite hydrogel system that also promotes wound angiogenesis and inhibits the development of inflammation through sustained release of Cu2+ and EGCG. The prepared SilMA/HAMA/Cu-EGCG hydrogels showed significant inhibitory effects on E. coli and promoted the proliferation and migration of L929 fibroblasts. In addition, the results of tube-forming experiments using matrix gels showed that the SilMA/HAMA/CuEGCG group had significant tubule formation and branching points, which was attributed to the fact that Cu2+ could increase the expression levels of PDGF-BB and VEGF and promote angiogenesis. In vivo whole-layer infected wound healing experiments confirmed its angiogenic and inflammation-modulating effects. Accelerated collagen deposition and accelerated wound healing were also observed, suggesting that this controlled-release antimicrobial composite hydrogel has great potential for application in chronic wound healing.150
Application of MPNs in Novel Therapies
Currently, clinical management of chronic wounds includes mechanical debridement, autolytic debridement, hyperbaric oxygen therapy, and the use of antimicrobial and anti-inflammatory drugs. These therapies may not only cause wound recurrence and expansion but also tend to trigger drug resistance, which prevents wound recovery and remodeling. In recent years, there has been a gradual increase in the number of novel therapies applied to chronic wounds. These drug-free therapies avoid drug resistance and can exert their therapeutic effects by other means, such as physical and chemical. Some novel therapies that play a therapeutic role based on ROS, such as CDT, PDT, and SDT, can better promote wound healing by locally modulating ROS levels at the wound site.151–154 In addition, PTT utilizes a photothermal agent that absorbs the energy of near-infrared (NIR) light and converts it into heat, effectively killing bacteria by disrupting the structure of their membranes, thus facilitating the wound’s timely entry into the recovery remodeling phase.155,156 Compared with traditional therapeutic drugs, MPNs have the dual characteristics of metal ions and polyphenols, which can not only exert the properties of each component to achieve antibacterial, anti-inflammatory, and oxidative stress relief, but also interact with each other, and achieve the therapeutic effect of “1+1>2” through the novel therapeutic approach.
Chemodynamic Therapy Based on MPNs
Chemodynamic therapy (CDT) uses the chronic wound microenvironment Fenton reaction to produce highly toxic hydroxyl radicals (·OH) to achieve bactericidal treatment. Fenton and its associated reactions have attracted widespread interest in several fields due to the discovery by Henny J. Fenton that H2O2 can be activated by Fe2+ to oxidize tartaric acid.157,158 The reaction between Fe2+ and hydrogen peroxide (Fe2+ + hydrogen peroxide → Fe3+ + ·OH + hydroxide) can produce highly active ·OH. ·OH with high redox potential (E (·OH /H2O) = 2.8 V) can regulate cellular metabolism in the chronic wound microenvironment and act as a bactericidal agent to repel infection. Therefore, MPN materials containing multivalent metal ions based on this design can achieve the treatment of chronic wound infections by CDT and have negligible resistance to bacteria. For example, in Fenton-reactive nanosystems mediated by transition metal ion materials (eg, Fe2+ and Cu2+), high-activity low-valence state ions are oxidized to low-activity high-valence state ions (eg, Fe2+ is oxidized to Fe3+ and Cu+ is oxidized to Cu2+). As polyphenols contain multiple hydroxyl groups, they can exhibit reducing properties, and when containing 1,2,3-trihydroxyl or 1,3,5-trihydroxyl arrangement (eg, epicatechin gallate, epigallocatechin gallate, and gallic acid), the polyphenols are more antioxidant and have superior reducing properties.122 Therefore, when the polyphenols with good reducing properties are introduced to construct MPNs, the transition metal ions can be realized from the high valence state (Fe3+) to the low valence state (Fe2+), which enhances the conversion of low-activity Fenton chemical ions to the high-activity chemical ions and improves the Fenton effect.
However, it is hindered by constant body temperature, lack of Fe2+ ions in infected tissues, and insufficient H2O2 levels. To improve the therapeutic efficiency of CDT, Xu et al developed an infection microenvironment-responsive nanotherapy using dual near-infrared (NIR)/dihydroartemisinin (DHA) enhanced antimicrobial CDT. The antimicrobial nanoagent Fe2O3@DHA@MPN (FDM) was synthesized by wrapping α-Fe2O3 loaded with DHA in an MPN shell. The MPN component in this system played a multifunctional role. First, pH-induced degradation of MPN can induce the simultaneous release of TA and Fe2+ ions; second, MPN degradation can achieve the release of DHA at the site of bacterial infection, while the breakage of the weak internal peroxide bridge of DHA in the presence of Fe2+ ions lead to the generation of toxic ROS (alkoxyl radicals) mediating the onset of CDT. Third, Fe3+ ions generated in H2O2-mediated CDT can be reduced to Fe2+ ions by TA to achieve the sustainability of DHA-mediated CDT. Fourth, MPN coatings with excellent photothermal properties can be used for PTT and NIR-enhanced CDT. Fifth, the TA-mediated bacterial adhesion effect can enhance the bactericidal effect of nanoparticles.56
Due to the high systemic glucose concentration in diabetic patients, the sterile healing of infected skin wounds is severely hampered, while glucose oxidase can produce gluconate and H2O2 while consuming glucose, lowering the pH of the wound microenvironment while increasing the H2O2 level, thus achieving enhanced CDT. Based on this, Shi et al designed a smart natural product for high glucose wound microenvironment activation and synthesized GOx-GA-Fe nanoenzyme (GGFzyme). GGFzyme can consume high concentrations of glucose and produce hydrogen peroxide in hyperglycemic wounds. The conversion of glucose to gluconic acid lowers the pH of the wound microenvironment, thereby increasing the catalytic activity of the nanase (GA-Fe) and the natural enzyme (GOx), promoting efficient catalytic production of ·OH and O2- against pathogenic bacteria. GGFzyme is useful in eradicating methicillin-resistant staphylococcus aureus infections, relieving hyperglycemia, alleviating excess H2O2 caused by oxidative stress, and effectively promoting hyperglycemic wound healing in vivo.57
Photodynamic Therapy Based on MPNs
Photodynamic therapy (PDT) is a new technology that uses photodynamic action for the diagnosis and treatment of diseases. The basis of its action is photodynamic action, ie the reaction of cells to chemical agents, light, and oxygen.159,160 The process is that laser irradiation at a specific wavelength excites a photosensitizer absorbed by the tissue, and the excited state of the photosensitizer in turn transfers energy to the surrounding oxygen, generating highly reactive monomorphic oxygen, which reacts with adjacent biological macromolecules in an oxidative manner, producing cytotoxic effects that lead to cell damage and even death.161 The advantage of PDT over conventional therapies is its ability to deliver precise and effective treatment with minimal side effects. As far as the current study is concerned, the metal–phenolic network is not able to produce PDT therapy directly, but it can be used to achieve the treatment of chronic wound infections by augmenting PDT.59
Titanium dioxide is considered a promising PDT material, but its wide optical band gap (3.2–3.2 eV) allows it to be excited only by UV light, which has limited penetration into the skin, greatly limiting its clinical applications. To address this issue, Cheng et al proposed a novel ligand-metal charge transfer (LMCT)-mediated PDT strategy for titanium dioxide (TiO2) using polydopamine (PDA) involved in the formation of mesoporous TiO2@PDA nanoparticles (mTiO2@PDA NPs). In this nanoparticle, the catechol group of PDA enables it to tightly attach to TiO2 in a colloidal environment and also to form LMCT bridges, which are excited by 808 nm irradiation to transfer electrons to TiO2 for PDT treatment. Combining the SDT) of TiO2 and the photothermal therapeutic properties of PDA, this simple structure mTiO2@PDA can achieve PDT/PTT/SDT synergistic antimicrobial therapy under the dual excitation of NIR and ultrasound, which can achieve good antimicrobial effect and rapid repair of infected wounds76 (Figure 6). This study showed that PDA molecules tend to form coordination bonds between the oxygen atom of catechol and the titanium atom of titanium dioxide. Hydrogen atoms in the other hydroxyl groups in catechol can form hydrogen bonds with the unsaturated oxygen in titanium dioxide. This leads to a promising research direction that electron transfer between tightly bound polyphenols and metal oxides can be achieved, resulting in enhanced PDT effects based on MPNs.
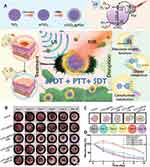 |
Figure 6 Application of MPNs in novel therapies. (A) Schematic diagram of NIR/US triggered PDT/PTT/SDT synergistic antibacterial action of mTiO2@PDA. (B) Images of skin wounds in each group at day 1 to day 10 (scale bar: 4 mm). (C) Changes of skin wound areas of different groups over time. (D) Representative changes of wound sizes over time. Reprinted from Cheng S, Qi ML, Li W, et al. Dual-Responsive Nanocomposites for Synergistic Antibacterial Therapies Facilitating Bacteria-Infected Wound Healing. Adv Healthc Mater. 2023;12(6):2202652, Copyright 2023, with permission from John Wiley and Sons.76 |
Sonodynamic Therapy Based on MPNs
Ultrasound (US) is a non-invasive method to safely deliver physical energy to deep tissues of the body and has been widely used clinically for controlled drug delivery, vasodilation, and SDT.162,163 Ultrasound-triggered methods can achieve the on-demand release of antimicrobial agents when used for antimicrobial therapy, reducing the side effects of antimicrobial agents on normal organs.154 In some studies, MPNs have been used as carriers of “acoustic sensitizers” to enable SDT treatment of chronic wound infections.
Ultrasound-driven Ag ion release is a promising strategy for antimicrobial therapy. In the study of Lu et al, they synthesized a P18-polyethylene glycol (PEG)-polyphenol polymer and then used the polyphenol to reduce Ag ions to Ag NPs for the preparation of P18-Ag NPs. The prepared PEG-P18-Ag NPs can produce ROS under ultrasonic conditions due to SDT. Such antimicrobial materials do not exert bacterial killing in vivo or in vitro The PEG-P18-Ag NPs exhibited strong bacterial inhibition against multidrug-resistant bacteria both in vivo and in vitro after ultrasonic activation. The promotion of ROS plays a key role in the bacterial killing process induced by PEG P18- Ag NPs. The results of this study provide a new approach for next-generation antimicrobial therapy.58
Photothermal Therapy Based on MPNs
Photothermal therapy (PTT) is a new minimally invasive treatment method that has been developed rapidly in recent years. It uses some photothermal agents (PTAs) with strong absorption in the near-infrared (NIR) light region to effectively convert the absorbed light energy into thermal energy under NIR irradiation to effectively kill bacteria by disrupting cell membranes, denaturing proteins and enzymes, cavitating cells, and evaporating cellular fluids to achieve therapeutic purposes.155,164 The ability of photothermal transduction is induced by electronic leaps between molecular/atomic orbital energy levels.165 Many studies have shown that MPNs also have photothermal effects, and their photothermal properties and photothermal stability are related to the species of metal ions and polyphenols. For example, the complexes of iron and polyphenols have strong absorption in the NIR region due to ligand interactions leading to electron leaps and atomic orbital splitting, which makes electron leaps after photothermal possible, and thus are suitable for PTT.
To realize PTT-dominated antimicrobial applications, Zhang et al constructed a series of core-shell GNRs@MPN nanodrugs by encapsulating GNRs in MPNs using a simple one-pot coordination reaction. They noticed that the photothermal conversion efficiency (η) of the procyanidin-constructed GNRs@MPNs was 44.0% by experimental comparison, and the antimicrobial performance was also superior to that of the GNRs made of tannins (η=38.14%), epigallocatechin gallate (η=29.29%), or simple GNRs (η=12.24%). The experiments on the mouse skin trauma model showed that the prepared GNRs@MPN also had excellent bactericidal properties in vivo and could promote the regeneration of wound epidermal tissues. This study provides suggestions for designing more MPNs with excellent photothermal properties71 (Figure 7).
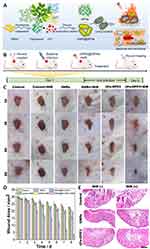 |
Figure 7 Application of MPNs in novel therapies. (A) Preparation scheme and antimicrobial mechanism of GNRs@MPNs. (B) The scheme of in vivo anti-infection treatment with GNRs@MPNs in a mice model. (C) Photographs of the MRSA-infected wound treated with different formulations for 8 days (scale bar, 1 mm). (D) The bar graph of relative wound areas infected by MRSA after various treatments. (E) The tissue photomicrographs of MRSA-infected wounds with H&E staining after different treatments (scale bar, 0.5 mm). Reprinted from Zhang CY, Huang LJ, Sun DW, Pu HB. Interfacing metal-polyphenolic networks upon photothermal gold nanorods for triplex-evolved biocompatible bactericidal activity. J Hazard Mater. 2022;426:127824, Copyright © 2021 The Author(s). Published by Elsevier B.V. Creative Commons CC-BY.71 |
In addition, Lin et al attempted to prepare a chitin-polyphenol-metal hydrogel framework using a series of polyphenols (gallic acid, tannic acid, quercetin, pyrrole aldehyde, and protocatechuic acid) and metal substrates (Fe, Cu, Ti, Zn) and verified their photothermal properties and therapeutic effects, respectively. The experimental results showed that the chitin-TA-Cu hydrogel exhibited significant photothermal antibacterial activity, and the photothermal effects of Cu and hydrogel materials could also synergistically promote cell proliferation and angiogenesis, ultimately accelerating the healing of infected wounds.73
He et al designed a multifunctional hydrogel (OGF) with ROS scavenging and photothermal antimicrobial activity based on oxidized dextran (Odex), gallic acid grafted gelatin (GAG), and trivalent iron ions for the treatment of infected wounds in diabetic mice. The hydrogels are double cross-linked by dynamic Schiff base bonds formed between the aldehyde group in Odex and the amino group in GAG and metal-ligand bonds formed between the iron ions and the polyphenol groups or carboxyl groups in GAG, resulting in good injectability, self-healing and adhesion properties of the resulting OGF hydrogels. Due to the efficient photothermal effect of the iron ion/polyphenol chelate, the hydrogel can rapidly and completely kill Staphylococcus aureus and Escherichia coli within 3.5 min under near-infrared light radiation. In addition, the combined hydrogel has good antioxidant, hemostatic, and biocompatible properties. It also significantly accelerated the complete re-epithelialization of S. aureus-infected wounds in diabetic mice within 18 days by eliminating infection, reducing oxidative stress and inflammation, and promoting angiogenesis.61
Conclusion and Outlook
In recent years, metal–phenolic networks have gradually increased in various applications such as anti-inflammatory,50,51 antibacterial,36 and antitumor.166 Since they can functionally combine the dual advantages of metal ions and polyphenols in structures that can be prepared in various forms such as nanoparticles, gels, and coatings, and structures with multiple therapeutic effects can be designed based on MPNs, research on this material has gradually increased. Some articles have summarized their applications in cancer diagnosis,64,167,168 antibacterial,41 etc., while the summary of MPNs in chronic wound treatment is still incomplete. In this paper, we compile how MPNs have been designed by researchers for application in chronic wound treatment in recent years. Due to the selectivity of MPN components, MPNs based on their use with multiple therapies for chronic wound treatment are also very beneficial for wound repair and remodeling, so this is also a direction of interest for researchers.
However, there are some shortcomings in the current studies of MPNs applied to chronic wounds. First, the design systems in some studies are more complex and the synthesis steps are cumbersome, which is not conducive to industrial production and clinical translation. Second, the issue of the potential safety of MPNs and their composites has not been carefully discussed. Third, the molecular mechanism of MPNs used in anti-inflammatory, antibacterial, and vascular neovascularization-promoting therapies is still unclear, but this is crucial for us to gain a deeper understanding and expand the application of MPNs. Fourth, the quantitative efficacy of MPNs applied to therapies such as CDT, PTT, and PDT is uncertain and there are no quantitative pharmacological studies, which is, however, a critical issue before clinical translation.71 Furthermore, regarding oxidative stress in chronic wounds, there is still a debate on exactly how ROS levels should be adjusted to accommodate the wound recovery process.77
Due to the great number of advantages of MPNs, there are many directions for their future research. First, exploring more natural and artificial polyphenolic materials with specific properties to design some MPNs with a simple synthesis process for clinical translation. Second, there is an urgent need for large-scale studies to evaluate the long-term safety of MPNs and their composites, to provide a comprehensive understanding of their biocompatibility, potential adverse reactions, and complications. Thirdly, in-depth research should be carried out to clarify the molecular mechanisms by which MPNs exert their therapeutic effects, to lay the foundation for expanding the application of MPNs. Fourth, to establish assays, pharmacokinetic, and pharmacodynamic investigation protocols for MPNs-based drug delivery systems in preparation for clinical translation and individualized drug delivery of MPNs-based drugs. Fifth, to design materials with better ROS response for rational wound oxidative stress regulation. For example, designing multifunctional materials that can perform different therapeutic roles as chronic wound recovery progresses.169,170 In the early stages of chronic wound treatment, the main role is to target the bacterial infection and inflammatory imbalance of the wound, while when the wound enters the proliferative and remodeling phase, it can achieve the promotion of neovascularization, collagen deposition, and remodeling of the ECM. Currently, the platforms of combined therapeutic strategies, multi-response delivery vehicles, and natural biomaterials with MPNs as the main body of the structure are developing rapidly and many research results have been achieved. Although there is still a long way to go from conceptual design to actual clinical therapeutic products, metal–phenolic networks have provided biomedical practice with unique ways to design functional nanomaterials and new means to overcome long-standing scientific and technological problems in the clinic, and it is believed that the research on MPNs can also be taken to the next level and ultimately benefit human health.
Abbreviations
ROS, reactive oxygen species; MPNs, metal–phenolic networks; TA, tannins; FDA, Food and Drug Administration; CDT, chemodynamic therapy; PDT, photodynamic therapy; SDT, sonodynamic therapy; PTT, photothermal therapy, Ag, silver; Au, gold, Re, rare earth; La, lanthanum; Gd, gadolinium; Tb, terbium; CAT, catechins; Fe, iron; Cu, copper; Zn, zinc; EGCG, epigallocatechin gallate; EGC, epigallocatechin gallate; ECG, epigallocatechin gallate; GA, gallic acid; OPC, proanthocyanidin; GAG, gallic acid grafted gelatin; kCA, k-type carrageenan; PDA, polydopamine; VEGF, vascular endothelial growth factor; NIR, near infrared light; DHA, dihydroartemisinin; Ti, titanium; Co, cobalt; Ni, nickel; Yb, ytterbium; BWT, black catechin; Ca, calcium; CS, chitosan; PEG, polyethylene glycol; Ga, gallium; ECM, extracellular matrix, TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; MMP, metallo-matrix protease; DNA, deoxyribonucleic acid; Pt, platinum; Pd, palladium; Al, aluminum; Sm, aluminum; SA, sodium alginate; AgNPs, silver nanoparticles; H2O2, hydrogen peroxide; E. coli, Escherichia coli; TGF-α, transforming growth factor-α; TGF-β, transforming growth factor-β; bFGF, basic fibroblast growth factor; PDGF, platelet derived growth factor; IL-17, interleukin-17; iNOS, inducible nitric oxide synthase; BSA, bovine serum albumin; NF-κB, activate nuclear factor κB; MAPk, mitogen-activated protein kinase; AGEs, advanced glycosylation end products; ·OH, hydroxyl radicals; LMCT, ligand-metal charge transfer; US, Ultrasound; PTAs, photothermal agents; S. aureus, Staphylococcus aureus; Odex, oxidized dextran.
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China (No. 81973253), the China Postdoctoral Science Foundation (No. 2021M702633), the Key R&D Program Project for Shaanxi Province (No. 2022SF-081), The authors thank the Instrument Analysis Center of Xi’an Jiaotong University for help.
Disclosure
There are no conflicts of interest to declare.
References
1. Joorabloo A, Liu TQ. Recent advances in nanomedicines for regulation of macrophages in wound healing. J Nanobiotechnol. 2022;20(1):407. doi:10.1186/s12951-022-01616-1
2. Ji JY, Ren DY, Weng YZ. Efficiency of multifunctional antibacterial hydrogels for chronic wound healing in diabetes: a comprehensive review. Int J Nanomed. 2022;17:3163–3176. doi:10.2147/IJN.S363827
3. Schilrreff P, Alexiev U. Chronic inflammation in non-healing skin wounds and promising natural bioactive compounds treatment. Int J Mol Sci. 2022;23(9):4928. doi:10.3390/ijms23094928
4. Sharifi S, Hajipour MJ, Gould L, Mahmoudi M. Nanomedicine in healing chronic wounds: opportunities and challenges. Mol Pharmaceut. 2021;18(2):550–575. doi:10.1021/acs.molpharmaceut.0c00346
5. Blanco-Fernandez B, Castano O, Mateos-Timoneda MA, Engel E, Perez-Amodio S. Nanotechnology approaches in chronic wound healing. Adv Wound Care. 2021;10(5):234–256. doi:10.1089/wound.2019.1094
6. Serra R, Grande R, Butrico L, et al. Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev Anti Infe. 2015;13(5):605–613. doi:10.1586/14787210.2015.1023291
7. Ibberson CB, Whiteley M. The social life of microbes in chronic infection. Curr Opin Microbiol. 2020;53:44–50. doi:10.1016/j.mib.2020.02.003
8. Maheswary T, Nurul AA, Fauzi MB. The insights of Microbes’ Roles in wound healing: a comprehensive review. Pharmaceutics. 2021;13(7):981. doi:10.3390/pharmaceutics13070981
9. Xu ZJ, Liang B, Tian JZ, Wu J. Anti-inflammation biomaterial platforms for chronic wound healing. Biomater Sci. 2021;9(12):4388–4409. doi:10.1039/D1BM00637A
10. Matter MT, Probst S, Lauchli S, Herrmann IK. Uniting drug and delivery: metal oxide hybrid nanotherapeutics for skin wound care. Pharmaceutics. 2020;12(8):780. doi:10.3390/pharmaceutics12080780
11. Teot L, Ohura N. Challenges and management in wound care. Plast Reconstr Surg. 2021;147(1s–1):9s–15s. doi:10.1097/PRS.0000000000007628
12. Thomas DC, Tsu CL, Nain RA, Arsat N, Fun SS, Lah NA. The role of debridement in wound bed preparation in chronic wound: a narrative review. Ann Med Surg. 2021;71:102876. doi:10.1016/j.amsu.2021.102876
13. Nuutila K, Eriksson E. Moist wound healing with commonly available dressings. Adv Wound Care. 2021;10(12):685–698. doi:10.1089/wound.2020.1232
14. Chin JD, Zhao L, Mayberry TG, Cowan BC, Wakefield MR, Fang YJ. Photodynamic therapy, probiotics, acetic acid, and essential oil in the treatment of chronic wounds infected with Pseudomonas aeruginosa. Pharmaceutics. 2023;15(6):1721. doi:10.3390/pharmaceutics15061721
15. Oluwole DO, Coleman L, Buchanan W, Chen T, La Ragione RM, Liu LX. Antibiotics-free compounds for chronic wound healing. Pharmaceutics. 2022;14(5):1021. doi:10.3390/pharmaceutics14051021
16. Zhang ZB, Guo JD, He YX, et al. An injectable double network hydrogel with hemostasis and antibacterial activity for promoting multidrug-resistant bacteria infected wound healing. Biomater Sci. 2022;10(12):3268–3281. doi:10.1039/D2BM00347C
17. He Y, Chang Q, Lu F. Oxygen-releasing biomaterials for chronic wounds breathing: from theoretical mechanism to application prospect. Mater Today Bio. 2023;20:100687. doi:10.1016/j.mtbio.2023.100687
18. Dai W, Dong YC, Han T, et al. Microenvironmental cue-regulated exosomes as therapeutic strategies for improving chronic wound healing. NPG Asia Mater. 2022;14(1):75. doi:10.1038/s41427-022-00419-y
19. Wang YZ, Armato U, Wu J. Targeting tunable physical properties of materials for chronic wound care. Front Bioeng Biotech. 2020;8:584.
20. Zhang X, Shu WT, Yu QH, Qu WR, Wang YN, Li R. Functional biomaterials for treatment of chronic wound. Front Bioeng Biotech. 2020;8:516. doi:10.3389/fbioe.2020.00516
21. Lopez-Goerne TM, Padilla-Godinez FJ, Castellanos M, Perez-Davalos LA. Catalytic nanomedicine: a brief review of bionanocatalysts. Nanomedicine. 2022;17(16):1131–1156. doi:10.2217/nnm-2022-0027
22. Zheng MH, Wang XC, Yue O, et al. Skin-inspired gelatin-based flexible bio-electronic hydrogel for wound healing promotion and motion sensing. Biomaterials. 2021;276:121026. doi:10.1016/j.biomaterials.2021.121026
23. Liu XH, Hou MD, Luo XM, et al. Thermoresponsive hemostatic hydrogel with a biomimetic nanostructure constructed from aggregated collagen nanofibers. Biomacromolecules. 2021;22(2):319–329. doi:10.1021/acs.biomac.0c01167
24. Nakipoglu M, Tezcaner A, Contag CH, Annabi N, Ashammakhi N. Bioadhesives with Antimicrobial Properties. Adv Mater. 2023;e2300840. doi:10.1002/adma.202300840
25. Khadem E, Kharaziha M, Bakhsheshi-Rad HR, Das O, Berto F. Cutting-edge progress in stimuli-responsive bioadhesives: from synthesis to clinical applications. Polymers. 2022;14(9):1709. doi:10.3390/polym14091709
26. Sierra-Sanchez A, Kim KH, Blasco-Morente G, Arias-Santiago S. Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. Npj Regen Med. 2021;6(1):35. doi:10.1038/s41536-021-00144-0
27. Przekora A. A concise review on tissue engineered artificial skin grafts for chronic wound treatment: can we reconstruct functional skin tissue in vitro? Cells. 2020;9(7):1622. doi:10.3390/cells9071622
28. Pormohammad A, Monych NK, Ghosh S, Turner DL, Turner RJ. Nanomaterials in wound healing and infection control. Antibiotics. 2021;10(5):473. doi:10.3390/antibiotics10050473
29. Ataide JA, Zanchetta B, Santos EM, et al. Nanotechnology-based dressings for wound management. Pharmaceuticals. 2022;15(10):1286. doi:10.3390/ph15101286
30. Girija AR, Balasubramanian S, Cowin AJ. Nanomaterials-based drug delivery approaches for wound healing. Curr Pharm Design. 2022;28(9):711–726. doi:10.2174/1381612828666220328121211
31. Dey AD, Yousefiasl S, Kumar A, et al. miRNA-encapsulated abiotic materials and biovectors for cutaneous and oral wound healing: biogenesis, mechanisms, and delivery nanocarriers. Bioeng Transl Med. 2023;8(1):e10343. doi:10.1002/btm2.10343
32. Lin Y, Chen Z, Liu Y, Wang J, Lv W, Peng R. Recent advances in nano-formulations for skin wound repair applications. Drug Des Devel Ther. 2022;16:2707–2728. doi:10.2147/DDDT.S375541
33. Gowda BHJ, Mohanto S, Singh A, et al. Nanoparticle-based therapeutic approaches for wound healing: a review of the state-of-The-art. Mater Today Chem. 2023;27:101319.
34. Butenko S, Miwa H, Liu YZ, Plikus MV, Scumpia PO, Liu WF. Engineering immunomodulatory biomaterials to drive skin wounds toward regenerative healing. Csh Perspect Biol. 2023;15(5):a041242.
35. Rios-Galacho M, Martinez-Moreno D, Lopez-Ruiz E, Galvez-Martin P, Marchal JA. An overview on the manufacturing of functional and mature cellular skin substitutes. Tissue Eng Part B. 2022;28(5):0131.
36. Li Y, Miao Y, Yang LN, et al. Recent advances in the development and antimicrobial applications of metal-phenolic networks. Adv Sci. 2022;9(27):2202684. doi:10.1002/advs.202202684
37. Ejima H, Richardson JJ, Liang K, et al. One-step assembly of coordination complexes for versatile film and particle engineering. Science. 2013;341(6142):154–157. doi:10.1126/science.1237265
38. Yang B, Zhou S, Zeng J, et al. Super-assembled core-shell mesoporous silica-metal-phenolic network nanoparticles for combinatorial photothermal therapy and chemotherapy. Nano Res. 2020;13(4):1013–1019. doi:10.1007/s12274-020-2736-6
39. Dong ZL, Hao Y, Li QG, et al. Metal-polyphenol-network coated CaCO(3)as pH-responsive nanocarriers to enable effective intratumoral penetration and reversal of multidrug resistance for augmented cancer treatments. Nano Res. 2020;13(11):3057–3067. doi:10.1007/s12274-020-2972-9
40. Dong JT, Chen W, Feng JG, et al. Facile, smart, and degradable metal-organic framework nanopesticides gated with Fe-III-tannic acid networks in response to seven biological and environmental stimuli. Acs Appl Mater Inter. 2021;13(16):19507–19520. doi:10.1021/acsami.1c04118
41. Guo ZH, Xie WS, Lu JS, et al. Tannic acid-based metal phenolic networks for bio-applications: a review. J Mater Chem B. 2021;9(20):4098–4110. doi:10.1039/D1TB00383F
42. Huang H, Li P, Liu CL, et al. pH-Responsive nanodrug encapsulated by tannic acid complex for controlled drug delivery. Rsc Adv. 2017;7(5):2829–2835. doi:10.1039/C6RA26936B
43. Wang YA, Zhang JW, Zhao Y, et al. Innovations and challenges of polyphenol-based smart drug delivery systems. Nano Res. 2022;15(9):8156–8184. doi:10.1007/s12274-022-4430-3
44. Guo YX, Sun Q, Wu FG, Dai YL, Chen XY. Polyphenol-containing nanoparticles: synthesis, properties, and therapeutic delivery. Adv Mater. 2021;33(22):2007356. doi:10.1002/adma.202007356
45. Ejima H, Richardson JJ, Caruso F. Metal-phenolic networks as a versatile platform to engineer nanomaterials and biointerfaces. Nano Today. 2017;12:136–148. doi:10.1016/j.nantod.2016.12.012
46. Slika H, Mansour H, Wehbe N, et al. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed Pharmacother. 2022;146:112442. doi:10.1016/j.biopha.2021.112442
47. Zhang MS, Wang L, Jin H, et al. Employing single valency polyphenol to prepare metal-phenolic network antitumor reagents through FeOOH assistance. J Control Release. 2023;358:612–625. doi:10.1016/j.jconrel.2023.05.020
48. Yan J, Wang GH, Xie LS, et al. Engineering radiosensitizer-based metal-phenolic networks potentiate STING pathway activation for advanced radiotherapy. Adv Mater. 2022;34(10):2105783. doi:10.1002/adma.202105783
49. Duan JW, Chen ZG, Liang XY, et al. Construction and application of therapeutic metal-polyphenol capsule for peripheral artery disease. Biomaterials. 2020;255:120199. doi:10.1016/j.biomaterials.2020.120199
50. Wei H, Qin J, Huang QX, et al. Epigallocatechin-3-gallate (EGCG) based metal-polyphenol nanoformulations alleviates chondrocytes inflammation by modulating synovial macrophages polarization. Biomed Pharmacother. 2023;161:114366. doi:10.1016/j.biopha.2023.114366
51. Wei ZW, Wang LY, Tang CQ, et al. Metal-phenolic networks nanoplatform to mimic antioxidant defense system for broad-spectrum radical eliminating and endotoxemia treatment. Adv Funct Mater. 2020;30(49):2002234. doi:10.1002/adfm.202002234
52. Shahidi F, Yeo J. Bioactivities of phenolics by focusing on suppression of chronic diseases: a review. Int J Mol Sci. 2018;19(6):1573. doi:10.3390/ijms19061573
53. Zheng D, Huang C, Huang H, et al. Antibacterial mechanism of curcumin: a review. Chem Biodivers. 2020;17(8):e2000171. doi:10.1002/cbdv.202000171
54. Zhang SC, Chai QH, Man ZT, et al. Bioinspired nano-painting on orthopedic implants orchestrates periprosthetic anti-infection and osseointegration in a rat model of arthroplasty. Chem Eng J. 2022;435:134848. doi:10.1016/j.cej.2022.134848
55. Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19(3):686. doi:10.3390/ijms19030686
56. Xu Y, Xiao L, Chen J, et al. alpha-Fe(2)O(3) based nanotherapeutics for near-infrared/dihydroartemisinin dual-augmented chemodynamic antibacterial therapy. Acta Biomater. 2022;150:367–379. doi:10.1016/j.actbio.2022.07.047
57. Shi S, Zhang QP, Sun H, et al. Glucose oxidase-integrated metal-polyphenolic network as a microenvironment-activated cascade nanozyme for hyperglycemic wound disinfection. Acs Biomater Sci Eng. 2022;8(12):5145–5154. doi:10.1021/acsbiomaterials.2c00985
58. Lu C, Tian Y, Tian H, et al. An ultrasound activable metal-phenolic network nano-antibiotics for in vivo on-site infection therapy. Sci China Mater. 2023;66(1):395–406. doi:10.1007/s40843-022-2125-1
59. Lee HP, Gaharwar AK. Light-responsive inorganic biomaterials for biomedical applications. Adv Sci. 2020;7(17):2000863. doi:10.1002/advs.202000863
60. Wang HC, Wang DY, Huangfu HM, et al. Branched AuAg nanoparticles coated by metal-phenolic networks for treating bacteria-induced periodontitis via photothermal antibacterial and immunotherapy. Mater Design. 2022;224:111401. doi:10.1016/j.matdes.2022.111401
61. He YM, Liu KY, Guo S, et al. Multifunctional hydrogel with reactive oxygen species scavenging and photothermal antibacterial activity accelerates infected diabetic wound healing. Acta Biomaterialia. 2023;155:199–217. doi:10.1016/j.actbio.2022.11.023
62. Fu MM, Zhao YT, Wang Y, et al. On-demand removable self-healing and pH-responsive europium-releasing adhesive dressing enables inflammatory microenvironment modulation and angiogenesis for diabetic wound healing. Small. 2023;19(3):2205489. doi:10.1002/smll.202205489
63. Chang YF, Cui PF, Zhou SW, et al. Metal-phenolic network for cancer therapy. J Drug Deliv Sci Tec. 2023;81:104194. doi:10.1016/j.jddst.2023.104194
64. Zhang Z, Xie LS, Ju Y, Dai YL. Recent advances in metal-phenolic networks for cancer theranostics. Small. 2021;17(43):2100314. doi:10.1002/smll.202100314
65. Xie WS, Guo ZH, Zhao LY, Wei Y. Metal-phenolic networks: facile assembled complexes for cancer theranostics. Theranostics. 2021;11(13):6407–6426.
66. Xie Y, Chen SQ, Peng X, et al. Alloyed nanostructures integrated metal-phenolic nanoplatform for synergistic wound disinfection and revascularization. Bioact Mater. 2022;16:95–106. doi:10.1016/j.bioactmat.2022.03.004
67. Qiao YS, Zhang Q, Wang Q, et al. Synergistic anti-inflammatory coating “Zipped Up” on polypropylene hernia mesh. Acs Appl Mater Inter. 2021;13(30):35456–35468. doi:10.1021/acsami.1c09089
68. Qin Y, Wang JP, Qiu C, Hu Y, Xu XM, Jin ZY. Self-assembly of metal-phenolic networks as functional coatings for preparation of antioxidant, antimicrobial, and pH-sensitive-modified starch nanoparticles. Acs Sustain Chem Eng. 2019;7(20):17379–17389. doi:10.1021/acssuschemeng.9b04332
69. Li JJ, Han JN, Yu WQ, Wang KY, Liu Z, Liu Y. Alginate-modulated continuous assembly of iron/tannic acid composites as photothermally responsive wound dressings for hemostasis and drug resistant bacteria eradication. Int J Biol Macromol. 2023;242(2):124886. doi:10.1016/j.ijbiomac.2023.124886
70. Xu YY, Cai YJ, Xia Y, et al. Photothermal nanoagent for anti-inflammation through macrophage repolarization following antibacterial therapy. Eur Polym J. 2023;186:111840. doi:10.1016/j.eurpolymj.2023.111840
71. Zhang CY, Huang LJ, Sun DW, Pu HB. Interfacing metal-polyphenolic networks upon photothermal gold nanorods for triplex-evolved biocompatible bactericidal activity. J Hazard Mater. 2022;426:127824. doi:10.1016/j.jhazmat.2021.127824
72. Huang LJ, Sun DW, Wu ZH, Pu HB, Wei QY. Reproducible, shelf-stable, and bioaffinity SERS nanotags inspired by multivariate polyphenolic chemistry for bacterial identification. Anal Chim Acta. 2021;1167:338570. doi:10.1016/j.aca.2021.338570
73. Lin XH, Zhang H, Li SS, et al. Polyphenol-driving assembly for constructing chitin-polyphenol-metal hydrogel as wound dressing. Carbohyd Polym. 2022;290:119444. doi:10.1016/j.carbpol.2022.119444
74. Zeng ZW, Guo CP, Lu DH, Geng ZJ, Pei DT, Yu S. Polyphenol-metal functionalized hydrogel dressing with sustained release, antibacterial, and antioxidant properties for the potential treatment of chronic wounds. Macromol Mater Eng. 2022;307(10):2200262. doi:10.1002/mame.202200262
75. Chen ZG, Duan JW, Diao YP, et al. ROS-responsive capsules engineered from EGCG-Zinc networks improve therapeutic angiogenesis in mouse limb ischemia. Bioact Mater. 2021;6(1):1–11. doi:10.1016/j.bioactmat.2020.07.013
76. Cheng S, Qi ML, Li W, et al. Dual-responsive nanocomposites for synergistic antibacterial therapies facilitating bacteria-infected wound healing. Adv Healthc Mater. 2023;12(6):2202652. doi:10.1002/adhm.202202652
77. Anh HTP, Huang CM, Huang CJ. Intelligent metal-phenolic metallogels as dressings for infected wounds. Sci Rep. 2019;9:11562. doi:10.1038/s41598-019-47978-9
78. Liu L, Xiao X, Li X, et al. Immobilization of ytterbium by plant polyphenols for antibiofilm materials with highly effective activity and long-term stability. Ind Eng Chem Res. 2020;59(41):18558–18566. doi:10.1021/acs.iecr.0c03534
79. Chen JW, Qiu LP, Li QL, Ai J, Liu HQ, Chen QH. Rapid hemostasis accompanied by antibacterial action of calcium crosslinking tannic acid-coated mesoporous silica/silver Janus nanoparticles. Mat Sci Eng C Mater. 2021;123:111958. doi:10.1016/j.msec.2021.111958
80. Xu G, Xu N, Ren TJ, et al. Multifunctional chitosan/silver/tannic acid cryogels for hemostasis and wound healing. Int J Biol Macromol. 2022;208:760–771. doi:10.1016/j.ijbiomac.2022.03.174
81. Hu QS, Nie Y, Xiang J, et al. Injectable sodium alginate hydrogel loaded with plant polyphenol-functionalized silver nanoparticles for bacteria-infected wound healing. Int J Biol Macromol. 2023;234:123691. doi:10.1016/j.ijbiomac.2023.123691
82. Esfahani AG, Lazazzera B, Draghi L, et al. Bactericidal activity of gallium-doped chitosan coatings against staphylococcal infection. J Appl Microbiol. 2019;126(1):87–101. doi:10.1111/jam.14133
83. Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver nanoparticles and their antibacterial applications. Int J Mol Sci. 2021;22(13):7202. doi:10.3390/ijms22137202
84. Kalantari K, Mostafavi E, Afifi AM, et al. Wound dressings functionalized with silver nanoparticles: promises and pitfalls. Nanoscale. 2020;12(4):2268–2291. doi:10.1039/C9NR08234D
85. Azharuddin M, Zhu GH, Das D, et al. A repertoire of biomedical applications of noble metal nanoparticles. Chem Commun. 2019;55(49):6964–6996.
86. Slavin YN, Asnis J, Hafeli UO, Bach H. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol. 2017;15(1):65. doi:10.1186/s12951-017-0308-z
87. Dryden M. Reactive oxygen species: a novel antimicrobial. Int J Antimicrob Ag. 2018;51(3):299–303. doi:10.1016/j.ijantimicag.2017.08.029
88. Behzad F, Naghib SM, Kouhbanani MAJ, Tabatabaei SN, Zare Y, Rhee KY. An overview of the plant-mediated green synthesis of noble metal nanoparticles for antibacterial applications. J Ind Eng Chem. 2021;94:92–104. doi:10.1016/j.jiec.2020.12.005
89. Subramaniam T, Fauzi MB, Lokanathan Y, Law JX. The role of calcium in wound healing. Int J Mol Sci. 2021;22(12):6486. doi:10.3390/ijms22126486
90. Immler R, Simon SI, Sperandio M. Calcium signalling and related ion channels in neutrophil recruitment and function. Eur J Clin Invest. 2018;48(S2):e12964. doi:10.1111/eci.12964
91. Berridge MJ, Bootman MD, Lipp P. Calcium - a life and death signal. Nature. 1998;395(6703):645–648. doi:10.1038/27094
92. Salvo J, Sandoval C. Role of copper nanoparticles in wound healing for chronic wounds: literature review. Burns Trauma. 2022;10:tkab047. doi:10.1093/burnst/tkab047
93. Zhang ZH, Li XJ, Sang SY, et al. Polyphenols as plant-based nutraceuticals: health effects, encapsulation, nano-delivery, and application. Foods. 2022;11(15):2189. doi:10.3390/foods11152189
94. Wang H, Wang CP, Zou Y, Hu JJ, Li YW, Cheng YY. Natural polyphenols in drug delivery systems: current status and future challenges. Giant-Amsterdam. 2020;3:100022. doi:10.1016/j.giant.2020.100022
95. Li L, Peng PL, Ding N, Jia WH, Huang CH, Tang Y. Oxidative stress, inflammation, gut dysbiosis: what can polyphenols do in inflammatory bowel disease? Antioxidants. 2023;12(4):967. doi:10.3390/antiox12040967
96. Kim KH, Ki MR, Min KH, Pack SP. Advanced delivery system of polyphenols for effective cancer prevention and therapy. Antioxidants. 2023;12(5):1048. doi:10.3390/antiox12051048
97. Liu W, Cui X, Zhong Y, Ma R, Liu B, Xia Y. Phenolic metabolites as therapeutic in inflammation and neoplasms: molecular pathways explaining their efficacy. Pharmacol Res. 2023;193:106812. doi:10.1016/j.phrs.2023.106812
98. Rana A, Samtiya M, Dhewa T, Mishra V, Aluko RE. Health benefits of polyphenols: a concise review. J Food Biochem. 2022;46(10):e14264. doi:10.1111/jfbc.14264
99. Meccariello R, D’Angelo S. Impact of polyphenolic-food on longevity: an Elixir of life. An overview. Antioxidants. 2021;10(4):507. doi:10.3390/antiox10040507
100. Li AN, Li S, Zhang YJ, Xu XR, Chen YM, Li HB. Resources and biological activities of natural polyphenols. Nutrients. 2014;6(12):6020–6047. doi:10.3390/nu6126020
101. Jafari H, Ghaffari-Bohlouli P, Niknezhad SV, et al. Tannic acid: a versatile polyphenol for design of biomedical hydrogels. J Mater Chem B. 2022;10(31):5873–5912. doi:10.1039/d2tb01056a
102. Kim S, Philippot S, Fontanay S, et al. pH- and glutathione-responsive release of curcumin from mesoporous silica nanoparticles coated using tannic acid-Fe(III) complex. Rsc Adv. 2015;5(110):90550–90558. doi:10.1039/C5RA16004A
103. Shen GZ, Xing RR, Zhang N, Chen CJ, Ma GH, Yan XH. Interfacial cohesion and assembly of bioadhesive molecules for design of long term stable hydrophobic nanodrugs toward effective anticancer therapy. Acs Nano. 2016;10(6):5720–5729. doi:10.1021/acsnano.5b07276
104. Mihai MM, Preda M, Lungu I, Gestal MC, Popa MI, Holban AM. Nanocoatings for chronic wound repair-modulation of microbial colonization and biofilm formation. Int J Mol Sci. 2018;19(4):1179. doi:10.3390/ijms19041179
105. Falanga V, Isseroff RR, Soulika AM, et al. Chronic wounds. Nat Rev Dis Primers. 2022;8(1):50. doi:10.1038/s41572-022-00377-3
106. Singer AJ. Healing mechanisms in cutaneous wounds: tipping the balance. Tissue Eng Part B. 2022;28(5):0114.
107. Liang YP, He JH, Guo BL. Functional hydrogels as wound dressing to enhance wound healing. Acs Nano. 2021;15(8):12687–12722. doi:10.1021/acsnano.1c04206
108. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665–706. doi:10.1152/physrev.00067.2017
109. Nour S, Imani R, Chaudhry GR, Sharifi AM. Skin wound healing assisted by angiogenic targeted tissue engineering: a comprehensive review of bioengineered approaches. J Biomed Mater Res A. 2021;109(4):453–478. doi:10.1002/jbm.a.37105
110. Huang JW, Heng SJ, Zhang WL, et al. Dermal extracellular matrix molecules in skin development, homeostasis, wound regeneration and diseases. Semin Cell Dev Biol. 2022;128:137–144. doi:10.1016/j.semcdb.2022.02.027
111. Potekaev NN, Borzykh OB, Medvedev GV, et al. The role of extracellular matrix in skin wound healing. J Clin Med. 2021;10(24):5947. doi:10.3390/jcm10245947
112. Diller RB, Tabor AJ. The role of the Extracellular Matrix (ECM) in wound healing: a review. Biomimetics. 2022;7(3):87. doi:10.3390/biomimetics7030087
113. Lanau Roig A, Fabrellas N, Sáez Rubio G, Wilson K. Tiempo de cicatrización de las heridas crónicas, a propósito de un estudio de prevalencia e incidencia. Enfermería Global. 2017;16(46):445–463. doi:10.6018/eglobal.16.2.251311
114. Monika P, Chandraprabha MN, Rangarajan A, Waiker PV, Murthy KCN. Challenges in healing wound: role of complementary and alternative medicine. Front Nutr. 2022;8:791899. doi:10.3389/fnut.2021.791899
115. Berthiaume F, Hsia HC. Regenerative approaches for chronic wounds. Annu Rev Biomed Eng. 2022;24(1):61–83. doi:10.1146/annurev-bioeng-010220-113008
116. Tao Y, Ju EG, Ren JS, Qu XG. Bifunctionalized mesoporous silica-supported gold nanoparticles: intrinsic oxidase and peroxidase catalytic activities for antibacterial applications. Adv Mater. 2015;27(6):1097–1104. doi:10.1002/adma.201405105
117. Fang G, Li WF, Shen XM, et al. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat Commun. 2018;9(1):129. doi:10.1038/s41467-017-02502-3
118. Hu WC, Younis MR, Zhou Y, Wang C, Xia XH. In situ fabrication of ultrasmall gold nanoparticles/2D MOFs hybrid as nanozyme for antibacterial therapy. Small. 2020;16(23):2000553. doi:10.1002/smll.202000553
119. Wang ZZ, Dong K, Liu Z, et al. Activation of biologically relevant levels of reactive oxygen species by Au/g-C3N4 hybrid nanozyme for bacteria killing and wound disinfection. Biomaterials. 2017;113:145–157. doi:10.1016/j.biomaterials.2016.10.041
120. Guo JL, Ping Y, Ejima H, et al. Engineering multifunctional capsules through the assembly of metal-phenolic networks. Angew Chem Int Edit. 2014;53(22):5546–5551. doi:10.1002/anie.201311136
121. Pires F, Santos JF, Bitoque D, et al. Polycaprolactone/Gelatin nanofiber membranes containing EGCG-loaded liposomes and their potential use for skin regeneration. Acs Appl Bio Mater. 2019;2(11):4790–4800. doi:10.1021/acsabm.9b00524
122. Cherepanov PV, Rahim MA, Bertleff-Zieschang N, et al. Electrochemical behavior and redox-dependent disassembly of gallic Acid/Fe metal-phenolic networks. Acs Appl Mater Inter. 2018;10(6):5828–5834. doi:10.1021/acsami.7b19322
123. Arakawa H, Maeda M, Okubo S, Shimamura T. Role of hydrogen peroxide in bactericidal action of catechin. Biol Pharm Bull. 2004;27(3):277–281. doi:10.1248/bpb.27.277
124. Huang TW, Lu HT, Ho YC, Lu KY, Wang P, Mi FL. A smart and active film with tunable drug release and color change abilities for detection and inhibition of bacterial growth. Mat Sci Eng C Mater. 2021;118:111396. doi:10.1016/j.msec.2020.111396
125. Chen S, Yan Y, Yu Y, et al. Ferric ions as a catalytic mediator in metal-EGCG network for bactericidal effect and pathogenic biofilm eradication at physiological pH. Adv Mater Interfaces. 2021;8(23):2101605. doi:10.1002/admi.202101605
126. Kelson AB, Carnevali M, Truong-Le V. Gallium-based anti-infectives: targeting microbial iron-uptake mechanisms. Curr Opin Pharmacol. 2013;13(5):707–716. doi:10.1016/j.coph.2013.07.001
127. Li FP, Liu FX, Huang K, Yang SB. Advancement of gallium and gallium-based compounds as antimicrobial agents. Front Bioeng Biotech. 2022;10(10):827960. doi:10.3389/fbioe.2022.827960
128. Zhao RL, Liang HLN, Clarke E, Jackson C, Xue ML. Inflammation in chronic wounds. Int J Mol Sci. 2016;17(12):2085. doi:10.3390/ijms17122085
129. Kharaziha M, Baidya A, Annabi N. Rational design of immunomodulatory hydrogels for chronic wound healing. Adv Mater. 2021;33(39):2100176. doi:10.1002/adma.202100176
130. Sim SL, Kumari S, Kaur S, Khosrotehrani K. Macrophages in skin wounds: functions and therapeutic potential. Biomolecules. 2022;12(11):1659. doi:10.3390/biom12111659
131. Chang M, Nguyen TT. Strategy for treatment of infected diabetic foot ulcers. Accounts Chem Res. 2021;54(5):1080–1093. doi:10.1021/acs.accounts.0c00864
132. Chen C, Liu TF, Tang YY, Luo GX, Liang GP, He WF. Epigenetic regulation of macrophage polarization in wound healing. Burns Trauma. 2023;11:tkac057. doi:10.1093/burnst/tkac057
133. Sousa AB, Aguas AP, Barbosa MA, Barbosa JN. Immunomodulatory biomaterial-based wound dressings advance the healing of chronic wounds via regulating macrophage behavior. Regen Biomater. 2022;9:rbac065. doi:10.1093/rb/rbac065
134. Li MR, Hou Q, Zhong LZ, Zhao YL, Fu XB. Macrophage related chronic inflammation in non-healing wounds. Front Immunol. 2021;12:681710. doi:10.3389/fimmu.2021.681710
135. Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10(5):520–529. doi:10.7150/ijbs.8879
136. Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10(11):1618. doi:10.3390/nu10111618
137. Corrêa FRS, Schanuel FS, Moura-Nunes N, Monte-Alto-Costa A, Daleprane JB. Brazilian red propolis improves cutaneous wound healing suppressing inflammation-associated transcription factor NFκB. Biomed Pharmacother. 2017;86:162–171. doi:10.1016/j.biopha.2016.12.018
138. He QQ, Yuan S, Tang H, et al. Safeguarding osteointegration in diabetic patients: a potent “Chain Armor” coating for scavenging ROS and macrophage reprogramming in a microenvironment-responsive manner. Adv Funct Mater. 2021;31(31). doi:10.1002/adfm.202101611
139. Li DY, Li JR, Wang SW, Wang QM, Teng W. Dually crosslinked Copper-Poly (tannic acid) nanoparticles with microenvironment-responsiveness for infected wound treatment. Adv Healthc Mater. 2023;12(17):2203063. doi:10.1002/adhm.202203063
140. Zhao XD, Pei DN, Yang YX, et al. Green tea derivative driven smart hydrogels with desired functions for chronic diabetic wound treatment. Adv Funct Mater. 2021;31(18):2009442. doi:10.1002/adfm.202009442
141. van der Vliet A, Janssen-Heininger YMW. Hydrogen peroxide as a damage signal in tissue injury and inflammation: murderer, mediator, or messenger? J Cell Biochem. 2014;115(3):427–435. doi:10.1002/jcb.24683
142. Wagener FADTG, Carels CE, Lundvig DMS. Targeting the redox balance in inflammatory skin conditions. Int J Mol Sci. 2013;14(5):9126–9167. doi:10.3390/ijms14059126
143. He ZC, Luo HT, Wang ZT, Chen DF, Feng Q, Cao XD. Injectable and tissue adhesive EGCG-laden hyaluronic acid hydrogel depot for treating oxidative stress and inflammation. Carbohyd Polym. 2023;299:120180. doi:10.1016/j.carbpol.2022.120180
144. Li WQ, Li W, Wan YL, Wang LF, Zhou T. Preparation, characterization and releasing property of antibacterial nano-capsules composed of e-PL-EGCG and sodium alginate-chitosan. Int J Biol Macromol. 2022;204:652–660. doi:10.1016/j.ijbiomac.2022.01.123
145. Chakraborty R, Borah P, Dutta PP, Sen S. Evolving spectrum of diabetic wound: mechanistic insights and therapeutic targets. World J Diabetes. 2022;13(9):696–716. doi:10.4239/wjd.v13.i9.696
146. Rai V, Moellmer R, Agrawal DK. Stem cells and angiogenesis: implications and limitations in enhancing chronic diabetic foot ulcer healing. Cells. 2022;11(15):2287. doi:10.3390/cells11152287
147. Liu E, Gao HJ, Zhao YJ, et al. The potential application of natural products in cutaneous wound healing: a review of preclinical evidence. Front Pharmacol. 2022;13:900439. doi:10.3389/fphar.2022.900439
148. Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliver Rev. 2019;146:97–125.
149. Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22(5):569–578. doi:10.1111/wrr.12205
150. Liu NB, Zhu SJ, Deng YZ, et al. Construction of multifunctional hydrogel with metal-polyphenol capsules for infected full-thickness skin wound healing. Bioact Mater. 2023;24:69–80. doi:10.1016/j.bioactmat.2022.12.009
151. Li XY, Gao P, Tan JY, et al. Assembly of metal phenolic/catecholamine networks for synergistically anti-inflammatory, antimicrobial, and anticoagulant coatings. Acs Appl Mater Inter. 2018;10(47):40844–40853. doi:10.1021/acsami.8b14409
152. Choi J, Sun IC, Hwang HS, Yoon HY, Kim K. Light-triggered photodynamic nanomedicines for overcoming localized therapeutic efficacy in cancer treatment. Adv Drug Deliver Rev. 2022;186:114344. doi:10.1016/j.addr.2022.114344
153. Zhao PR, Li HY, Bu WB. A forward vision for chemodynamic therapy: issues and opportunities. Angew Chem Int Edit. 2023;62(7):e202210415. doi:10.1002/anie.202210415
154. Fan LH, Muhammad AI, Ismail BB, Liu DH. Sonodynamic antimicrobial chemotherapy: an emerging alternative strategy for microbial inactivation. Ultrason Sonochem. 2021;75:105591. doi:10.1016/j.ultsonch.2021.105591
155. Chen Y, Gao YJ, Chen Y, Liu L, Mo AC, Peng Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J Control Release. 2020;328:251–262. doi:10.1016/j.jconrel.2020.08.055
156. Xie G, Wang X, Mo M, Zhang LB, Zhu JT. Photothermal hydrogels for promoting infected wound healing. Macromol Biosci. 2023;23(2):2200378. doi:10.1002/mabi.202200378
157. Zhou YF, Fan SY, Feng LL, Huang XL, Chen XY. Manipulating intratumoral Fenton chemistry for enhanced chemodynamic and chemodynamic-synergized multimodal therapy. Adv Mater. 2021;33(48):2104223. doi:10.1002/adma.202104223
158. Tian QW, Xue FF, Wang YR, et al. Recent advances in enhanced chemodynamic therapy strategies. Nano Today. 2021;39:101162. doi:10.1016/j.nantod.2021.101162
159. Liu ZY, Xie ZJ, Li WT, et al. Photodynamic immunotherapy of cancers based on nanotechnology: recent advances and future challenges. J Nanobiotechnol. 2021;19(1):160. doi:10.1186/s12951-021-00903-7
160. Xiong K, Wei FM, Chen Y, Ji LN, Chao H. Recent progress in photodynamic immunotherapy with metal-based photosensitizers. Small Methods. 2022;7(5):2201403. doi:10.1002/smtd.202201403
161. Awad M, Thomas N, Barnes TJ, Prestidge CA. Nanomaterials enabling clinical translation of antimicrobial photodynamic therapy. J Control Release. 2022;346:300–316. doi:10.1016/j.jconrel.2022.04.035
162. Son S, Kim JH, Wang XW, et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem Soc Rev. 2020;49(11):3244–3261. doi:10.1039/C9CS00648F
163. Zhang Z, Li B, Xie LS, et al. Metal-phenolic network-enabled lactic acid consumption reverses immunosuppressive tumor microenvironment for sonodynamic therapy. Acs Nano. 2021;15(10):16934–16945. doi:10.1021/acsnano.1c08026
164. Han ZH, Gao MJ, Wang ZH, Peng LC, Zhao YB, Sun L. pH/NIR-responsive nanocarriers based on mesoporous polydopamine encapsulated gold nanorods for drug delivery and thermo-chemotherapy. J Drug Deliv Sci Tec. 2022;75:103610. doi:10.1016/j.jddst.2022.103610
165. Zhang CT, Li J, Yang CX, et al. A pH-sensitive coordination polymer network-based nanoplatform for magnetic resonance imaging-guided cancer chemo-photothermal synergistic therapy. Nanomed-Nanotechnol. 2020;23:102071. doi:10.1016/j.nano.2019.102071
166. Liu P, Shi XY, Zhong SH, et al. Metal-phenolic networks for cancer theranostics. Biomater Sci. 2021;9(8):2825–2849. doi:10.1039/D0BM02064H
167. Dai Q, Geng HM, Yu Q, Hao JC, Cui JW. Polyphenol-based particles for theranostics. Theranostics. 2019;9(11):3170–3190. doi:10.7150/thno.31847
168. Fan JX, Zheng DW, Mei WW, et al. A metal-polyphenol network coated nanotheranostic system for metastatic tumor treatments. Small. 2017;13(48):1702714. doi:10.1002/smll.201702714
169. Du XC, Jia BQ, Wang WJ, et al. pH-switchable nanozyme cascade catalysis: a strategy for spatial-temporal modulation of pathological wound microenvironment to rescue stalled healing in diabetic ulcer. J Nanobiotechnol. 2022;20(1):12. doi:10.1186/s12951-021-01215-6
170. Ding XY, Li G, Zhang P, Jin E, Xiao CS, Chen XS. Injectable Self-healing hydrogel wound dressing with cysteine-specific on-demand dissolution property based on tandem dynamic covalent bonds. Adv Funct Mater. 2021;31(19):2011230. doi:10.1002/adfm.202011230
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.